Abstract
Background /Aims
The serum pepsinogen (PG) assay is used to screen subjects at high risk for gastric cancer. Currently, there are few studies on the PG levels for the detection of Helicobacter pylori infection. This study aimed to determine the PG assay findings for detecting ongoing infection.
Methods
Asymptomatic subjects who underwent a 13C-urea breath test (13C-UBT) on the day of gastroscopy and serum assay for cancer screening were included. Subjects with a recent intake of acid suppressants or antibiotics, gastrectomy, or renal failure were excluded. H. pylori infection was defined as a positive 13C-UBT result.
Results
Among the 500 included subjects, 167 (33.4%) had current infection. The serum PG II levels of > 12.95 ng/mL (area under the curve [AUC] = 0.930, sensitivity 86.5%, specificity 90.7%) and PG I/II ratios of < 4.35 (AUC = 0.875, sensitivity 86.8%, specificity 79.6%) were related to infection. The PG I/II ratios were inversely correlated with age (r = -0.160, p = 0.039). The cutoff values of PG I/II ratios were lower in older subjects aged ≥ 50 years (< 4.05; AUC = 0.875, sensitivity 80.7%, specificity 88.2%) than in younger subjects aged < 50 years (< 4.35; AUC = 0.873, sensitivity 77.4%, specificity 88.9%).
Conclusions
Serum PG II levels > 12.95 ng/mL and PG I/II ratios < 4.35 suggest ongoing infection in asymptomatic subjects; therefore, H. pylori confirmation tests (i.e., 13C-UBT) should be considered under these conditions. Stricter criteria are required in older subjects aged ≥ 50 years (PG I/II ratio < 4.05) to detect ongoing infection than younger subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diagnosing and treating Helicobacter pylori are important to prevent gastric cancer [1]. Invasive diagnostic methods using endoscopic biopsies often show false negative findings due to the uneven distribution of bacteria in the stomach [2], whereas serology tests often show false positive test findings [3]. Among these H. pylori tests, the urea breath test (UBT) and monoclonal stool antigen test show the highest sensitivities (97.7–100% and 96.0–100%) and specificities (97.9–100% and 97.0–100%) for diagnosing ongoing infection [4].
Serum pepsinogen (PG) and anti-H. pylori immunoglobulin G (IgG) are used to screen subjects at high-risk subjects for gastric cancer [5]. The PG I level decreases with gastric corpus atrophy; therefore, low PG I levels and low PG I/II ratios are useful for detecting subjects with a high risk for intestinal-type gastric cancer [6]. On the other hand, high PG II levels are useful in East Asia for detecting subjects at high risk for diffuse-type gastric cancer [7, 8]. Compared to the studies on detecting high-risk gastric cancer subjects [5,6,7,8,9], there are few studies on serum PG assay findings for detecting ongoing H. pylori infection. A study showed that PG II levels of ≥ 12 ng/mL or PG I/II ratios of ≤ 4.0 are useful for detecting infection, based on Giemsa staining findings of 283 subjects [9]. Another study reported that PG II levels of ≥ 10.25 ug/L indicate ongoing infection, based on the serology test results of 940 seropositive subjects and 1182 seronegative subjects [10]. Either serology test or Giemsa staining was used to confirm ongoing infection, but neither the UBT nor the stool antigen test has been used as a confirmatory test in any study.
In this study, we hypothesized that a PG level or a PG I/II ratio indicating ongoing infection in asymptomatic subjects would help diagnose and treat H. pylori infection through the timely recommendation and performance of confirmatory tests (i.e., UBT, stool antigen test). We tried to determine the cutoff values of serum PG levels for detecting ongoing H. pylori infection in asymptomatic subjects based on 13C-UBT findings. Furthermore, we attempted to establish the significant variables related to PG assay findings in asymptomatic subjects.
Methods
Study Subjects
This was a single-center, cross-sectional study based on data from asymptomatic Koreans who underwent gastric cancer screening tests between September 2012 and December 2020. Subjects were included when 13C-UBT was performed on the same day as the gastroscopy and serum assays. Subjects with abnormal serum creatinine levels, previous gastrectomy, or recent intake of certain drugs (i.e., antibiotics, acid suppressants) were excluded. This study was approved by the Institutional Review Board of Konkuk University Medical Center (2021–02-021), and all subjects signed informed consent forms before the tests.
Serum Assays
Venous blood was sampled before gastroscopy after 12 h of fasting. Serum PG levels were measured using a latex-enhanced turbidimetric immunoassay (HBi, Anyang, Korea), as described previously [11]. Gastric corpus atrophy was diagnosed if the serum PG I level was ≤ 70 ng/mL and PG I/II ratio was ≤ 3.0. For serology assay, Chorus H. pylori IgG assay (DIESSE Diagnostica Senese, Siena, Italy), which showed an acceptable accuracy (sensitivity 100%, specificity 75.4%) in Koreans, was used for the serology tests [12].
Upper Gastrointestinal Endoscopy
Endoscopic examination was performed using either GIF-H290/-H260 (Olympus, Tokyo, Japan) or EG-2990i (Pentax, Tokyo, Japan) endoscopes. Based on the Kyoto classification scoring system for gastritis [13], the background mucosa of the stomach was scored from 0 to 8. Atrophy (A) was scored as 0 (no atrophy or limited atrophy confined to the antrum), 1 (closed-type atrophy with a visible atrophic border in the lesser curvature side of the body on the retroflexed view), or 2 (open-type atrophy invading the cardia and/or fundus). Intestinal metaplasia (IM) was scored as 0 (none), 1 (limited IM confined to the antrum), or 2 (extensive IM invading the body). Hypertrophic rugae (H) and nodular gastritis (N) were scored as either 0 (absent) or 1 (present). Diffuse redness (DR) in the corpus was scored as 0 (none), 1 (mild), or 2 (severe).
Confirmation of H. pylori Infection Status
13C-UBT was performed with the aid of the 13C-UBT (POCone®, Otsuka Electronics Co., Ltd.) as described in our previous study [14]. A cutoff value of ≥ 2.5% was considered the gold standard test for diagnosing H. pylori infection in this study. Past infection was diagnosed if the non-infected subject had a history of eradication, gastric corpus atrophy (PG I ≤ 70 ng/mL and PG I/II ≤ 3.0), H. pylori-related changes on gastroscopy (xanthoma, advanced atrophic gastritis over the angle [A1 or A2], or metaplastic gastritis [IM1 or IM2]), or abnormal gastric biopsy findings suggesting past infection (i.e., atrophy, intestinal metaplasia, xanthoma). To exclude subjects with unintended eradication, H. pylori-naive subjects were diagnosed only when the regular arrangement of collecting venules was observed in the angle in subjects with negative test findings in both the invasive and noninvasive H. pylori test findings [15].
Statistical Analysis
Differences between the infected and non-infected subjects were analyzed using the t-test and Chi-square test for continuous and categorical variables. For variables with asymmetrical distribution, Kruskal–Wallis test and Fisher’s exact test were used for continuous and categorical variables, respectively. Differences between the H. pylori-naive, infected, and past-infected subjects were analyzed by ANOVA with Bonferroni correction for continuous variables and Chi-square test with Bonferroni correction for categorical variables. Categorical variables were presented as number and proportion (%) of the subjects. Continuous variables with symmetrical distribution were presented as mean ± standard deviation, and those with asymmetrical distribution were presented as median with minimum and maximum values. Furthermore, logistic regression analysis was done to verify significant variables related to ongoing H. pylori infection. Findings were presented as odds ratio (OR) with 95% confidence intervals (CIs). To determine cutoff values for detecting ongoing infection, receiver operating characteristic (ROC) curve analysis was performed. Area under the curve (AUC) was provided with sensitivity, specificity, and standard error (SE). Correlation analysis was done to verity the links between significant variables and serum assay findings. Findings were presented as Pearson correlation coefficient (r) values. PASW statistics version 24.0 (SPSS Inc., Chicago, IL, USA) was used for analysis, and p-values below 0.05 were considered significant.
Results
Differences Between H. pylori-Infected and Non-infected Subjects
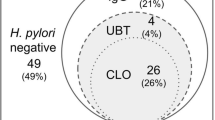
Among the 500 asymptomatic subjects, 167 (33.5%) showed positive 13C-UBT findings, whereas 333 (66.5%) showed negative 13C-UBT findings (Fig. 1). The mean age (45.8 ± 11.6 vs. 41.8 ± 11.1, p < 0.001) and proportion of diabetes mellitus patients (8.4% vs. 3.9%, p = 0.037) differed between the 167 infected and 333 non-infected subjects (Table 1).
The infected subjects had higher mean serum PG I levels (73.5 ± 26.6 ng/mL vs. 51.6 ± 20.4 ng/mL, p < 0.001) and PG II levels (21.8 ± 9.5 ng/mL vs. 9.9 ± 5.7 ng/mL, p < 0.001) compared to the non-infected subjects. Furthermore, the mean PG I/II ratio of the infected group was lower than that of the non-infected group (3.6 ± 1.2 vs. 5.7 ± 1.4, p < 0.001). Gastric corpus atrophy (PG I ≤ 70 ng/mL and PG I/II ≤ 3.0) was more common in the infected subjects than in the non-infected subjects (17.4 vs. 3.6%, p < 0.001).
Differences Between H. pylori-Naive, -Infected, and Past-Infected Subjects
Among the 333 non-infected subjects, 70 had a history of infection. Specifically, 17 had eradication history, 12 had gastric corpus atrophy, and 11 had intestinal metaplasia and/or atrophy based on the biopsied gastric specimens. Meanwhile, xanthoma, advanced atrophic gastritis (A1 or A2), or metaplastic gastritis (IM1 or IM2) were observed during gastroscopy in the remaining 30 subjects.
The subjects with past infection were older (52.4 ± 9.8 years) than the H. pylori-infected (45.5 ± 11.7 years, p < 0.001) and -naive (39.1 ± 9.6 years, p < 0.001) subjects. There were no differences in the proportion of males between the past-infected (68.6%), infected (65.1%), and naive (65.1%) subjects (p = 0.853). The mean PG I and PG II levels of the H. pylori-naive group (50.8 ± 17.2 ng/mL and 9.1 ± 4.1 ng/mL) were lower than those of the infected (73.2 ± 26.7 ng/mL and 21.7 ± 9.6 ng/mL, all p < 0.001) and past-infected (54.8 ± 29.7 ng/mL and 12.7 ± 9.3 ng/mL, all p < 0.001) groups. The mean PG I/II ratio of the H. pylori-naive group (5.9 ± 1.3) was higher than that of the infected (3.7 ± 1.2, p < 0.001) and past-infected (4.9 ± 1.6, p < 0.001) groups. Gastric corpus atrophy was observed in 17.1% (12/70) of the past-infected and 17.4% (29/167) of the infected groups. Meanwhile, it was not observed in the H. pylori-naive group (0/263, p < 0.001).
Independent Variables Related to Ongoing Infection
H. pylori infection was associated with high serum PG I levels (OR 1.044, 95% CIs 1.033—1.055, p < 0.001), high PG II levels (OR 1.302, 95% CIs 1.241–1.367, p < 0.001), and low PG I/II ratios (OR 0.285, 95% CIs 0.227–0.358, p < 0.001). It was also associated with age (OR 1.031, 95% CIs 1.014–1.049, p < 0.001) and diabetes mellitus (OR 0.444, 95% CIs 0.204–0.968, p = 0.041) on univariate analysis (Table 2). On multivariate analysis, only serum PG II level (OR 1.400, 95% CIs 1.307–1.500, p < 0.001) and PG I/II ratio (OR 0.289, 95% CIs 0.230–0.364, p < 0.001) were the independent risk factors for ongoing infection.
Cutoff Values for Detecting Ongoing Infection
The cutoff value of PG II level for detecting ongoing H. pylori infection was above 12.95 ng/mL (AUC = 0.930, SE = 0.012, sensitivity 86.5%, specificity 90.7%), as shown in Fig. 2a. Moreover, the cutoff value of the PG I/II ratio was below 4.35 (AUC = 0.875, SE = 0.017, sensitivity 86.8%, specificity 79.6%), as shown in Fig. 2b.
Receiver operating characteristic (ROC) curves for determining the cutoff values for ongoing H. pylori infection A The cutoff value for serum PG II level for detecting ongoing infection was > 12.95 ng/mL (area under the curve [AUC] = 0.930, standard error [SE] = 0.012, sensitivity 86.5%, specificity 90.7%). B The cutoff value of the PG I/II ratio was < 4.35 (AUC = 0.875, SE = 0.017, sensitivity 86.8%, specificity 79.6%)
When the 70 subjects with past infection were excluded, the cutoff values for the PG II level and PG I/II ratio were > 10.15 ng/mL (AUC = 0.951, SE = 0.011, sensitivity 97.6%, specificity 76.4%; Supplementary Fig. 1A) and < 4.45 (AUC = 0.910, SE = 0.016, sensitivity 80.2%, specificity 89.7%; Supplementary Fig. 1B), respectively. In comparison, when the 263 naive subjects were excluded, the cutoff values were > 12.95 ng/mL (AUC = 0.854, SE = 0.030, sensitivity 85.6%, specificity 77.8%; Supplementary Fig. 2A) and < 4.15 (AUC = 0.750, SE = 0.038, sensitivity 73.1%, specificity 73.6%; Supplementary Fig. 2B), respectively.
H. pylori-Infected Subjects with Low PG II Levels (≤ 12.95 ng/mL) and High PG I/II Ratios (≥ 4.35)
Among the 167 infected subjects, 124 showed a high serum PG II level (> 12.95 ng/mL) and a low PG I/II ratio of < 4.35, satisfying both of the cutoff values found in this study (Table 3). The other 19 subjects only satisfied the cutoff for the PG II level, while another ten subjects only satisfied the cutoff for the PG I/II ratio. The remaining 14 subjects showed a low PG II level of ≤ 12.95 ng/mL and a high PG I/II ratio of ≥ 4.35. The sensitivity was 91.6% (153/167) when using either PG II level > 12.95 ng/mL or PG I/II ratio < 4.35 for detecting ongoing infection. When using both cutoff values, the sensitivity was 74.3% (124/167).
Non-infected Subjects with High Serum PG II Levels (> 12.95 ng/mL) and Low PG I/II Ratios (< 4.35)
Among the 333 non-infected subjects, 273 showed a low serum PG II level (≤ 12.95 ng/mL) and a high PG I/II ratio (≥ 4.35), satisfying the cutoff values found in this study (Table 3). The other 16 subjects satisfied only high PG I/II ratios of ≥ 4.35, and another 25 subjects satisfied only low PG II levels of ≤ 12.95 ng/mL. The remaining 19 subjects showed a high PG II level (> 12.95 ng/mL) and a low PG I/II ratio (< 4.35). The specificity was 94.3% (314/333) when using either PG II level ≤ 12.95 ng/mL or PG I/II ratio ≥ 4.35 for detecting subjects without ongoing infection. When using both cutoff values, the specificity was 82.0% (273/333).
Cutoff Values According to the Age
There was a significant difference in the age between the 19 non-infected subjects who did not satisfy any of the cutoff values and the 273 non-infected subjects who satisfied both cutoff values (46.2 ± 9.8 vs. 40.1 ± 10.7, p = 0.032). Furthermore, an inverse correlation was found between age and serum PG I/II ratios (r =−0.160, p = 0.039, Fig. 3a), whereas no correlation was found between age and serum PG II levels (r = 0.083, p = 0.288, Fig. 3b).
Scatter plots with Pearson’s correlation coefficient (r) values to verify links between age and significant serum assay findings found by multivariate analysis A An inverse correlation was observed between the age and PG I/II ratio (r =−0.160, p = 0.039). B There was no correlation between the age and PG II levels (r = 0.083, p = 0.288)
Old age was also related to a high infection rate as described earlier in the results. Based on ROC curve analysis (Supplementary Fig. 3), the cutoff value of age for the lack of past infection was below 50.5 years (AUC = 0.706, SE = 0.029, sensitivity 65.7%, specificity 77.7%). In old subjects aged ≥ 50 years (n = 150), the cutoff value of PG I/II ratio for detecting ongoing infection was < 4.05 (AUC = 0.875, SE = 0.032, sensitivity 80.7%, specificity 88.2%; Fig. 4a). In comparison, the cutoff value of PG I/II ratio was < 4.35 (AUC = 0.873, SE = 0.021, sensitivity 77.4%, specificity 88.9%; Fig. 4b) in young subjects aged < 50 years (n = 350).
ROC curves for determining the cutoff values for ongoing infection in old (≥ 50 years old) and young (< 50 years old) subjects A In older subjects aged ≥ 50 years (n = 150), the cutoff value of PG I/II ratio was < 4.05 (AUC = 0.875, SE = 0.032, sensitivity 80.7%, specificity 88.2%). B In younger subjects aged < 50 years (n = 350), the cutoff value of PG I/II ratio was < 4.35 (AUC = 0.873, SE = 0.021, sensitivity 77.4%, specificity 88.9%)
Discussion
In this study, we found that high serum PG II levels (> 12.95 ng/mL) and low PG I/II ratios (< 4.35) are useful in detecting H. pylori infection in asymptomatic Koreans. Using these criteria, only 5.7% (19/333) of the non-infected subjects and 8.4% (14/167) of the infected subjects showed discrepancies with the 13C-UBT findings. Interestingly, lower PG I/II ratios were useful in detecting ongoing infection in older subjects aged ≥ 50 years (< 4.05) and in subjects with past infection (< 4.15) than in younger subjects aged < 50 years (< 4.35) and in H. pylori-naive subjects (< 4.45). There was an inverse correlation between age and serum PG I/II ratios, which seems to be associated with higher rates of past infection rates in the older population than in the younger population. These findings can be explained by previous studies that showed that PG levels change more dramatically in younger age because non-atrophic stomachs are more common in younger populations than in older populations [16, 17]. Therefore, older subjects and those with past infection required stricter criteria (lower PG I/II ratios) for detecting ongoing infection. Serum PG I/II ratio of < 4.35 was less accurate for detecting ongoing infection in elderly subjects aged ≥ 50 years and those with past infection.
The cutoff value of serum PG II level for detecting H. pylori infection was above 12.95 ng/mL in this study. East Asian studies show higher mean PG II levels than those from other countries, because the PG II levels increase when a patient is infected by a toxic H. pylori strain [18, 19]. Furthermore, the PG II levels are higher when latex agglutination methods are used for measurement instead of chemiluminescent magnetic particle immunoassay [20]. In their study, a PG II level of > 12 ng/mL or PG I/II ratio < 4.0 suggests infection when PG levels are measured by latex agglutination methods, while it is > 10 ng/mL (or PG I/II ratio < 5.0) when using chemiluminescent magnetic particle immunoassay. Another Japanese study showed that a PG II level of ≥ 12 ng/mL or a PG I/II ratio of ≤ 4.5 were suggestive of ongoing infection, based on the serological findings of 4,160 seropositive subjects and 323 seronegative subjects [21].
Among all serum PG assay findings, PG II level was mostly correlated with ongoing infection in our study, followed by the PG I/II ratio. Both PG I and PG II levels increase after H. pylori infection [22], with more prominent changes in the latter [23]. The PG I level varies among infected patients because it decreases with the progression of intestinal metaplasia and atrophy toward the corpus [24, 25]. Therefore, there is a rapid decrease in the serum PG II levels after eradication, followed by slow increases in the PG I levels and PG I/II ratios [26, 27]. Since the PG I/II ratios require a longer time than the PG II levels to reflect the infection status [28], the PG I/II ratios and PG I levels are used for detecting gastric cancers in subjects with past or chronic infection rather than those with ongoing infection [29].
There are limitations to our study. The Chorus H. pylori IgG testing was not performed in all included subjects at our center. Nonetheless, we could draw conclusions because 13C-UBT was considered as the gold standard for ongoing infection in this study. 13C-UBT is more accurate at diagnosing H. pylori infection than serology tests [4]; therefore, we found higher accuracy (sensitivity 91.6%, specificity 94.3%) compared to those of previous studies that used serology tests for detecting ongoing infection (sensitivity 71.6–95.1%, specificity 70.1–73.7%) [11, 21, 22].
In conclusion, a high serum PG II level (> 12.95 ng/mL) or a low serum PG I/II ratio (< 4.35) is suggestive of ongoing infection in asymptomatic subjects, associated with an excellent sensitivity of 91.6% and a specificity of 94.3%. H. pylori confirmation tests (i.e., UBT) should be performed under these conditions. Furthermore, in older subjects ≥ 50 years old, a low cutoff value of < 4.05 would be useful for PG I/II ratios to detect ongoing infection. Similarly, stricter criteria (PG I/II ratios < 4.15) would help detect ongoing infection in subjects with past infection.
References
Liou JM, Malfertheiner P, Lee YC, et al. Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM). Screening and eradication of Helicobacter pylori for gastric cancer prevention the Taipei global consensus. Gut 2020;69:2093–2112.
Kim JH, Lee SY, Lee SP, et al. The histologic detection of Helicobacter pylori in seropositive subjects is affected by pathology and secretory ability of the stomach. Helicobacter 2018;23:e12480.
Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, et al. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis 2019;38:55–66.
Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev 2018;3:CD012080.
Kwak MS, Chung GE, Chung SJ, Kang SJ, Yang JI, Kim JS. Predicting the development of gastric neoplasms in a healthcare cohort by combining Helicobacter pylori antibodies and serum pepsinogen: a 5-year longitudinal study. Gastroenterol Res Pract 2018;2018:8796165.
Cho JH, Jeon SR, Kim HG, Jin SY, Park S. The serum pepsinogen levels for risk assessment of gastric neoplasms: new proposal from a case-control study in Korea. Medicine (Baltimore) 2017;96:e7603.
Baek SM, Kim N, Kwon YJ, et al. Role of serum pepsinogen II and Helicobacter pylori status in the detection of diffuse-type early gastric cancer in young individuals in South Korea. Gut Liver 2020;14:439–449.
Ito M, Yoshihara M, Takata S, et al. Serum screening for detection of high-risk group for early-stage diffuse type gastric cancer in Japanese. J Gastroenterol Hepatol 2012;27:598–602.
Kiyohira K, Yoshihara M, Ito M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentration as a marker of Helicobacter pylori infection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol 2003;38:332–338.
He CY, Sun L, Gong Y, Xu Q, Dong N, Yuan Y. Serum pepsinogen II: a neglected but useful biomarker to differentiate between diseased and normal stomachs. J Gastroenterol Hepatol 2011;26:1039–1046.
Kwon H, Lee SY, Kim JH, et al. ABC classification is less useful for older Koreans born before 1960. Gut Liver 2019;13:522–530.
Lee SY, Moon HW, Hur M, Yun YM. Validation of western Helicobacter pylori IgG antibody assays in Korean adults. J Med Microbiol 2015;64:513–518.
Kim GH. Endoscopic findings of Kyoto classification of gastritis. Korean J Helicobacter Up Gastrointest Res 2019;19:88–93.
Kim JY, Lee SY, Kim JH, Sung IK, Park HS. Efficacy and safety of twice a day, bismuth-containing quadruple therapy using high-dose tetracycline and metronidazole for second-line Helicobacter pylori eradication. Helicobacter 2020;25:e12683.
Lee SY. Helicobacter pylori-negative gastric cancer. Korean J Helicobacter Up Gastrointest Res 2021;21:10–21.
Cho JH, Jeon SR, Jin SY, Park S. Analysis of factors associated with recovery of the serum pepsinogen ratio after Helicobacter pylori eradication: a long-term follow-up study in Korea. Scand J Gastroenterol 2019;54:1306–1314.
Haj-sheykholeslami A, Rakhshani N, Amirzargar A, Rafiee R, Shahidi SM, Nikbin B, Khosravi F, Massarrat S. Serum pepsinogen I, pepsinogen II, and gastrin 17 in relatives of gastric cancer patients: comparative study with type and severity of gastritis. Clin Gastroenterol Hepatol 2008;6:174–179.
Yuan L, Zhao JB, Zhou YL, et al. Type I and type II Helicobacter pylori infection status and their impact on gastrin and pepsinogen level in a gastric cancer prevalent area. World J Gastroenterol 2020;26:3673–3685.
Di Mario F, Moussa AM, Cavallaro LG, et al. Clinical usefulness of serum pepsinogen II in the management of Helicobacter pylori infection. Digestion 2004;70:167–172.
Kikuchi S, Kato M, Mabe K, et al. Optimal criteria and diagnostic ability of serum pepsinogen values for Helicobacter pylori infection. J Epidemiol 2019;29:147–154.
Kitamura Y, Yoshihara M, Ito M, et al. Diagnosis of Helicobacter pylori-induced gastritis by serum pepsinogen levels. J Gastroenterol Hepatol 2015;30:1473–1477.
Lorente S, Doiz O, Trinidad Serrano M, Castillo J, Lanas A. Helicobacter pylori stimulates pepsinogen secretion from isolated human peptic cells. Gut 2002;50:13–18.
Yu H, Liu Y, Jiang S, et al. Serum pepsinogen II levels are doubled with Helicobacter pylori infection in an asymptomatic population of 40,383 Chinese subjects. Medicine (Baltimore) 2021;100:e26562.
Cheng HC, Tsai YC, Yang HB, et al. The corpus-predominant gastritis index can be an early and reversible marker to identify the gastric cancer risk of Helicobacter pylori-infected nonulcer dyspepsia. Helicobacter 2017;22:e12385.
Iijima K, Sekine H, Koike T, Imatani A, Ohara S, Shimosegawa T. Serum pepsinogen concentrations as a measure of gastric acid secretion in Helicobacter pylori-negative and -positive Japanese subjects. J Gastroenterol 2005;40:938–944.
Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, et al. Pepsinogen II can be a potential surrogate marker of morphological changes in corpus before and after H. pylori eradication. Biomed Res Int 2014;2014:481607.
Gatta L, Di Mario F, Vaira D, et al. Quantification of serum levels of pepsinogens and gastrin to assess eradication of Helicobacter pylori. Clin Gastroenterol Hepatol 2011;9:440–442.
Ohkusa T, Miwa H, Nomura T, et al. Improvement in serum pepsinogens and gastrin in long-term monitoring after eradication of Helicobacter pylori: comparison with H. pylori-negative patients. Aliment Pharmacol Ther 2004;20:25–32.
Choi HS, Lee SY, Kim JH, et al. Combining the serum pepsinogen level and Helicobacter pylori antibody test for predicting the histology of gastric neoplasm. J Dig Dis 2014;15:293–298.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2016R1D1A1B02008937).
Author information
Authors and Affiliations
Contributions
Jong Hyeon Jeong and Sun-Young Lee carried out data curation, analysis, investigation, and writing. Sun-Young Lee was responsible for conceptualization, acquisition of funding, and project administration. All authors took part in methodology, resources, software, validation, and approval of final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No benefits have been received or will be received from a commercial party related to the content of this study.
Ethical approval
This study was approved by the Institutional Review Board of Konkuk University Medical Center (2021–02-021), and all subjects signed informed consent forms before the tests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Jeong, J.H., Lee, SY., Kim, J.H. et al. Useful Serum Pepsinogen Levels for Detecting Ongoing Helicobacter pylori Infection in Asymptomatic Subjects: A Cross-Sectional Study Based on 13C-urea Breath Test Findings. Dig Dis Sci 67, 5602–5609 (2022). https://doi.org/10.1007/s10620-022-07471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07471-2