Abstract
Purpose
The anti-epileptic drug vigabatrin is associated with reduction in light-adapted 30-Hz flicker electroretinogram (ERG) amplitude. Ophthalmological assessments, including ERGs, monitor retinal health during vigabatrin treatment. RETeval™ is a hand-held ERG device adapted for dilation-free ERG assessment. To evaluate the usefulness of RETeval™ for vigabatrin ERG assessment, we evaluated intra-visit reliability and clinical feasibility of RETeval™ ERG assessment in children under 3 years of age undergoing vigabatrin treatment.
Methods
In this prospective study, children underwent 30-Hz flicker ERG assessment with RETeval™ before routine vigabatrin monitoring including sedated-ERG using the Espion E2 Colour Dome. Intraclass correlation coefficient (ICC) statistics identified the degree of intra-visit reliability from two repeated measurements of the same participant within one testing session. The omega squared (ω2) statistic identified the level of association between RETeval™ and Espion light-adapted 30-Hz flicker responses.
Results
Nine children completed RETeval™ ERG testing. The intra-visit ICCs for the RETeval™ 30-Hz flicker amplitude (µV) were high: 0.81 (right eye) and 0.86 (left eye), while the implicit times (ms) were 0.79 (right eye) and 0.42 (left eye). The RETeval™ 30-Hz flicker amplitude was positively associated with the Espion 30-Hz flicker response (ω2 = 0.71). The Bland–Altman plot showed no bias in the mean difference of amplitudes between the two systems.
Conclusion
This is the first study to assess the utility of RETeval™ device in children under 3 years of age undergoing vigabatrin treatment. RETeval™ demonstrated high intra-visit reliability with responses consistent with the standard Espion ERG. RETeval™ may be beneficial for assessment of retinal toxicity in young children treated with vigabatrin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infantile Spasms (IS) affects 2–5 per 10,000 children [1,2,3]. IS presents as recurrent seizures, often leading to cognitive declines and premature mortality [4]. An effective anti-epileptic drug in young children is vigabatrin (SABRIL®, Lundbeck, Deerfield, IL) [5, 6]. Vigabatrin is associated with retinal toxicity causing bilateral concentric visual field loss [7, 8]. These visual field complications are correlated with other deficits including attenuation of the light-adapted (LA) 30-Hz flicker electroretinogram (ERG) [9].
Ophthalmological assessments, including ERGs, monitor retinal health during vigabatrin treatment. Currently, reliable paediatric visual-assessment techniques are limited [10]. ERG assessment following standards recommended by the International Society for Clinical Electrophysiology of Vision (ISCEV) [11] is typically an important tool for monitoring vigabatrin-associated effects in young children. This requires pupillary dilation and electrode placement in close proximity to the cornea [12]. Testing varies from centre to centre; some centres conduct testing while the child is awake, others use sedation of general anaesthesia to ensure compliance during testing. At the Hospital for Sick Children, ERGs are performed while children are sedated. The choice of sedation is to maintain compliance without the potential negative neurodevelopmental outcomes that have been associated with anaesthesia [13,14,15].
The RETeval™ system (LKC Technologies, Gaithersburg, MD, USA) is a non-invasive, hand-held ERG system not requiring pupillary dilation. RETeval™ uses skin electrodes and accommodates for changes in pupil diameter up to 6.5 mm by constantly altering the flash luminance (cd·s/m2) to stabilize retinal illuminance (Td·s) [16]. RETeval™ is useful in studies of retinal diseases and healthy children [17,18,19,20,21]. The current study aims to evaluate the clinical feasibility of using the RETeval™ device by assessing intra-visit reliability, association and agreement between RETeval™ and sedated ERG in awake children under 3 years undergoing vigabatrin treatment.
Methods
Research ethics approval
This study was approved by the Research Ethics Board (REB) at the Hospital for Sick Children and adhered to the guidelines of the tenets of the Declaration of Helsinki for clinical research involving human participants.
Participant recruitment
Patients were recruited during their scheduled Ophthalmology appointment, which includes the Espion (Espion E2 Color Dome, Diagnosys LLC) sedated ERG. Informed consent was signed for each patient by the parents and/or guardians.
Inclusion criteria were children ≤ 3 years of age who had been diagnosed with IS, and who were undergoing treatment with vigabatrin. Exclusion criteria were other known retinal diseases or medications other than vigabatrin known to affect the retina. Sex was not a determining factor for inclusion in this study.
RETeval™ ERG
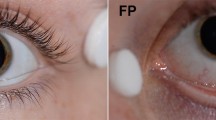
A graduate student (XJ) trained in the use of the device, recorded the LA 30-Hz flicker ERG protocol using the RETeval™. Children were placed into a comfortable supine position and wrapped in a blanket, while the skin underneath both eyes was gently scrubbed with Nuprep®. Recording electrodes were disposable RETeval™ sensor strips (LKC Technologies Inc., Gaithersburg, MD, USA) placed over the skin 2 mm below the lateral half of the lower eyelids of both eyes. The experimenter held the device in such a way that the hand-piece cusped around the eyes of the child (Fig. 1). A built-in automatic sensor tracked the pupils for imaging stabilization, and a built-in screen displayed the status of pupil tracking, such that testing could only begin once the pupil was detected by the system. Children had previously been exposed to ambient room lighting during the clinical intake and did not receive additional light adaptation. The test was conducted in each eye using a pre-set protocol (85 Td·s @ 28.3-Hz flicker with 848 Td background). The RETeval™, which housed the flickering light, was placed over one eye and testing always commenced with the right eye (OD) followed by the left eye (OS). Two repetitions were attempted on each eye to allow assessment of intra-visit reliability of the RETeval™ response. The device was repositioned following each trial while the electrodes remained on the skin, and the duration between two attempts was approximately 5–10 s. In the case of non-compliance or if the duration of the procedure was over 5 min, testing was discontinued.
RETeval™ hand-held ERG being operated on an infant under sedation. Real-time pupil tracking is shown on the screen of the device during testing. The LA 30-Hz flicker ERG waveforms are displayed on screen immediately following test completion. Repeated trials are shown as superimposed traces to provide instant appraisal of waveform reproducibility
Espion ERG
Following the RETeval™ ERG, the child was given mydriatic eye drops of 1% tropicamide (Mydriacyl®; Alcon Laboratories Inc., TX, USA) and 2.5% phenylephrine (Mydfrin®, Alcon Laboratories Inc, TX, USA) by the nurses to dilate the pupils. Chloral hydrate was administered orally for sedation (weight-dependent chloral hydrate dosage: 80 mg/kg up to 1 g maximum).
The ERG was performed using the Espion E2 Color Dome in accordance with ISCEV standards [11]. Following the administration of an eye drop topical anaesthetic, corneal electrodes (ERG-JET™, Fabrinal Eye Care, La Chaux-de-Fonds, Switzerland) were positioned over the cornea of each eye, and the ERG was conducted using Ganzfeld illumination.
Data analysis
Analysis of the amplitude of 30-Hz flicker ERG was the main concern of the current study. Vigabatrin ERG defects are associated with amplitude change and not implicit time [9, 12]. Implicit time data were evaluated for interest only. Intra-visit reliability of the RETeval™ response was evaluated from two repeated measurements of the same participant within one testing session. Analysis was conducted using the intraclass correlation coefficient (ICC) statistics (Statistical Package for Social Studies software, IBM, Chicago, IL). The association between the RETeval™ LA 30-Hz flicker ERG and the Espion ERG LA 30-Hz flicker responses was assessed with the partial omega squared (ω2) estimated in PROC GLM (SAS 9.4, Cary, NC); this statistical method accounted for multiple testing as data from all eyes were included in the analysis. The partial omega squared represents the proportion of shared variance between the two measures after controlling for the random subject effect. Agreement between RETeval™ and sedated ERG was evaluated by Bland–Altman test; for this analysis amplitudes were scaled using a scaling factor of 7.04; i.e. Espion ERG = RETeval ERG × 7.04. The scaling factor was determined through the linear regression of Espion ERG vs. RETeval ERG amplitude responses while setting the intercept at 0.
Results
Between November 2016 and March 2018, parents of twenty-nine children were approached for RETeval™ testing while waiting for sedation for their child’s impending Espion sedated-ERG; of these, nine (13.6 ± 6.7 months, range 6–27 months) completed the RETeval™ 30-Hz flicker ERG. The demographics data for these children are listed in Table 1. Seven of these nine children (77.8%) completed the intra-visit reliability assessments, with at least one eye having two repetitions of the 30-Hz flicker ERG response. For the twenty children who did not complete RETeval™ measurements, eighteen of them showed inadequate testing compliance due to excessive crying, removing of electrodes, and body movements. The remaining two children provided enough testing cooperation, however recordings were unsuccessful, as pupil detection by the device took longer than 5 min.
Intra-visit reliability
The intra-visit reliability of the RETeval™ 30-Hz flicker ERG response was assessed by the level of reproducibility between two repeated measurements within a single visit using ICC statistics. Seven right eyes from seven participants and five left eyes from five participants, which equated to 12 eyes from seven participants, provided two repetitions for intra-visit calculations. The intra-visit ICCs for the RETeval™ 30-Hz flicker ERG amplitude (µV) were 0.81 and 0.86 for the right and left eyes, respectively. The intra-visit ICCs for the 30-Hz flicker implicit time (ms) were 0.79 and 0.42 for the right and left eyes, respectively. Representative ERGs (Fig. 2a, b) from a 13-month old child show intra-visit reliability of the RETeval™ 30-Hz flicker response. The waveform morphology of the RETeval™ visually resembles the Espion ERG (Fig. 2c, d) within a span of 100 ms; the difference is in the response amplitude due to different types of electrodes used by the two systems (skin electrode vs. corneal electrode).
RETeval™ 30-Hz flicker ERG response in a 13-month child (ID 314) with Infantile Spasms (a = OD, b = OS). Espion ERG LA 30-Hz flicker response (c = OD, d = OS) of the same participant (ID 314) measured on the date of RETeval™ testing. Each waveform shown is the average ERG response from trial 1 and trial 2. The two superimposed same-session repeated waveforms demonstrate morphological similarity between the two RETeval™ responses and between RETeval™ and Espion ERG responses
Intra-visit reliability data are shown in Fig. 3; the values of the 30-Hz flicker responses on two trials are plotted against each other. Within this specific paediatric cohort, the flicker response amplitudes of both the Espion ERG and the RETeval™ ERG were not associated with change in age (Fig. 4). For the eyes with two repeated measurements, the average of the two trials was used for this calculation.
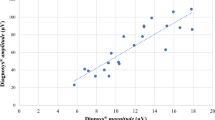
Clinical feasibility
The clinical feasibility of the RETeval™ 30-Hz flicker response was determined through comparisons with the Espion response. Nine children had at least one measurable RETeval™ recording. Thirteen eyes (n = 13) from nine children contributed to the calculation of cross-platform correlation. For the eyes with two repeated measurements, the average of the two trials was used for this calculation. The amplitude of the RETeval™ 30-Hz flicker response showed a positive association with the 30-Hz flicker response on Espion ERG (ω2 = 0.71, Fig. 5a). No correlation was found between the implicit times of the RETeval™ and Espion flicker ERGs (ω2 = 0, Fig. 5b). The Bland–Altman plot showed no bias in the mean difference of 30-Hz flicker amplitudes between the two ERG systems (Fig. 6); however, three eyes of three participants (ID316, 317, 325) showed larger differences in amplitudes between the two different recording devices. Both right and left eyes were used in this calculation, and for the eyes with two repeated measurements, the average of the two trials was used. One of these discrepancies (ID316) showed equivalently lower Espion ERG amplitude than the hand-held system, and two of these cases (ID317 and ID325) had lower RETeval™ ERG amplitudes than the Espion system. One of these participants (ID325) had a difference value outside of the limit of agreement (95% confidence interval).
Discussion
The aim of the present study was to investigate the reliability and clinical feasibility of the RETeval™ ERG for recording the 30-Hz flicker response in non-sedated children undergoing vigabatrin therapy.
Nine children completed RETeval™ assessment. Although low in view of the number of parents who had agreed to the procedure (N = 29), it was expected that children would be in an increased state of agitation as they were fasting in preparation for sedation. Two children cooperated initially with RETeval™, however, testing lasted longer than 5 min and was discontinued because of time constraints required to ensure the proper flow of clinical procedure.
Reliability
The intra-visit reliability established in the present study for the RETeval™ 30-Hz flicker protocol was high and comparable to previous reports using RETeval™ [20, 21]. Intra-visit reliability for the RETeval™ flicker ERG is strong in healthy adults between 20 and 24 years old (ICC = 0.92 for amplitude and 0.91 for implicit time) [20]. Concurrently, we collected RETeval™ data on a larger cohort between 11 months and 69 years (N = 92, median age = 20), and found an ICC of 0.82 for amplitudes and 0.53 for implicit time using the RETeval™ 30-Hz flicker response [21]. In the present cohort, the mean age was 13.6 months. The reductions in our reliability assessment, particularly the implicit time measures from the left eye, compared with other studies may be attributed to experimenter- and patient-dependent factors, including increased body movement of young children during recording, poor contact or adhesiveness of the skin electrodes and excessive shifting of the eyes causing loss of pupil detection. The waveform morphology of the RETeval™ flicker response is repeatable and moderately resembles the waveforms of the Espion ERG. The smaller amplitude signal of the RETeval™ skin electrode ERG is attributed to lower signal to noise ratio in comparison with the Espion ERG using corneal electrodes. An interesting aspect of waveform morphology is that consecutive RETeval™ traces (Fig. 2a, b) are identical and on the Espion system they are not (Fig. 2c, d). This is because RETeval™ uses a Fourier-based approach to record steady-state ERGs and the first eight harmonics are used to reconstruct the waveform [22]. In addition, the system’s auto-blink rejection excludes those data in the proximity of a blink. The Espion ERG acquires 100 ms sweeps with subsequent averaging. The consecutive Espion ERG flicker traces are not identical to each other as they are not periodic at the flicker frequency.
Clinical feasibility
Our study exhibited a strong positive correlation between the RETeval™ and the Espion ERG 30-Hz flicker amplitudes, and no correlation between the implicit time responses. The lack of correlations is expected as there is such a small range of values across participants. Clinical efficacy of the RETeval™ 30-Hz flicker response in adults is shown by reduction with known retinal diseases causing cone dysfunctions [23]. Likewise, in children with nystagmus and retinal dystrophy (5.6 ± 2.7 years), the RETeval™ 30-Hz flicker response amplitude is reduced compared with controls with nystagmus and no retinal dystrophy [17].
Although the correlation between the amplitudes is strong, the Bland–Altman analysis revealed interesting discrepancies. Three eyes of three participants showed large amplitude differences between RETeval™ and Espion ERG. In participant (ID316), the explanation is technical and not pathological; when the child was followed up 4 months later ERG amplitudes were within normal limits. In this participant, the upward rolling of the eye in Bell’s phenomenon may explain the result. In other cases (ID317 and ID325), when the RETeval™ ERG amplitudes were lower than the Espion ERG, head and eye movements during hand-held ERG testing may have resulted in less robust recordings and subsequent reductions in amplitude responses. Reports of cone-dominant ERG changes in infants and young children using the RETeval™ system are limited. To the best of our knowledge, the present study is the first that employs the RETeval™ 30-Hz flicker response to evaluate potential retinal defects in children < 3 years of age undergoing vigabatrin therapy for IS.
Limitations
There are several limitations for this study. The small sample size limits the generalizability and the power of these results. The age-expected normal range for the RETeval™ LA 30-Hz flicker amplitude is currently unknown for children younger than 3 years of age. Non-compliance, especially following fasting periods for the Espion sedated-ERG, reduced the tolerability of the RETeval™ flicker in children under 3 years of ages. Furthermore, the skin electrodes used by the RETeval™ system, designed for older children and adults, were sometimes difficult to be positioned at appropriate markers on the infant’s face. As a result, some children removed the electrodes from the skin underneath the eyelids.
Conclusion
This study was the first to demonstrate the reliability and feasibility of the RETeval™ LA 30-Hz flicker protocol in children under 3 years of ages undergoing vigabatrin treatment. Generally, RETeval™ 30-Hz flicker response was comparable to the Espion ERG. RETeval™ is a quick and sedation-free technique; therefore, it may prove to be a viable option for some children whose parents or physicians require more information on possible toxicity than might be provided by the fundus examination alone. RETeval™ may be useful for retinal assessment when the sedated ERG is contraindicated or when sedation is unavailable.
References
Riikonen R, Donner M (1979) Incidence and aetiology of infantile spasms from 1960 to 1976: a population study in Finland. Dev Med Child Neurol 21:333–343
Sidenvall R, Eeg-Olofsson O (1995) Epidemiology of infantile spasms in Sweden. Epilepsia 36:572–574
Rantala H, Putkonen T (1999) Occurrence, outcome, and prognostic factors of infantile spasms and Lennox-Gastaut syndrome. Epilepsia 40:286–289
Wong M, Trevathan E (2001) Infantile spasms. Pediatr Neurol 24:89–98
Chiron C, Dumas C, Jambaqué I et al (1997) Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy Res 26:389–395
Jones K, Go C, Boyd J et al (2015) Vigabatrin as first-line treatment for infantile spasms not related to tuberous sclerosis complex. Pediatr Neurol 53:141–145
Eke T, Talbot JF, Lawden MC (1997) Severe persistent visual field constriction associated with vigabatrin. Br Med J 314:180–181
Lawden MC, Eke T, Degg C et al (1999) Visual field defects associated with vigabatrin therapy. J Neurol Neurosurg Psychiatry 67:716–722
Harding GF, Wild JM, Robertson KA et al (2000) Separating the retinal electrophysiologic effects of vigabatrin: treatment versus field loss. Am J Ophthalmol 55:347–352
Good WV (2011) Measuring field loss in children administered vigabatrin: a problem in search of a solution. J AAPOS 15:411–412
McCulloch DL, Marmor MF, Brigell MG et al (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Westall CA, Wright T, Cortese F et al (2014) Vigabatrin retinal toxicity in children with infantile spasms: an observational cohort study. Neurology 83:2262–2268
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. BJA Br J Anaesth 105:i61–i68
Zhang H, Du L, Du Z et al (2015) Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. J Anesth 29:749–757
Davidson AJ, Becke K, De Graaff J et al (2015) Anesthesia and the developing brain: a way forward for clinical research. Paediatr Anaesth 25:447–452
Kato K, Kondo M, Sugimoto M et al (2015) Effect of pupil size on flicker ERGs recorded with RETeval system: new mydriasis-free full-field ERG system. Investig Ophthalmol Vis Sci 56:3684–3690
Grace SF, Lam BL, Feuer WJ et al (2017) Nonsedated handheld electroretinogram as a screening test of retinal dysfunction in pediatric patients with nystagmus. J AAPOS 21:384–388
Al-Otaibi H, Al-Otaibi MD, Khandekar R et al (2017) Validity, usefulness and cost of RET eval system for diabetic retinopathy screening. Transl Vis Sci Technol 6:3
Miura G, Nakamura Y, Sato E, Yamamoto S (2016) Effects of cataracts on flicker electroretinograms recorded with RETevalTM system: new mydriasis-free ERG device. BMC Ophthalmol 16:22
Asakawa K, Amino K, Iwase M et al (2017) New mydriasis-free electroretinogram recorded with skin electrodes in healthy subjects. Biomed Res Int 2017:1–7
Liu H, Ji X, Dhaliwal S et al (2018) Evaluation of light- and dark-adapted ERGs using a mydriasis-free, portable system: clinical classifications and normative data. Doc Ophthalmol 137:169–181
Davis CQ, Kraszewska O, Manning C (2017) Constant luminance (cd·s/m2) versus constant retinal illuminance (Td·s) stimulation in flicker ERGs. Doc Ophthalmol 134:75–87
Nakamura N, Fujinami K, Mizuno Y et al (2016) Evaluation of cone function by a handheld non-mydriatic flicker electroretinogram device. Clin Ophthalmol 10:1175–1185
Acknowledgements
This work was supported by the Vision Science Research program at the University of Toronto (Xiang Ji), and the SickKids Ophthalmology Research Fund (Dr. Carol Westall). LKC Technologies, Inc. provided the RETeval™ device and some sensor strips electrodes used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Statement of human rights
All research procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, X., McFarlane, M., Liu, H. et al. Hand-held, dilation-free, electroretinography in children under 3 years of age treated with vigabatrin. Doc Ophthalmol 138, 195–203 (2019). https://doi.org/10.1007/s10633-019-09684-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-019-09684-9