Abstract
Background
Sodium butyrate (NaB) is a short-chain fatty acid produced by intestinal microbial fermentation of dietary fiber, and has been shown to be effective in inhibiting ulcerative colitis (UC). However, how NaB regulates inflammation and oxidative stress in the pathogenesis of UC is not clear.
Aims
The purpose of this study was to use a dextran sulfate sodium salt (DSS)-induced murine colitis model, and determine the effects of NaB and the related molecular mechanisms.
Methods
Colitis model was induced in mice by administration of 2.5%(wt/vol) DSS. 0.1 M NaB in drinking water, or intraperitoneal injection of NaB (1 g/kg body weight) was given during the study period. In vivo imaging was performed to detect abdominal reactive oxygen species (ROS). Western blotting and RT-PCR were used to determine the levels of target signals.
Results
The results showed that NaB decreases the severity of colitis as determined by an improved survival rate, colon length, spleen weight, disease activity index (DAI), and histopathological changes. NaB reduced oxidative stress as determined by a reduction in abdominal ROS chemiluminescence signaling, inhibition of the accumulation of myeloperoxidase and malondialdehyde, and restoration of glutathione activity. NaB activated the COX-2/Nrf2/HO-1 pathway by increasing the expressions of COX-2, Nrf2, and HO-1 proteins. NaB inhibited the phosphorylation of NF-κB and activation of NLRP3 inflammasomes, and reduced the secretion of corresponding inflammatory factors. Furthermore, NaB promoted the occurrence of mitophagy via activating the expression of Pink1/Parkin.
Conclusions
In conclusion, our results indicate that NaB improves colitis by inhibiting oxidative stress and NF-κB/NLRP3 activation, which may be via COX-2/Nrf2/HO-1 activation and mitophagy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a chronic and recurrent form of inflammatory bowel disease (IBD). UC is associated with certain genetic factors, impaired intestinal barrier function, intestinal flora imbalance, and altered mucosal immune response [1]. Clinical manifestations of IBD include abdominal pain, weight loss, bloody diarrhea, and an increased risk of colorectal cancer (CRC) [2,3,4]. IBD is most common in Western countries, affecting more than 1 million people in the USA and more than 2.5 million in Europe. The number of persons with IBD is also increasing in Asia, South America, and the Middle East [5]. Currently, treatment of IBD is based on drugs including 5-aminosalicylic acid (5-ASA), glucocorticoids, immunomodulators, and immunotherapeutic agents; however, drug resistance and disease recurrence are common [6, 7].
Oxidative stress has long been known to be a pathogenic factor of UC [8]. Under normal circumstances, basal levels of reactive oxygen species (ROS) in the gut have bactericidal effects, and are involved in promoting intestinal stem cell proliferation to help to maintain gut homeostasis. However, dysregulation of antioxidant function under inflammatory conditions is accompanied by high levels of ROS, resulting in lipid peroxidation, protein dysfunction, and DNA mutations [9, 10]. In addition, ROS can act as a second messenger to activate intracellular nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways, and ultimately enhance the expression of various proinflammatory cytokines such as tumor necrosis factor (TNF)-α, and interleukin (IL)-1, IL-8, and IL-6 in intestinal epithelial cells [11, 12].
Nuclear factor erythrocyte 2-associated factor 2 (Nrf2) is a stress response transcription factor associated with a number of inflammatory mechanisms. Nrf2 can be activated by cyclooxygenase-2 (COX-2) and electrophilic oxo-derivatives (EFOX), and translocated to the nucleus during oxidative stress [13, 14], which induces the expression of many antioxidant and antiinflammatory genes. Heme oxygenase-1 (HO-1) is one of the downstream genes regulated by Nrf2, and plays a key role in defense against tissue inflammation and oxidative stress [15]. In vivo, Nrf2-knockout mice exposed to dextran sulfate sodium salt (DSS) exhibited more severe histopathological signs of colitis [16]. Conversely, promoting Nrf2 nuclear translocation and activating downstream antioxidant enzyme HO-1 expression can effectively alleviate the progression of colitis [17,18,19,20]. Thus, the antiinflammatory and antioxidant effects of the Nrf2 signaling pathway may provide a novel method to alleviate colitis.
The NLRP3 inflammasome is an innate, intracellular immune receptor. It is composed of the NLRP3 protein, the apoptosis-associated speck-like protein containing CARD (ASC), and pro-caspase-1 [21]. Many lines of evidence suggest that the NLRP3 inflammasome plays an important role in intestinal inflammation, such as promoting the secretion of intestinal inflammatory factors, triggering local immune responses, and initiating pyroptosis [22,23,24,25]. Activation of NLRP3 inflammasomes is induced by a variety of signals involving inflammation and oxidative stress, such as activation of NF-κB or transcription induction of NLRP3 itself, and signals that directly activate inflammasome assembly, including K+ outflow, increased calcium concentration in cytoplasm, ROS production, and mitochondrial damage [26,27,28].
Mitophagy is a process of degrading or removing damaged mitochondria that can clear excessive mitochondrial reactive oxygen species (mtROS) and mitochondrial DNA (mtDNA) produced during inflammation, and is mainly mediated by the Pink1/Parkin axis [29,30,31]. Nrf2 can also promote mitophagy through p62 and Pink1, thus maintaining mitochondrial homeostasis [32,33,34]. Guo et al. found that andrographolide-induced mitophagy prevented the occurrence of CRC by inhibiting NLRP3 inflammasomes [35]. Liu et al. found that ginsenoside Rd-induced mitophagy improved colitis by inhibiting the activation of NLRP3 inflammasomes [36]. However, more research is needed on the relations between mitophagy and NLRP3 inflammasomes in colitis.
A large number of studies have shown persons with colitis and those at high risk of developing colitis have reduced dietary fiber intake, or an imbalance of intestinal flora and their metabolites short-chain fatty acids (SCFAs) [37]. Sodium butyrate (NaB) is a product of dietary fiber fermented by intestinal flora, and has been shown to reduce the risk of colitis and may have therapeutic potential [38]. The effects of NaB mainly including immune regulation, improvement of intestinal flora, enhancement of intestinal barrier function, and promotion of intestinal epithelial cell proliferation [39,40,41]. Currently, the effect of NaB on NLRP3 inflammasomes is controversial. In a co-culture model of intestinal inflammation, the co-culture of gallic acid and butyric acid produced an antiinflammatory effect by inhibiting NLRP3 inflammasomes [42]. However, another study showed that the addition of inulin, which can be fermented into butyric acid, aggravated colitis in mice [43]. These differences may be due to the influence of other SCFAs in the experiment, different methods of establishing a model of colitis, and the complexity of NLRP3 inflammasome activation. Moreover, recent studies have demonstrated that different administration methods and concentrations of NaB may play a role in inhibiting or promoting inflammation in colitis [44, 45].
Thus, the purpose of this study was to establish a DDS-induced murine colitis model with symptoms similar to the active phase of human UC and determine the effects of oral and intraperitoneal administration of NaB on colitis symptoms, and explore the possible mechanisms.
Material and Methods
Preparation of DSS, NaB, and 5-ASA Solution
DSS powder (25 g) (Meilunbio, China) was dissolved in 1000 mL autoclaved tap water by making 1000 mL of 2.5% (wt/vol) DSS solution at a time. Then, 11 g NaB powder (Sigma Aldrich, USA) was dissolved in 10,000 mL autoclaved tap water or 2.5% DSS solution to form 0.1 M NaB solution. The solution can be stored at 4 °C for 4 weeks until use. Each mouse drank about 5 mL of solution per day. In addition, NaB and 5-ASA (Sigma Aldrich, USA) were dissolved in normal saline, respectively, and prepared into 66.7 mg/mL and 20 mg/mL solutions for intraperitoneal injection and gavage. Mice were given 150 μL solution per 20 g body weight by intraperitoneal injection or gavage.
Experimental Protocol
Six-week-old male C57BL/6 mice were purchased from the Medical Laboratory Animal Center of Guangdong Province (Foshan, China). All mice were acclimated for 1 week in a specific-pathogen-free (SPF) environment under the following conditions: temperature (22–25 °C), humidity (55–70%), and 12 h light–dark cycle. Standard chow and tap water were accessed ad libitum before intervention. After 1-week acclimation, mice were divided randomly into five groups: (1) control group (n = 8); (2) DSS model group (n = 12); (3) NaB (i.p.), DSS + 500 mg/kg NaB (intraperitoneal injection) group (n = 8); (4) NaB(p.o.), DSS + 0.1 M NaB (orally) group (n = 8); (5) 5-ASA, DSS + 150 mg/kg 5-ASA group (n = 8). The experimental period was divided into two periods: pretreatment (days 1–12) and treatment (days 13–23). During the pretreatment period, mice in NaB(p.o.) group and NaB(i.p.) group were given 0.1 M NaB in drinking water and an intraperitoneal injection of 500 mg/kg NaB solution once a day, respectively. The other mice normally drank the autoclaved tap water and received an intraperitoneal injection of normal saline daily. During the treatment period, the induction of acute DSS colitis was consistent as indicated previously. In mice except the control group, colitis was induced by 2.5% DSS for 8 d, followed by pure water for 3 d. In the NaB treatment groups, NaB was added to water or intraperitoneally injected as NaB solution daily. The positive drug control group was given 150 mg/mL 5-ASA intragastric administration every day after modeling.
Body Weight and Disease Activity Assessment
During the experiment, the diet, activity, spirit, and hair condition of mice in each group were observed daily, and the fecal traits of mice were also observed. The mice were weighed and recorded daily, and the survival rate and disease activity index (DAI) of mice were recorded daily after the modeling began. The calculation criteria were as follows: (1) body mass: 0 = no change; 1 = decrease (1–5%) of plasma weight; 2 = decrease (5–10%) in plasma weight; 3 = decrease (10–20%) in plasma weight; 4 = decrease the original body mass by > 20%. (2) Fecal traits: 0 = normal (strip-shaped stool); 1 = loose stool (unformed, rotten stool); 2 = not attached to the anal paste, scattered stool; 4 = dilute stool, can be attached to the anus watery stool. (3) Gastrointestinal bleeding: 0 = normal; 2 = fecal occult blood; 4 = gross blood stool. The DAI score is (1) + (2) + (3) combined/3.
In Vivo Imaging of Intestinal ROS
On the eighth day after DSS modeling, three mice from each group were randomly selected for intestinal ROS imaging. Before imaging, the abdomen of selected mice was shaved or chemically depilated, and 2 mg/mL L-012 (Fujifilm Wako Chemicals, Osaka, Japan) was prepared with fresh solution dissolved in normal saline.
Intraperitoneal injection was given at a dose of 20 mg/kg by 0.1 mL/10 g body weight. Mice were anesthetized with isoflurane (2%) and placed in a supine position of three mice at a time in a light-tight chamber of the multimode Small Animal Imaging System (FX Pro, Kodak, USA), with chard-coupled device precooling if necessary. Images were captured at 1–5 min after the injection of L-012 and the exposure time was adjusted based on the image quality. After taking photos, the mice were put back into the cage.
Sample Collection
Before the end of the experiment, animals were fasted for 12 h and sacrificed by cervical dislocation under anesthesia of 4% chloral hydrate (0.1 mL/kg). All blood was collected, part of which was used to prepare serum. The mouse was positioned supine on a surgical pad to perform a ventral midline incision with sterile surgical forceps and scissors, and the spleen was removed and weighed. The entire colon (from ileocecal junction to anus) was resected to determine the weight and length of the colon. The entire colon was placed in a sterile 10 cm Petri dish, and, using a steel gavage needle attached to a syringe, the stools were flushed out with sterile phosphate-buffered saline (PBS). Fragments of the colon were cut from the proximal and distal ends of the colon (0.5 cm long), and were quickly frozen in liquid nitrogen and stored at −80 °C for later analysis (RNA and protein separation).
Histopathological Examination
Samples of the colons and spleens were prepared for histological analysis. Samples were fixed in 10% formalin, paraffin embedded, sliced, and stained with hematoxylin and eosin (HE), and then observed under a light microscope. Histological scoring was performed in a blinded way by a pathologist (H.A.L.). Focally increased numbers of inflammatory cells in the lamina propria were scored as 1, confluence of inflammatory cells extending into the submucosa as 2, and transmural extension of the infiltrate as 3. For tissue damage, discrete lymphoepithelial lesions were scored as 1, mucosal erosions as 2, and extensive mucosal damage and/or extension through deeper structures of the bowel wall as 3. The two equally weighted subscores (cell infiltration and tissue damage) were added, and the combined histological colitis severity score ranged from 0 to 6 [59].
Reduced GSH Assay
Colon tissues were lysed with glutathione buffer and the supernatant was collected. The glutathione (GSH) content in the supernatant was assessed by the reduced glutathione assay kit (Solarbio, China, no. BC1175) according to the manufacturer’s instructions. The absorbance was measured at 450 nm (A450). The concentration of GSH was determined using a standard GSH calibration curve and was related to the quality of the tissue used.
MDA Measurement
MDA content in colon tissues was measured by the malondialdehyde kit (Wanleibio, China, no. WLA048a) according to the manufacturer’s instructions. In brief, colon tissues were lysed with MDA extraction solution. Lysates were centrifuged for 10 min at 8000g at 4 °C. The supernatant was used to measure the MDA content. The protein concentration (Cprot) was measured by the BCA Protein Assay Kit (Beyotime, no. P0012). The absorbances at 532 nm (A532) were measured by a microplate photometer.
MDA content in tissues (nM/mg prot) = (determination OD value − control OD value)/(standard OD value − blank OD value) × standard concentration (10 nM/mL) ÷ protein concentration of the sample to be tested (mg prot/mL).
Assessment of Myeloperoxidase (MPO)
MPO activity was assessed on inflammatory colonic mucosa using an MPO assay kit to quantify neutrophil infiltration, as described in the manufacturer’s instructions (Njjcbio, China, no. A044-1–1). Results showed active units per milligram of tissue.
RT-PCR
Total RNA was extracted from colon tissues by homogenizing in Trizol lysis (AG, Guangzhou, China) and used for synthesis of cDNA with PrimeScript RT reagent Kit (Takara, Tokyo, Japan) according to the manufacturer's instructions. The cDNA concentration and purity were determined using a NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, Wilmington, USA). Then, mRNA expression levels of the following genes were analyzed with quantitative real-time polymerase chain reaction (qPCR) assays using SYBR Premix Ex TaqTM II (AG, Guangzhou, China) in a real-time PCR detection system (LightCycler 96, Roche, USA). The primers of forward and reverse sequences are presented in Table 1. Data were calibrated to β-actin and calculated via the method of ΔΔCt, to determine the expression level of mRNA equaled to 2 −ΔΔCt.
Western Blot Analysis
The colon tissues were homogenized in RIPA lysis (BestBio biotechnology company, Shanghai, China) containing 1% PMSF (Sigma-Aldrich Company, USA) and 0.8% phosphatase inhibitors (KeyGEN biotechnology company, Nanjing, China). The homogenates were centrifuged at 14,000 r/min for 15 min at 4 °C to yield the supernatants considered as protein extracts for further analysis. After quantification of the protein concentration, the protein extracts were loaded and separated by electrophoresis on 10% and 12% SDS-PAGE gels, followed by transfer to polyvinylidene fluoride (PVDF) membranes at 100 V for 20–60 min. Then, the membranes were blocked with rapid and efficient Western sealing fluid (Genefist, Oxford, UK) for 10 min, incubated with primary antibody [Cell Signaling Technology, USA; ABclonal biotechnology company, Hubei, China; Abcam (Shanghai), China; Novus Biologicals (Shanghai), China] at 4 °C overnight and probed with secondary antibody (ABclonal biotechnology company, Hubei, China) conjugated with horseradish peroxidase for 2 h. Finally, immunoblots were visualized using enhanced chemiluminescence (Millipore, Burlington, America) and a Tanon-S200 imaging system (Shanghai, China). Bands were quantified and normalized to the loading control using ImageJ software (National Institutes of Health).
Statistical Analysis
Statistical analyses of different sets of experiments were performed using Prism 7 (GraphPad Software, Inc., La Jolla, CA). A two-tailed t-test or Mann–Whitney test were used in a single variable with two-group comparison, a one-way ANOVA with Tukey post test or Kruskal–Wallis test was used in single-variable comparisons with more than two groups, and a two-way ANOVA with Bonferroni post test was used for multivariable analyses. Differences with P < 0.05 were regarded as statistically significant.
Results
NaB Alleviates Symptoms of DSS-Induced Colitis
A summary of the experimental design is shown in Fig. 1A. During the pretreatment periods, there was no significant difference in the body weight of each group, indicating that daily intraperitoneal injection (i.p.) or oral administration of NaB had no effect on the body weight of mice (Fig. 1B). Weight loss after DSS treatment is an important clinical indicator suggesting the occurrence of colitis [46]. After DSS treatment, mice in the model group showed lethargy, decreased activity, and significant weight loss beginning on the fifth day of modeling. Although NaB (i.p. and oral) treatment alleviated weight loss to some extent (compared with 1 d before DSS treatment), there was no statistically significant difference compared with the model group (Fig. 1B, C).
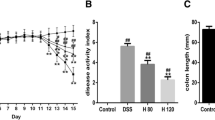
NaB administration alleviates symptoms of DSS-induced colitis. A A summary of the experimental design. B Body weight change during the whole experimental period. C Body weight lost (%) during the treatment period. D Mice survival rate. E DAI. F–G Photographs of the colon and colon length. H Spleen weight. All values are presented as the mean ± standard deviation. One-way ANOVA was used for statistical analysis (n = 8, 6, 7, 7, and 7 respectively). *P < 0.05, **P < 0.01, ***P < 0.001 versus model group. DSS dextran sulfate sodium, NaB sodium butyrate, i.p. intraperitoneal injection, p.o. oral
DSS can significantly increase mortality and DAI in mice, and these are two indicators for evaluating the severity of inflammation [46]. The criteria for the DAI were based on previous studies, and included weight change, stool consistency, and rectal bleeding [47]. Compared with mice in the model group, mice treated with NaB (i.p. and oral) and 5-ASA showed significant improvements in mortality rate and DAI score over the course of disease progression (Fig. 1D, E). In addition, 5-ASA and NaB (i.p. and oral) treatment significantly decreased colon shortening (Fig. 1F, G) and splenomegaly (Fig. 1H). There was no difference in the effects of administration of NaB i.p. and NaB orally with respect to the above indices. Taken together, these results suggest that both oral and intraperitoneal injection of NaB can effectively improve DSS-induced colitis in mice.
NaB Reduces Histopathological Changes in DSS-Induced Colitis
To further confirm the effect of NaB on inflammation and tissue damage, colon and spleen tissue sections of each group were stained and examined histologically. The tissue structure and cytology of the colon and spleen in the control group were normal without obvious injury or inflammation. Mice in the model group had significant changes in the pathological morphology of the colon and spleen, including severe diffuse destruction of the upper colonic cortex and infiltration of a large number of mononuclear and multinuclear cells in the colonic mucosa and submucosa, mostly at the distal end of the colon. The spleen was infiltrated with inflammatory cells, and the boundary between red pulp and white pulp was not clear. Inflammatory cell infiltration and tissue damage were significantly reduced in the colon and spleen of mice treated with NaB (i.p. and oral) and 5-ASA compared with mice in the model group (Fig. 2A–C). These results suggest that NaB can protect against DSS-induced histological changes and inflammatory cell infiltration.
NaB Attenuates Oxidative Stress and Activates the COX-2/Nrf2/HO-1 Pathway in DSS-Induced Colitis
Severe oxidative stress can occur in the model mice, and it has been reported that oxidative stress can lead to the release of multiple inflammatory cytokines in the intestinal tract resulting in damage of intestinal epithelial cells, as well as excessive activation of programmed cell death [47].To evaluate the extent of intestinal oxidative stress, a chemofluorescent probe L-012, which detects ROS and reactive nitrogen species (RNS), was used for imaging in vivo. Compared with control mice, increased L-012 luminescence intensity was observed in the abdomen of model mice, while a less intense abdominal signal was observed in mice treated with NaB (i.p. and oral) and 5-ASA (Fig. 3A). This result is consistent with our detection of myeloperoxidase (MPO), which is an enzyme present in neutrophils that produces ROS during inflammation (Fig. 3B).
NaB attenuates oxidative stress and activates the COX-2/Nrf2/HO-1 pathway in DSS-induced colitis. A Anesthetized mouse imaged 1–5 min post L-012 injection (20 mg/kg, i.p.). B–D The levels of MPO, MDA, and GSH in colon tissue. All values are presented as the mean ± standard deviation. E–G COX-2, Nrf2, and HO-1 mRNA levels in colon tissue. H COX-2, Nrf2, and HO-1 protein levels in colon tissue were detected by Western blotting. I–K Quantification of colonic relative expression of COX-2/β-actin, Nrf2/β-actin, and HO-1/β-actin. One-way ANOVA was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001 versus model group. DSS dextran sulfate sodium, NaB sodium butyrate, i.p. intraperitoneal injection, p.o. oral
In addition, there is increasing evidence that lipid peroxidation contributes to the progression of colitis, and may induce ferroptosis [48]. Therefore, intestinal lipid peroxide malondialdehyde (MDA) and reduced glutathione(GSH) levels were measured to determine colonic redox status. Compared with the control group, DSS treatment resulted in a significant increase in lipid peroxidation products and depletion of the antioxidant GSH. NaB (i.p. and oral) and 5-ASA treatment significantly reduced lipid peroxidation products and restored GSH levels (Fig. 3C, D).
Nrf2/HO-1 is a classic antiinflammatory and antioxidant signaling pathway, which is closely related to the development of colitis. COX-2 is well known for its role in inflammation, but some studies have also shown that COX-2 exerts antioxidant and antiinflammatory effects by activating Nrf2 [48]. To further explore the possible molecular mechanism of NaB in the treatment of colitis, we evaluated the effects of NaB on the COX-2/Nrf2/HO-1 signaling pathway. Polymerase chain reaction (PCR) studies showed that NaB (i.p. and oral) and 5-ASA treatment effectively inhibited the DSS-induced upregulation of COX-2, and restored Nrf2/HO-1 expression to a nearly normal level (Fig. 3E–G). Western blotting showed that NaB (i.p. and oral) and 5-ASA treatment did not inhibit the upregulation of COX-2 at the protein level, but effectively restored the expression of Nrf2 and HO-1(Fig. 3H–K). These results may suggest a dual role of COX-2 in inflammation, and demonstrated that NaB can activate COX-2/Nrf2/HO-1 signaling to attenuate oxidative stress in DSS-induced colitis.
NaB Reduces Inflammatory Cytokine Secretion and NF-κB/NLRP3 Activation
NF-κB is a key transcription factor for inflammation-related genes, and has been shown to exacerbate colitis by activating NLRP3 inflammasomes [26]. To investigate whether NaB plays an antiinflammatory role by inhibiting NF-κB/NLRP3, PCR and Western blotting were used to detect related inflammatory factors and signaling pathways. PCR showed that NF-κB expression was induced by DSS treatment, followed by significantly higher expression of IL-6 and TNF-α (Fig. 4A–C). The expressions of NLRP3 inflammasome-related genes (NLRP3, IL-8, IL-β) were also significantly increased in the model group (Fig. 4D–F). Treatment with NaB (i.p. and oral) and 5-ASA effectively inhibited the expression of NF-κB/NLRP3 and inflammatory factors. Western blotting was used to determine the expression levels of proteins involved in NF-κB signaling (NF-κB p65 and p-NF-κB p65) and NLRP3 inflammasomes (Caspase-1, cleaved Caspase-1, and NLRP3) in DSS-induced colitis. The results showed that NF-κB/NLRP3 was upregulated in the model group and inhibited in the NaB (i.p. and oral) and 5-ASA treatment groups, including phosphorylation of NF-κB p65 and formation of cleaved Caspase-1 (Fig. 4G–J). Thus, it may be speculated that NaB attenuates DSS-induced colitis by inhibiting the activation of NF-κB/NLRP3 in the colon.
NaB administration reduces inflammatory cytokine secretion and NF-κB/NLRP3 activation. A–F IL-6, TNF-α, NF-κB, IL-1β, IL-18, and NLRP3 mRNA levels in colon tissue. G NF-κB p65, p-NF-κB p65, NLRP3, Caspase-1, and cleaved-Caspase-1 protein levels in colon tissue were detected by Western blotting. H–J Quantification of the colonic relative expression of p-NF-κB p65/total NF-κBp65, NLRP3/β-actin, and cleaved Caspase-1/β-actin. One-way ANOVA was used for statistical analysis (n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus model group. DSS dextran sulfate sodium, NaB sodium butyrate, i.p. intraperitoneal injection, p.o. oral
NaB Promotes Pink1/Parkin-Driven Mitophagy in DSS-Induced Colitis
Nrf2 has been shown to be involved in mitophagy, which can reduce the accumulation of mtROS and mtDNA and thus negatively regulate NLRP3 inflammasomes [28]. However, this process has been rarely examined in colitis.
The current view is that the Pink1/Parkin signaling pathway mediates mitophagy. The damaged mitochondrial surface accumulates large amounts of Pink1/Parkin, which mediates mitochondrial ubiquitination. Then, p62 protein binds to both ubiquitin and LC3 II to target autophagosomes and promote the clearance of ubiquitinated mitochondria. In this study, the expressions of mitophagy-related proteins p62, LC3 II, Pink1, and Parkin were determined by PCR and Western blotting. NaB (i.p. and oral) and 5-ASA treatment effectively upregulated Pink1 and Parkin expression compared with the model group (Fig. 5A–E). Compared with the model group, LC3 II was increased and p62 was decreased in the NaB (i.p. and oral) and 5-ASA treatment groups, indicating that autophagy flow was activated (Fig. 5C, F, G). Overall, these results suggest that Pink1/Parkin-driven mitophagy is involved in the NaB attenuation of DSS-induced colitis.
NaB promotes Pink1/Parkin-driven mitophagy in DSS-induced colitis. A, B Pink1 and Parkin mRNA levels in colon tissue. C Pink1, Parkin, p62, and LC3 II protein levels in colon tissue were detected by Western blotting. D–G Quantification of the colonic relative expression of Pink1/β-actin, Parkin/β-actin, p62/β-actin, and LC3 II/β-actin. One-way ANOVA was used for statistical analysis (n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus model group. DSS dextran sulfate sodium, NaB sodium butyrate, i.p. intraperitoneal injection, p.o. oral
Discussion
IBD is a chronic immune-mediated disease of the gastrointestinal tract, and includes two subtypes, UC and Crohn's disease (CD). Although their common features are persistent diarrhea and abdominal pain, important differences exist with regard to disease pathology. Inflammation with UC is mainly observed in the mucosa and submucosa of the colon, whereas inflammation in CD is usually transmural and discontinuous, and may affect any part of the gastrointestinal tract. In Western countries, UC is more prevalent than CD (120–200/100,000 versus 50–200/100,000 persons, respectively), and occurs at an older age [49]. Administration of drinking with 2–5% DSS has proved to be a reliable method for establishing a colitis model in mice. Treated mice develop characteristic symptoms including weight loss, bloody diarrhea, colon ulcerations, epithelial cell loss, and neutrophil infiltration, which are similar to some features of flares in human UC [50]. Therefore, 2.5% DSS in drinking water was used in this study to establish a murine colitis model to examine the effects of NaB intervention and explore potential mechanisms.
Weight loss, increased DAI, shortened colon length, and histopathological changes are the most common indicators of UC severity. In addition, mortality was also used to describe the severity of colonic inflammation in this study. However, there were no statistical differences in body weight loss among all DSS-treated groups. This may be due to the increased mortality of mice in the model group at the later stage of the experiment, which led to the withdrawal of low-body weight mice from the experiment, and thus they were not included in the statistical analysis. NaB alleviated the severity of colitis as determined by an improved survival rate, increased colon length, spleen weight, and DAI, and a decrease in pathological histopathological changes. Overall, these results indicate that NaB has potential for the prevention and treatment of colitis. It is worth noting that in previous studies on the effect of NaB on colitis in mice, NaB was mostly administered by drinking water or with feed, and there were inconsistencies among the study results. It has been suggested that NaB produced by fermentable fiber in the intestinal tract increases IL-10 inhibition and congenital immune deficiency (Tlr5-knockout) colitis in murine models by activating inflammasome NLRP3 [50]. Berndt et al. showed that oral administration of 4% wt/vol NaB exacerbated DSS-induced colitis in a mouse model, while intraperitoneal injection of 1 g/kg/d NaB reduced the inflammatory response, possibly because oral administration of NaB upregulated the production of inflammatory factors in lamina dendritic cells, leading to an intensified response to DSS [44]. Vieira et al. showed that oral NaB at a lower dose (0.5% wt/vol) can alleviate acute colitis [45]. In this study, we found that oral administration of 0.1 M NaB or intraperitoneal injection of 1 g/kg NaB can relieve colitis in mice. The results are broadly consistent with those of previous studies, because 0.1 M is approximately equal to 1.1% wt/vol. It has been suggested that oral administration of a low concentration of NaB can achieve treatment of colitis and reduce adverse reactions. However, given the short half-life of oral NaB, it may be more practical to use more stable NaB pro-drugs for treatment [51].
Targeting proinflammatory and pro-oxidative pathways is regarded as an effective way to treat colitis. In this study, we demonstrated the effects of NaB on oxidative stress, inflammatory cytokine secretion, and related inflammatory signaling pathways. To detect the protective effect of NaB on oxidative stress, we used L-012 for imaging mice in vivo. In the human oral cavity and blood, and in the mouse abdominal cavity, L-012 reacts with ROS produced by activated neutrophils to produce strong chemiluminescence, and DSS can significantly enhance this luminescence signal in the colitis model [52]. The results showed that NaB effectively reduced the ROS luminescence signal in the abdomen of mice with colitis, although this result was not statistically analyzed because there were only three cases in each group and individual differences were large. NaB also reduced the accumulation of MPO and MDA, suggesting that it could reduce the occurrence of neutrophil-induced oxidative stress and lipid peroxidation.
To verify the role of NaB in the inflammatory response, we examined the expression of NF-κB and NLRP3 inflammasome-related proteins and inflammatory factors. NF-κB is one of the key factors of intestinal mucosal immune regulation. In colitis patients, the amount of activated NF-κB is significantly associated with the severity of intestinal inflammation [53]. p65, also known as RelA, is a key subunit of NF-κB. Phosphorylation and nuclear transport of p65 are markers of NF-κB activation. Our results showed that NaB treatment effectively inhibited the phosphorylation of NF-κB-p65, and significantly inhibited the expression of related inflammatory factors TNF-α and IL-6. Activation of NF-κ B and accumulation of ROS have been reported to enhance NLRP3 inflammasome expression in colitis. Activation of NLRP3 inflammasomes in turn recruit and activate Caspase-1. Activated Caspase-1 cleaves pro-IL-1β and pro-IL-18 into their biologically active forms, thereby promoting the progression of colitis [22,23,24,25]. Our study suggests that NaB plays an antiinflammatory role by inhibiting NLRP3 inflammasome activation, a finding that has been controversial in previous studies. However, our results showed that NaB can decrease NLRP3 and cleaved Caspase-1 expression in DSS-induced colitis, which is consistent with the palliative effect of NaB on colitis. In addition, based on our results and previous studies [54,55,56], we suggest that NaB inhibits the expression of inflammatory cytokines and activates NF-κB/NL RP 3, which may act on macrophages and intestinal epithelial cells.
Nrf2 is known to promote antiinflammatory processes by coordinating recruitment of inflammatory cells and regulating gene expression through antioxidant response elements (ARE). Nrf2 is normally bound to the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), and subsequently ubiquitinated [57]. During inflammation, Nrf2 migrates to the nucleus and modulates HO-1 expression to counteract oxidative stress [15]. To explore the possible mechanisms whereby NaB attenuates inflammation and oxidative stress, we examined Nrf2-related signaling pathways. The results showed that NaB significantly restored Nrf2/HO-1 expression in mice with colitis. COX-2 is often described as a proinflammatory mediator, but was upregulated by NaB in our study. However, considering that COX-2-dependent electrophilic oxygen derivative (EFOX) molecules have been shown to act as antiinflammatory mediators by activating Nrf2-dependent antioxidant response elements [13, 14], we believe that COX-2 can be cautiously considered to be one of the signals regulating Nrf2 in colitis.
Mitophagy is necessary to maintain mitochondrial homeostasis and eliminate intracellular excess ROS. Several studies have shown that mitophagy removes NLRP3 inflammasome activators, such as intracellular DAMP, mtROS, and mtDNA, thereby reducing inflammasome activation and IL-18 secretion. In contrast, NLRP3 inflammasomes are overactivated when Pink1/Parkin deficiency leads to the accumulation of damaged mitochondria [31, 58]. We found that NaB promoted the expression of Pink1 and Parkin, key proteins of mitophagy. Changes of LC3 II and p62 also confirmed the occurrence of mitophagy. In general, NaB may regulate antioxidant signals and mitophagy via NrF2 to alleviate inflammation in colitis, which has not been described previously (Fig. 6).
Conclusions
The results of this study demonstrated that NaB (0.1 M orally) and NaB (1 g/kg i.p.) exhibit a protective and preventive effect against DSS-induced colitis by inhibiting oxidative stress and NF-κB/NLRP3 activation, which may be via the COX-2/Nrf2/HO-1 pathway and mitophagy. Overall, our results suggest that NaB may be a promising candidate to treat UC.
Abbreviations
- NaB:
-
Sodium butyrate
- DSS:
-
Sulfate sodium salt
- UC:
-
Ulcerative colitis
- IBD:
-
Inflammatory bowel disease
- CRC:
-
Colorectal cancer
- SCFAs:
-
Short-chain fatty acids
- p.o.:
-
Orally
- i.p.:
-
Intraperitoneally
- DAI:
-
Disease activity index
- ROS:
-
Reactive oxygen species
- mtROS:
-
Mitochondrial reactive oxygen species
- mtDNA:
-
Mitochondrial DNA
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2:
-
Nuclear factor erythrocyte 2-associated factor 2
- COX-2:
-
Cyclooxygenase-2
- EFOX:
-
Electrophilic oxo-derivatives
- HO-1:
-
Heme oxygenase-1
- MPO:
-
Myeloperoxidase
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- ASC:
-
Apoptosis-associated speck-like protein containing CARD
References
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J. Ulcerative colitis. Lancet (London, England) 2017;389:1756–1770.
Feuerstein JD, Moss AC, Farraye FA. Ulcerative colitis. Mayo Clin Proc 2019;94:1357–1373.
Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol 2020;64:51–60.
D.E. O'Sullivan, R.L. Sutherland, S. Town, et al., Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clinical gastroenterology and hepatology 2021.
Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature reviews. Gastroenterology & hepatology 2015;12:720–727.
Cottone M, Renna S, Modesto I, Orlando A. Is 5-ASA still the treatment of choice for ulcerative colitis? Curr Drug Targets 2011;12:1396–1405.
Baker DE, Kane S. The short- and long-term safety of 5-aminosalicylate products in the treatment of ulcerative colitis. Reviews in gastroenterological disorders 2004;4:86–91.
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016;2016:4350965.
Wang Z, Li S, Cao Y, Tian X, Zeng R, Liao D, Cao D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid Med Cell Longev 2016;2016:9875298.
Aviello G, Knaus UG. ROS in gastrointestinal inflammation: rescue or sabotage? Brit J Pharmacol 2017;174:1704–1718.
Masi A, Fortini P, Krokidis MG et al. Increased levels of 5’,8-Cyclopurine DNA lesions in inflammatory bowel diseases. Redox Biol 2020;34:101562.
Morgan MJ, Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 2011;21:103–115.
Groeger AL, Cipollina C, Cole MP et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol 2010;6:433–441.
Chen C. COX-2’s new role in inflammation. Nat Chem Biol 2010;6:401–402.
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and molecular life sciences: CMLS 2016;73:3221–3247.
Khor TO, Huang M, Kwon KH, Chan JY, Reddy BS, Kong A. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 2006;66:11580–11584.
Y. Chen, P. Zhang, W. Chen, G. Chen, Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett, 2020, 225.
Li J, Wang H, Zheng Z et al. Mkp-1 cross-talks with Nrf2/Ho-1 pathway protecting against intestinal inflammation. Free radical biology & medicine 2018;124:541–549.
Wang R, Luo Y, Lu Y, Wang D, Wang T, Pu W, Wang Y. Maggot extracts alleviate inflammation and oxidative stress in acute experimental colitis via the activation of Nrf2. Oxid Med Cell Longev 2019;2019:4703253.
Mei Y, Wang Z, Zhang Y et al. FA-97, a new synthetic caffeic acid phenethyl ester derivative, ameliorates DSS-induced colitis against oxidative stress by activating Nrf2/HO-1 pathway. Front Immunol 2019;10:2969.
Próchnicki T, Latz E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab 2017;26:71–93.
Bauer C, Duewell P, Mayer C et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010;59:1192–1199.
Huber S, Gagliani N, Zenewicz LA et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012;491:259–263.
Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti T. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010;32:379–391.
Zhen Y, Zhang H. NLRP3 Inflammasome and inflammatory bowel disease. Front Immunol 2019;10:276.
M. Cornut, E. Bourdonnay, T. Henry, Transcriptional regulation of inflammasomes. Int J Mol Sci, 2020, 21.
Shimada K, Crother TR, Karlin J et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012;36:401–414.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221–225.
Priault M, Salin B, Schaeffer J, Vallette FM, di Rago J, Martinou J. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ 2005;12:1613–1621.
Matsuda N, Sato S, Shiba K et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. The Journal of cell biology 2010;189:211–221.
Y. Xu, J. Shen, Z. Ran, Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy, 2020, 16.
Xiao L, Xu X, Zhang F et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 2017;11:297–311.
Ryoo I, Kwak M. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol Appl Pharm 2018;359:24–33.
Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free radical biology & medicine 2015;88:179–188.
Guo W, Sun Y, Liu W et al. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy 2014;10:972–985.
Liu C, Wang J, Yang Y et al. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem Pharmacol 2018;155:366–379.
Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? Ebiomedicine 2021;66:103293.
D. Parada Venegas, M.K. De la Fuente, G. Landskron, et al., Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol, 2019, 10, 277.
X. Dou, N. Gao, D. Yan, A. Shan, Sodium butyrate alleviates mouse colitis by regulating gut microbiota dysbiosis. Animals, 2020, 10.
Park J, Kotani T, Konno T et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. Plos One 2016, 11, e156334
Silva JPB, Navegantes-Lima KC, Oliveira ALB et al. Protective mechanisms of butyrate on inflammatory bowel diseas. Curr Pharm Design 2018;24:4154–4166.
Luzardo-Ocampo I, Loarca-Piña G, Gonzalez De Mejia E. Gallic and butyric acids modulated NLRP3 inflammasome markers in a co-culture model of intestinal inflammation. Food and chemical toxicology 2020;146:111835.
Singh V, Yeoh BS, Walker RE et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019;68:1801–1812.
Berndt BE, Zhang M, Owyang SY et al. Butyrate increases IL-23 production by stimulated dendritic cells. American journal of physiology. Gastrointestinal and liver physiology 2012;303:G1384–G1392.
Vieira ELM, Leonel AJ, Sad AP et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. The Journal of nutritional biochemistry 2012;23:430–436.
J.J. Kim, M.S. Shajib, M.M. Manocha, W.I. Khan, Investigating intestinal inflammation in DSS-induced model of IBD. Journal of visualized experiments : JoVE, 2012.
Liu Y, Tang B, Wang F et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020;10:5225–5241.
Xu M, Tao J, Yang Y et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis 2020;11:86.
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–1794.
Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 2017;12:1295–1309.
Donovan JD, Bauer L, Fahey GC, Lee Y. In vitro digestion and fermentation of microencapsulated tributyrin for the delivery of butyrate. J Food Sci 2017;82:1491–1499.
Asghar MN, Emani R, Alam C et al. In vivo imaging of reactive oxygen and nitrogen species in murine colitis. Inflamm Bowel Dis 2014;20:1435–1447.
Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med 2008;263:591–596.
Jiang L, Wang J, Liu Z, Jiang A et al. Sodium butyrate alleviates lipopolysaccharide-induced inflammatory responses by down-regulation of NF-κB, NLRP3 signaling pathway, and activating histone acetylation in bovine macrophages. Front Vet Sci 2020;5(7):579674.
Li X, Wang C, Zhu J et al. Sodium butyrate ameliorates oxidative stress-induced intestinal epithelium barrier injury and mitochondrial damage through AMPK-mitophagy pathway. Oxid Med Cell Longev 2022;2022(29):3745135.
Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 2018;49:190–205.
Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochimica et biophysica acta. Molecular basis of disease 2017;1863:585–597.
Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol 2020;11:591803.
Bauer C, Duewell P, Mayer C et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010;1192–1199.
Funding
This study was supported by National Natural Science Foundation of China (No. 81773429) and Natural Science Foundation of Guangdong Province, China (No. 2022A1515011631).
Author information
Authors and Affiliations
Contributions
Study idea, design and manuscript preparation: S.S., Z.B., and Q.Z. Data collection and interpretation: Z.B., Y.Q., and Y.Y. Experiment performance and data analysis: Z.B., X.S., L.L., H.L., and L.M. Final correction and review: W.L, L.Z., and S.S.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Animal welfare statement
All animal experiments followed the Guidelines for the Care and Use of Laboratory Animals, and the study was approved by Ethics Committee of Southern Medical University and Guangdong Medical Laboratory Animal Center (C202011-1). The animal studies are done according to ethical procedures and experimental protocols were approved in accordance with the Southern Medical University Experimental Animal Ethics Committee (L2019074). All experiments also complied with the Chinese regulations regarding animal experimentation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bian, Z., Zhang, Q., Qin, Y. et al. Sodium Butyrate Inhibits Oxidative Stress and NF-κB/NLRP3 Activation in Dextran Sulfate Sodium Salt-Induced Colitis in Mice with Involvement of the Nrf2 Signaling Pathway and Mitophagy. Dig Dis Sci 68, 2981–2996 (2023). https://doi.org/10.1007/s10620-023-07845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07845-0