Abstract
Background
Recent investigations have proposed the potential role of gamma-aminobutyric acid (GABA) in regulating motility and immunity of the gastrointestinal system.
Aims
We aimed to investigate the anti-inflammatory effects of ivermectin (IVM) through GABAB receptors following acetic acid-induced colitis in rats.
Methods
In a controlled experimental study, we enrolled 78 male Wistar rats (13 groups; 6 rats/group). After colitis induction using acetic acid (4%), IVM, baclofen (a standard GABAB agonist) or the combination of both agents was delivered to rats orally (by gavage), with the same dosage continued for 5 days. The control group received the vehicle, and prednisolone (a standard anti-inflammatory agent) was administered in a separate group as the positive control. Colon samples were collected on the sixth day for histopathological evaluations and measurement of myeloperoxidase (MPO) activity, TNF-α levels, and p-NF-ĸB p65, COX-2 and iNOS expression levels.
Results
The greatest recovery was found after administering IVM 0.5, baclofen 0.5, or IVM 0.2 + baclofen 0.2 mg/kg/day (ulcer index [UI] = 1.4 ± 0.4, 1.7 ± 0.6, and 1.4 ± 0.3, respectively; p < 0.001 vs. the control [UI = 6.5 ± 0.7]). Histopathological evaluations revealed a significant decrease in the inflammation severity in the three above-mentioned groups. P-NF-ĸB p65, COX-2, and iNOS expression, MPO activity, and TNF-α levels also decreased dramatically following treatment with IVM 0.5, baclofen 0.5, or the combination therapy (p < 0.001 vs. the control).
Conclusions
IVM exerted promising anti-inflammatory effects in treating acetic acid-induced colitis in rats. Its synergistic effect with baclofen also signified the possible involvement of GABAB receptors in this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a chronic bowel relapsing–remitting inflammatory condition within the gastrointestinal (GI) tract [1,2,3]. Although the etiology of IBD is still unclear [4], recent extensive studies have indicated that a complex interaction between the ambient environment, genetic susceptibility, microbial agents, and immunoregulatory factors might be involved in the pathogenesis of the disease [5,6,7]. Concerning immunoregulatory factors, some evidence has revealed that nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) is overexpressed in IBD, which in turn induces a wide variety of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) [8]. Myeloperoxidase (MPO) activity also increases significantly in IBD, indicative of neutrophil accumulation [8].
Recent investigations have proposed a potential role of gamma-aminobutyric acid (GABA) in the neuroimmune dialogue between the enteric nervous system and the intestinal mucosal immune system. GABA seems to have a significant role in regulating motility, secretion, and immunity of the GI system [9, 10]. Emerging evidence indicates that GABA has many other beneficial effects, especially ameliorating immune and inflammatory responses. In the colon tissue, epithelial cells are enriched with a functional GABAergic signaling system. Studies also report that many species of commensal bacteria residing in the gut can produce GABA [11]. In addition, lower concentrations of GABA have been found in the colonic mucosa of patients diagnosed with ulcerative colitis [9].
Ivermectin (IVM), a broad-spectrum anthelmintic agent, has been found to possess distinct anti-inflammatory properties, and it is known to affect cellular and humoral immune responses. Evidence suggests that IVM could act through GABA receptors and ligand-gated channels, such as glutamate-gated chloride channels [10]. In addition, IVM has been utilized in a wide variety of conditions, from regulating glucose and cholesterol levels in diabetic mice to suppressing malignant cells proliferation and inhibition of viral replication. Studies have revealed that IVM-mediated inhibition of inflammatory cytokines production may occur through the suppression of the NF-κB pathway [12]. Concerning the potential anti-inflammatory role of IVM, the present study aimed to evaluate its beneficial effects in treating acetic acid-induced colitis in rats and to investigate the possible role of the GABAergic signaling pathway in this respect. This study will also provide a better understanding of the role of the GABAB receptors in IBD and could pave the way for new clinical applications of IVM to modulate inflammatory conditions by interacting with the GABA signaling pathway.
Materials and Methods
Animals
Male Wistar rats weighing 200–220 g were obtained from the animal house of the Pharmacology Department, Tehran University of Medical Sciences (Tehran, Iran). Animals were housed under controlled environmental conditions; they were kept in translucent polycarbonate cages at an ambient temperature of 23 ± 2 °C in a 12-h/12-h light/dark cycle, and had free access to clean tap water and adequate food before any experiment. Rats underwent general anesthesia before any painful procedure, surgery, or sampling. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8523, revised in 2011) and Institutional Animal Care and Use Committee (IACUC) guidebook [13]. In addition, the Ethics in Medical Research Committee of Tehran University of Medical Sciences approved all the study protocols (No. IR.TUMS.MEDICINE.REC.1399.135). Animals were euthanized at the sampling time by administering lethal doses of anesthetic agents, as described later in the manuscript.
Reagents
Ketamine 10% and xylazine 2% were purchased from Alfasan (Woerden, Netherlands) and were injected intraperitoneally to induce general anesthesia in rats. Acetic acid was purchased from Merck (Darmstadt, Germany), and prednisolone was obtained from Sigma-Aldrich (St. Louis, MO, USA). IVM was received as a gift from Gilaranco (Rasht, Iran), and baclofen was purchased from Shimi Darou Kowsar (Tehran, Iran). To dissolve IVM, normal saline (0.9% NaCl) and propylene glycol (total concentration of 10% [v/v]), as solvent, were utilized. Baclofen was dissolved in dimethyl sulfoxide (DMSO), purchased from Sigma-Aldrich (St. Louis, MO, USA) and subsequently diluted with normal saline (final concentration of DMSO: 0.4% v/v). Prednisolone was dissolved in normal saline. Prednisolone, IVM, and baclofen were delivered to rats orally through a flexible plastic gavage tube.
Acetic Acid-Induced Colitis Model
The model was performed as described previously by Rashidian et al. [14]. Briefly, rats were starved for 24 h before colitis induction while having free access to clean tap water. On the day of colitis induction, rats were given general anesthesia, and 2 mL of 4% acetic acid solution was delivered into the rat colons through a rectal tube in an upside-down position, using an 8-cm flexible plastic catheter with a diameter of 2 mm, inserted into the rectum. Rats were kept in this position for an extra minute to prevent any leakage. Subsequently, they were kept warm under a lamp until complete recovery from anesthesia. Then, the first dose of each reagent was administered orally (by gavage) to each rat based on the experimental groups.
Experimental Design and Sampling Procedure
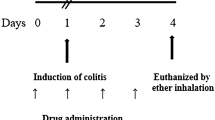
Rats were randomly divided into different experimental groups (six rats each). After the induction of colitis, treatment with different doses of reagents was initiated. The first doses were administered at around the second hour post-induction, and drug administrations were then continued for 5 days (one bolus dose each day with the same dose as the day of colitis induction). On the sixth day, rats were euthanized by administering high doses of ketamine and xylazine [15], and tissue sampling was performed as follows: the ventral surface of each rat was incised at the midline; the colon was explored, and the last 8 cm of the colon, including the rectum, was transected. The 8-cm section was incised at the midline and washed with ice-cold normal saline, and macroscopic photographs were obtained. The ulcerated area was detected by a dark-colored change and thickening of the tissue as compared to the normal colon; a full-thickness sample (1.5 cm in length) was then obtained, including the intermediate margin of the ulcerated/necrotic area and the grossly healthy tissue. This segment was then halved in width into two segments. One section was frozen immediately using a liquid nitrogen tank and transferred to a − 80 °C freezer to be used for further investigations, including western blot analysis, enzyme-linked immunosorbent assay (ELISA), and colorimetric assay. The other section was stored in 10% formaldehyde (for at least 48 h) to be prepared for histopathological studies. Figure 1 shows the timeline of the current study and the experimental groups.
Accordingly, the final experiments consisted of 13 groups (6 rats each) considering different doses of reagents:
-
Sham group: no colitis induction + vehicle/day (normal saline + propylene glycol 10% v/v + DMSO 4% v/v)
-
Control group (acetic acid): colitis induced + vehicle/day
-
Prednisolone group (standard positive control): colitis induced + prednisolone 1 mg/kg/day
-
IVM dose–response groups: colitis induced + IVM 0.05, 0.1, 0.2, 0.5, or 1 mg/kg/day in five separate groups
-
Baclofen (as a standard GABAB agonist) dose–response groups: colitis induced + baclofen 0.2, 0.5, 1, or 2 mg/kg/day in four separate groups
-
IVM + baclofen group: colitis induced + coadministration of sub-effective dose of IVM and sub-effective dose of baclofen—this group was designed to investigate any possible synergistic effect (described in the results section and Online Resource 1).
Although the main aim of the present study was investigating the anti-inflammatory role of IVM through the GABAB signaling pathway, we examined the role of GABAA in this treatment separately by injecting bicuculline—a standard GABAA antagonist—intraperitoneally before administering the effective dose of IVM (0.5 mg/kg/day), presented briefly in Online Resource 1.
We chose IVM and baclofen dosage based on previous experiments in rats suggesting the most effective doses; they were then adjusted based on the experiments performed in this study. IVM was initiated with a dosage of 0.05 mg/kg and then increased to investigate the dose–response pattern; baclofen was initiated with 1 mg/kg, and the effectiveness of higher and lower doses was explored [10, 16]. Prednisolone was also administered based on previous reports [17].
Disease Activity Index (DAI)
During the experiments and treatment period, body weight changes, diarrhea, and rectal bleeding were evaluated in all groups. We scored each parameter based on a previously described method for calculating the disease activity index (DAI) [18, 19]. Briefly, changes in body weight were scored as 0 = no weight loss, 1 = 1–5%, 2 = 6–10%, 3 = 11–15%, and 4 = > 15% weight loss. Stool consistency was also scored as 0 = normal, 2 = loose, and 4 = diarrhea, and rectal bleeding was scored as 0 = normal, 2 = occult bleeding, and 4 = gross bleeding. These three scores were recorded for each rat in each study group. Subsequently, DAI was calculated for each rat by averaging these three scores. The mean of DAIs was then calculated and compared among the study groups.
Macroscopic Evaluation of Rats’ Colons and Ulcer Index (UI) Calculation
After colon dissection, an observer blinded to the treatments evaluated each colon and scored the severity of ulcers based on the following scale: 0 = no macroscopic change observed; 1 = only mucosal erythema could be seen; 2 = mild mucosal edema, slight bleeding, or slight erosion; 3 = moderate edema, bleeding ulcers or prominent erosions; 4 = “severe” ulceration, erosions, edema, and tissue necrosis [20]. The ulcerated/necrotic area was calculated based on the obtained macroscopic photographs using ImageJ software version 1.53a (National Institutes of Health, USA), reported as cm2. The ulcer index (UI) was then calculated as follows [21]:
-
Ulcer index (UI) = Ulcer area (cm2) + Ulcer severity grade.
Histopathological Examination and Scoring
As described earlier, colon specimens were fixed using 10% formalin and were dehydrated, embedded in paraffin wax, processed, and cut into 4-µm-thick sections. They were then stained with hematoxylin and eosin (H&E). Colon sections were assessed by an expert pathologist, blinded to the treatments, who scored tissues based on the inflammation severity. Inflammation severity was graded on a scale of 0–5 as described previously [22] (Table 1). Random measurements were also repeated regarding inflammation severity to assess intra-observer reliability, calculated to be 0.91 (95% CI 0.88–0.93).
Western Blot Analysis for p-NF-κB p65, COX-2, and iNOS Expression
To investigate the inflammatory response in the intestinal tissue of rats subjected to colitis induction and treated with IVM, baclofen, or a combination of these two drugs, western blot analysis for p-NF-κB p65, prostaglandin-endoperoxide synthase 2 (COX-2), and inducible nitric oxide synthase (iNOS/NOS2) was performed via the following protocol:
Frozen colon samples were defrosted and homogenized in lysis buffer (500 µL Tris–HCL, pH = 8; 0.003 g EDTA; 0.08 g NaCl; 0.025 g sodium deoxycholate; 0.01 g sodium dodecyl sulfate [SDS]; protease inhibitor cocktail [one tablet]; 10 µL Triton [NP40 1%]). Tissues were then centrifuged at 4 °C and 12,000×g for 10 min in an Eppendorf 5415R centrifuge (Hamburg, Germany), and the supernatant consisting of protein was extracted and stored in a − 20 °C freezer. Protein concentration was assessed by bicinchoninic acid assay (BCA; Bradford), and bovine serum albumin (BSA) was utilized as the standard protein in the procedure. Briefly, after preparing the samples using a sample buffer (0.6 mL Tris; pH = 6.8; 2.5 mg glycerol; 0.5 mg β-mercaptoethanol; 0.01 g bromophenol blue; 0.2 g SDS), electrophoresis was performed on polyacrylamide gels. Following electrophoresis, protein bands were transferred to polyvinylidene fluoride (PVDF) membranes, and blocking was performed using a blocking solution consisting of 2% non-fat dried milk in TBS-T buffer for 1 h 15 min at room temperature. After blocking, incubation with primary antibodies, including β-actin (C4) mouse monoclonal antibody (1:300 dilution; sc-47778; Santa Cruz Biotechnology, Inc., TX, USA), p-NF-κB p65 (27.Ser 536) mouse monoclonal antibody (1:300 dilution; sc-136548; Santa Cruz Biotechnology), COX-2 (H-3) mouse monoclonal antibody (1:300 dilution; sc-376861; Santa Cruz Biotechnology), and anti-NOS2 (C-11) mouse monoclonal antibody (1:300 dilution; sc-7271; Santa Cruz Biotechnology), was performed for 16 to 18 h. After subsequent washing with TBS-T buffer (20 mL Tris–HCL; 8 g NaCl; 1 mL Tween 20 10% v/v; 100 mL distilled water) three times for 15 min each, secondary antibodies were added, including mouse m-IgGκ BP-HRP antibody (1:1000 dilution; sc-516102; Santa Cruz Biotechnology) and mouse anti-rabbit IgG-HRP antibody (1:1000 dilution; sc-2357; Santa Cruz Biotechnology), and the mixture was shaken at room temperature, followed by washing three times with TBS-T buffer (15 min each time). The protein bands were then detected via the chemiluminescent western blotting technique using photographic films.
Myeloperoxidase (MPO) Activity Colorimetric Assay
MPO activity was assessed using the MPO Colorimetric Activity Assay Kit (Sigma-Aldrich, St. Louis, MO, USA; Catalog Number: MAK068). In this assay, MPO catalyzes the formation of hypochlorous acid, which then forms taurine chloramine by reacting with taurine. Taurine chloramine subsequently reacts with the chromophore TNB and results in the formation of DTNB. Each unit (1 U) of MPO activity is defined as the amount of enzyme that hydrolyzes the substrate and forms taurine chloramine to consume 1.0 µmol of TNB per minute at room temperature. The sample preparation and MPO activity assay were performed according to the protocol provided by the manufacturer, the final absorbance was measured at 412 nm, and the final MPO activity was adjusted for each sample’s protein concentration, reported as mU/mg protein in this manuscript.
ELISA for Measurement of TNF-α Levels
Quantitative assessment of TNF-α was performed using a rat TNF-α ELISA kit (DuoSet ELISA Development Systems, R&D Systems, Minneapolis, MN, USA; Catalog Number: DY510), according to the protocol provided by the manufacturer. The optical density was then determined with a microplate reader set at 450 nm. The final concentrations were adjusted for the protein concentration of the samples, and the final results are reported as pg/mg protein.
Statistical Analysis
The final results are reported as mean ± standard deviation (SD). Group differences regarding UI, DAI, p-NF-κB p65, COX-2, and iNOS expression levels, MPO activity, and TNF-α level were analyzed using parametric one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A p value < 0.05 was considered statistically significant. All the statistical analyses were performed using GraphPad Prism version 8.2.1 software for macOS (GraphPad Software, San Diego, CA, USA).
Results
Disease Activity Index (DAI)
We found severe diarrhea and weight loss in rats who underwent acetic acid-induced colitis in the control group. Rectal bleeding was also noticeable in the control group compared to the others. The highest DAI was found in this group; however, prednisolone 1 mg/kg/day, IVM 0.1 mg/kg/day or more, baclofen 0.2 mg/kg/day or more, and the combination therapy of IVM 0.2 + baclofen 0.2 mg/kg/day significantly improved the DAI compared to the control group (p < 0.001) (Fig. 2).
Macroscopic Evaluation of Rats’ Colons and Ulcer Index (UI)
In the control group, signs of significant mucosal injury could be seen grossly in the colon tissue after dissection, including edema, hyperemia, wall thickness, ulceration, and necrosis (UI = 6.5 ± 0.7; Fig. 3). However, colitis signs improved markedly in groups treated with effective doses of IVM and baclofen, almost the same as the prednisolone group. The dose–response pattern of IVM and baclofen are illustrated in Fig. 3. IVM 0.2 and baclofen 0.2 were considered sub-effective doses that led to a relative improvement in the colon tissue, based on macroscopic evaluations and the UIs of 3.0 ± 0.9 and 2.4 ± 0.6, respectively. However, the IVM 0.5 (UI = 1.4 ± 0.4) and baclofen 0.5 (UI = 1.7 ± 0.6) mg/kg/day were the optimum effective doses (p < 0.001 vs. the control group) and showed no necrosis or severe ulceration, and no edema or wall thickening could be found grossly. A further increase in the dosage of IVM or baclofen did not produce a substantial change in the final results, and no significant difference was found between higher doses and the IVM 0.5 and baclofen 0.5 (p > 0.05). The difference between effective doses of IVM or baclofen and the prednisolone group was not statistically significant (prednisolone group UI = 1.3 ± 0.5; p > 0.05). Coadministration of IVM 0.2 and baclofen 0.2 also significantly affected the colon tissue, leading to recovery from colitis (UI = 1.4 ± 0.3; p < 0.001 compared to the control group), showing a synergistic pattern (Online Resource 1).
Macroscopic appearance of the last 8 cm of the rat colons (including rectum) at the sixth day following acetic acid-induced colitis, treated with vehicle (I, control), ivermectin (IVM) 0.05 mg/kg/day (II), IVM 0.1 mg/kg/day (III), IVM 0.2 mg/kg/day (IV), IVM 0.5 mg/kg/day (V), IVM 1 mg/kg/day (VI), prednisolone 1 mg/kg/day (VII), baclofen 0.2 mg/kg/day (VIII), baclofen 0.5 mg/kg/day (IX), baclofen 1 mg/kg/day (X), baclofen 2 mg/kg/day (XI), and IVM 0.2 + baclofen 0.2 mg/kg/day (XII). Ulcer indices are shown in the chart; **P ≤ 0.01 and ***P ≤ 0.001 compared to the control group
Histopathological Examination
Sample tissues obtained from the control group (acetic acid) indicated extensive ulceration and necrosis of either the entire colon wall or part of it, along with irregular villus mucosal surface and extensive transmural inflammatory cells infiltration (score = 4.8 ± 0.4). In addition, colon layers were almost indistinguishable from each other under a light microscope. In contrast, the IVM 0.5, baclofen 0.5, and IVM 0.2 + baclofen 0.2 groups showed the most significant effect in lowering histopathological scores and the grade of inflammation, with scores of 0.3 ± 0.5, 0.2 ± 0.4, and 0.3 ± 0.5, respectively (p < 0.001 compared to the control group). Histopathological scores in the IVM 0.5, baclofen 0.5, and IVM 0.2 + baclofen 0.2 groups were lower than those in the prednisolone group (p = 0.04, 0.01, and 0.04, respectively), indicating better improvements. In these three groups, mucosa, submucosa, muscularis propria, and serosa were distinguishable almost as well as the normal colon tissue, with no or mild inflammatory cells infiltration. The IVM 0.2 + baclofen 0.2 group had significantly lower scores than either IVM 0.2 or baclofen 0.2 administration alone (p < 0.001) (Fig. 4).
Histopathological sections of rat colons following acetic acid-induced colitis treated with vehicle (I, control), prednisolone 1 mg/kg/day (II), IVM 0.5 mg/kg/day (III), and IVM 0.2 + baclofen 0.2 mg/kg/day (IV) at ×200 magnification stained with hematoxylin and eosin (H&E). Acetic acid stimulation led to extensive mucosal damage (control group), such that colon layers could not be distinguished from each other. Significant transmural inflammatory cells infiltration was also observed, and the normal structure of crypts and goblet cells was disrupted. Effective doses of IVM (0.5 mg/kg/day) and prednisolone (1 mg/kg/day) attenuated histopathological damage by diminishing infiltration of inflammatory cells, and crypt and goblet cell structures were almost normal in these groups. Mucosa, submucosa, muscularis propria, and serosa could be seen with almost normal structures. The same was found with the coadministration of IVM and baclofen simultaneously (IVM 0.2 + baclofen 0.2 mg/kg/day). The chart provides histopathological scores (based on the inflammatory responses) for the study groups; lower scores indicate better recovery from colitis injury and milder inflammation. The results are illustrated based on the mean ± SD for each group; **p ≤ 0.01 and ***p ≤ 0.001 compared to the control group. Arrows: (1) inflammatory cells, (2) depth of a crypt, and (3) goblet cells
Western Blot Analysis for p-NF-κB p65, COX-2, and iNOS Expression
Following the induction of colitis with acetic acid, the expression level of p-NF-κB p65 increased significantly compared to that in the sham group (p < 0.001). After the administration of the effective dose of IVM (0.5 mg/kg/day) or baclofen (0.5 mg/kg/day), p-NF-κB p65 decreased markedly in comparison with the control group (p < 0.05 and < 0.001, respectively). The lowest expression level was recorded in the combination therapy with IVM 0.2 + baclofen 0.2 mg/kg/day (p < 0.001 vs. the control and IVM 0.5 groups and p < 0.05 vs. the baclofen 0.5 group) (Fig. 5).
Western blot analysis for a p-NF-ĸB p65, b COX-2, and c iNOS expression in the sham (no colitis induction) and control groups compared to the effective therapies ivermectin (IVM) 0.5 mg/kg/day, baclofen 0.5 mg/kg/day, and IVM 0.2 + baclofen 0.2 mg/kg/day. Densities were normalized to B-actin band density. The results are illustrated based on the mean ± SD for each group; *p ≤ 0.05 and ***p ≤ 0.001 compared to the control group; ###p ≤ 0.001 compared to the sham group
Almost the same pattern was found in the expression of COX-2 and iNOS. After colitis induction, the expression level of both factors increased significantly compared to the sham group (p < 0.001). However, COX-2 and iNOS expression decreased markedly in the intestinal tissue of rats receiving IVM (0.5 mg/kg/day), baclofen (0.5 mg/kg/day), or a combination of IVM 0.2 + baclofen 0.2 mg/kg/day (p < 0.001 vs. the control).
Myeloperoxidase Activity and TNF-α Levels in Colon Tissues
Colorimetric assessment of MPO activity, as an indicator of granulocytic infiltration, revealed a considerable decrease in MPO activity in groups treated with IVM 0.5 or baclofen 0.5 or the combination therapy, in comparison with the control group (p < 0.001). MPO activity in the sham group was calculated as 163.2 ± 8.6 mU/mg protein. It was measured as 160.3 ± 9.6 mU/mg protein in the prednisolone group, in contrast to the control group with estimated MPO activity of 317.9 ± 7.7 mU/mg protein (p < 0.001 vs. the control group and p > 0.5 vs. the sham group). Meanwhile, in the IVM 0.5, baclofen 0.5, and IVM 0.2 + baclofen 0.2 groups it was 220.7 ± 10.5, 155.0 ± 6.5, and 167.1 ± 8.6 mU/mg protein, respectively (p < 0.001). The IVM 0.2 + baclofen 0.2 group also showed markedly lower MPO activity than both the IVM 0.2 and IVM 0.5 groups (p < 0.001), but no difference was found in comparison with baclofen alone (0.2 or 0.5; p > 0.05) (Fig. 6).
TNF-α level, a pivotal proinflammatory cytokine, showed the same pattern as MPO activity among different groups, and the control group had extremely high levels of TNF-α (116.2 ± 14.0 pg/mg protein) compared to the treatment groups. Effective doses of IVM and baclofen, administered separately or in combination therapy, both significantly decreased TNF-α levels following colitis induction. Estimated TNF-α levels were 6.5 ± 3.3 pg/mg protein in the prednisolone group and 11.1 ± 4.2, 4.6 ± 3.3, and 9.8 ± 3.4 pg/mg protein in the IVM 0.5, baclofen 0.5, and IVM 0.2 + baclofen 0.2 groups (p < 0.001 vs. the control group), respectively (Fig. 6).
Discussion
Acetic acid-induced colitis can be used as a standard experimental model of ulcerative colitis [23]. Additionally, corticosteroids have been used in numerous animal studies as a standard treatment to reverse the devastating effect of acetic acid [24, 25]. In this study, IVM showed a promising effect in treating acetic acid-induced colitis in rats in terms of inflammatory biomarkers and both macroscopic and microscopic features. This study revealed that IVM 0.5 mg/kg/day could decrease DAI, UI, p-NF-κB p65, COX-2, and iNOS expression, MPO activity, and TNF-α levels. These desired effects were also observed with baclofen (a standard GABAB agonist) 0.5 mg/kg/day and synergistically with the combination of IVM 0.2 mg/kg/day and baclofen 0.2 mg/kg/day.
IVM, an old anthelmintic drug, was recently studied by Tabary et al. in an experimental model of skin flap, showing a robust anti-inflammatory effect and improving flap survival [10]. The authors also proposed the GABAergic pathway as a possible mechanism for the beneficial anti-inflammatory effect of IVM on skin flap survival. GABA is the major inhibitory neurotransmitter in the mature central nervous system, acting on two distinct subtypes of receptors: GABAA and GABAB [26, 27]. GABAA receptors are hetero-pentameric chloride ion channels, whereas the GABAB subtype is a G protein-coupled receptor [27]. The presence of both subunits was previously shown in colon tissue [28, 29].
Recent studies have also indicated that GABA is involved in inflammatory events and immune cell activity, since its receptors have been found on macrophages, dendritic cells, and T-cells [30]. Both GABAA and GABAB seem to play a pivotal role in attenuating the inflammatory condition; GABAA reduces macrophage cytokine production and inhibits T-cell proliferation, while GABAB reduces TNF-α production and decreases interleukin (IL-6 and IL-12) release from microglial cells. Concerning IBD, the role of GABAA is still a matter of controversy. In a study by Dudley et al., it was found that the GABAA agonist topiramate could alleviate colitis in rats. In contrast, activation of GABAA led to worsening colitis in mice models in a study by Ma et al. [28]. However, concerning the role of GABAB in treating colitis, there are still too few data available. In the present study, we targeted GABAB receptors by administering baclofen (GABAB agonist) to investigate the possible involvement of the GABAB pathway in the anti-inflammatory effect of IVM in colitis and the simultaneous coadministration of these two agents. Both agents showed a significant and promising effect in the treatment of acetic acid-induced colitis in rats. Also, in a separate group investigating the probable role of GABAA, we found no reliable or significant effect regarding the macroscopic and microscopic parameters used in the present study, and it did not significantly reverse the positive effects of IVM (see Online Resource 1).
It is worth noting that inflammation is among the most important events justifying the beneficial role of IVM in the treatment of colitis. We found lower levels of TNF-α and NF-κB, as well as reduced MPO activity, after the administration of effective doses of IVM or baclofen, all of which are major inflammatory factors known to be involved interactively in the pathogenesis and treatment of IBD [31]. The anti-inflammatory effect of IVM was described previously by Tabary et al. [10]. Briefly, they showed that IVM could diminish the inflammatory condition by decreasing the production and release of IL-1b and TNF-α following skin flap surgery in rats. They also indicated that GABAergic pathway activation could be a possible mediator for this anti-inflammatory effect. A study by Duthey et al. also demonstrated a new role for GABAB receptor activation in alleviating inflammation [32]. They used baclofen in both in vitro and in vivo models of allergic contact dermatitis. In vitro, baclofen reduced chemotaxis of human peripheral blood mononuclear cells. In addition, baclofen significantly reduced signs of inflammation and recruitment of neutrophils, monocytes, and lymphocytes into the skin in vivo. The current study also indicated that IVM exerts an acceptable anti-inflammatory effect after induction of colitis in rats.
TNF-α is believed to play an important role in the pathogenesis of colitis, as many drugs target TNF-α or its receptor in IBD treatment [33,34,35]. We found lower levels of TNF-α after the administration of IVM or baclofen, which implies that TNF-α might be a crucial target. In vitro, TNF-α secretion was the only reduced cytokine, among numerous others, after the administration of baclofen in treating allergic contact dermatitis, as described previously [32].
Transcription factor NF-κB seems to be one of the major regulatory components of dysregulated cytokine production and signaling mechanisms in the pathogenesis of IBD. Macrophages and epithelial cells isolated from inflamed gut specimens from IBD patients indicated higher levels of NF-κB p65 [36]. A study by Liu et al. showed that baclofen suppressed the increased expression of p-NF-κB p65 in satellite glial cells (SGCs) treated with lipopolysaccharide (LPS) in vitro [37]. They also concluded that baclofen could attenuate inflammatory orofacial pain by modulating IL-1β. These findings are in line with ours, indicating that NF-κB expression is reduced after administration of IVM or baclofen in acetic acid-induced colitis models. It is worth mentioning that although prednisolone revealed better MPO and TNF-α activity than IVM 0.2 + baclofen 0.2 mg/kg/day, this difference did not reach statistical significance. On the other hand, the prednisolone mechanism might differ from that of IVM; for example, prednisolone inhibited NF-κB in patients with inflammatory bowel disease [38]. However, there might be considerable differences between prednisolone and IVM on their downstream action. Finally, a longer duration of treatment might be needed for the maximal effect of IVM, which needs further investigation in future studies.
COX-2, a proinflammatory enzyme, is induced in both animal models of IBD and patients with IBD [39]; the same has been observed for iNOS [40]. Experimental evidence also shows that simultaneous inhibition of COX-2 and iNOS might have a promising role in treating chemically induced colitis [41]. In line with previous studies, our findings revealed that COX-2 and iNOS expression increased in rats following colitis induction, while a significant reduction was observed after treatment with IVM, baclofen, or the combination of both drugs.
This study was designed as an experimental study, and all the limitations related to experimental and animal studies are also applicable to ours. In addition, some recent animal colitis models in the literature have utilized agents other than acetic acid, especially 2,4,6-trinitrobenzene sulfonic acid (TNBS) [42]; however, at the time it was only possible for us to use acetic acid to perform the colitis model. Studies have also reported that the acetic acid-induced colitis model is comparable to TNBS in the case of colitis induction [43]. The effect of IVM and baclofen could be investigated in TNBS-induced colitis in rats in further studies. Moreover, evaluation of the role of the GABAA receptor in the anti-inflammatory role of IVM in colitis was not within the main scopes of our study. The role of this receptor can be investigated in further studies, and its controversial role based on previous studies necessitates the conduct of a comprehensive study at the molecular level to investigate its role in greater depth.
Conclusions
This study showed that controlling the inflammatory condition in acetic-acid colitis in rats could be achieved through activation of the GABAB signaling pathway by administering IVM or baclofen or the combination therapy using both agents. The combination therapy helps us administer lower doses of these agents, which can lower the risk of any possible side effects of higher doses of IVM or baclofen. This could also pave the way to utilize these agents in the clinical setting safely. Further studies still need to be conducted to investigate the possibility of utilizing these agents in the clinic. This study also signified the anti-inflammatory role of GABA, which allows researchers to investigate its role in other organs.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Molodecky NA, Soon S, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42.
Tasdemir S, Parlakpinar H, Vardi N, Kaya E, Acet A. Effect of endogen-exogenous melatonin and erythropoietin on dinitrobenzene sulfonic acid–induced colitis. Fundam Clin Pharmacol 2013;27:299–307.
Xavier R, Podolsky D. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–434.
Podolsky DK. Inflammatory bowel disease. N Engl J Med 1991;325:928–937.
Monteleone G, Pallone F, MacDonald TT. Emerging immunological targets in inflammatory bowel disease. Curr Opin Pharmacol 2011;11:640–645.
Braus NA, Elliott DE. Advances in the pathogenesis and treatment of IBD. Clin Immunol 2009;132:1–9.
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. The Lancet 2007;369:1641–1657.
Dejban P, Sahraei M, Chamanara M, Dehpour A, Rashidian A. Anti-inflammatory effect of amitriptyline in a rat model of acetic acid-induced colitis: the involvement of the TLR4/NF-kB signaling pathway. Fundam Clin Pharmacol. 2020.
Motavallian A, Bouzari S, Zamani E, Karimian P, Dabirian S, Molavi M, Torshkooh FA. An investigation of the anti-inflammatory effects of gabapentin on acetic acid-induced colitis in rats. Mol Biol Rep. 2021. https://doi.org/10.1007/s11033-021-06357-2.
Tabary M, Aryannejad A, Noroozi N, Tavangar SM, Jafari RM, Araghi F, Dadkhahfar S, Dehpour AR. Ivermectin increases random-pattern skin flap survival in rats: the novel role of GABAergic system. J Surg Res 2021;259:431–441.
Aggarwal S, Ahuja V, Paul J. Attenuated GABAergic signaling in intestinal epithelium contributes to pathogenesis of ulcerative colitis. Dig Dis Sci 2017;62:2768–2779. https://doi.org/10.1007/s10620-017-4662-3.
Yan S, Ci X, Chen N, Chen C, Li X, Chu X, Li J, Deng X. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflam Res 2011;60:589–596.
Risks NIoHOfPfR, Association AREN. Institutional Animal Care and Use Committee Guidebook. vol 92. US Department of Health and Human Services, Public Health Service, National. 1992.
Rashidian A, Muhammadnejad A, Dehpour A-R, Mehr SE, Akhavan MM, Shirkoohi R, Chamanara M, Mousavi S-E, Rezayat S-M. Atorvastatin attenuates TNBS-induced rat colitis: the involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology 2016;24:109–118.
Underwood W, Anthony R. AVMA guidelines for the euthanasia of animals. Retrieved March 2020;2013:2020–2021.
Franek M, Vaculin S, Rokyta R. GABA~ B receptor agonist baclofen has non-specific antinociceptive effect in the model of peripheral neuropathy in rat. Physiol Res 2004;53:351–355.
Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J, di Stasi LC. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chemico Biol Interact 2012;195:76–85.
Yousefi-Ahmadipour A, Rashidian A, Mirzaei MR, Farsinejad A, PourMohammadi-Nejad F, Ghazi-Khansari M, Ai J, Shirian S, Allahverdi A, Saremi J. Combination therapy of mesenchymal stromal cells and sulfasalazine attenuates trinitrobenzene sulfonic acid induced colitis in the rat: The S1P pathway. J Cell Physiol 2019;234:11078–11091.
El-Salhy M, Umezawa K. Anti-inflammatory effects of novel AP-1 and NF-κB inhibitors in dextran-sulfate-sodium-induced colitis in rats. Int J Mol Med 2016;37:1457–1464.
Deshmukh C, Veeresh B, Pawar A. Protective effect of Emblica officinalis fruit extract on acetic acid induced colitis in rats. J Herbal Med Toxicol 2010;4:83–87.
Rashidian A, Mehrzadi S, Ghannadi AR, Mahzooni P, Sadr S, Minaiyan M. Protective effect of ginger volatile oil against acetic acid-induced colitis in rats: a light microscopic evaluation. J Integr Med 2014;12:115–120.
Rezayat SM, Dehpour A-R, Motamed SM, Yazdanparast M, Chamanara M, Sahebgharani M, Rashidian A. Foeniculum vulgare essential oil ameliorates acetic acid-induced colitis in rats through the inhibition of NF-kB pathway. Inflammopharmacology 2018;26:851–859.
Jurjus AR, Khoury NN, Reimund J-M. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods 2004;50:81–92.
Rashidian A, Keshavarz-Bahaghighat H, Abdollahi A, Chamanara M, Faghir-Ghanesefat H, Hoseini-Ahmadabadi M, Dehpour AR. Agmatine ameliorates acetic acid-induced colitis in rats: involvement of nitrergic system. Immunopharmacol Immunotoxicol 2019;41:242–249.
Rashidian A, Rashki A, Abdollahi A, Haddadi N-S, Chamanara M, Mumtaz F, Dehpour AR. Dapsone reduced acetic acid-induced inflammatory response in rat colon tissue through inhibition of NF-kB signaling pathway. Immunopharmacol Immunotoxicol 2019;41:607–613.
Roberts E, Frankel S. γ-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem 1950;187:55–63.
Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Pharmacol Rev 2008;60:243–260.
Ma X, Sun Q, Sun X, Chen D, Wei C, Yu X, Liu C, Li Y, Li J. Activation of GABAA receptors in colon epithelium exacerbates acute colitis. Front Immunol 2018;9:987.
Uezono Y, Kaibara M, Hayashi H, Kawakami S, Enjoji A, Kanematsu T, Taniyama K. Characterization of GABAB receptor in the human colon. J Pharmacol Sci 2004;94:211–213.
Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids 2013;45:87–94.
Piechota-Polanczyk A, Fichna J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Archiv Pharmacol 2014;387:605–620.
Duthey B, Hübner A, Diehl S, Boehncke S, Pfeffer J, Boehncke WH. Anti-inflammatory effects of the GABAB receptor agonist baclofen in allergic contact dermatitis. Exp Dermatol 2010;19:661–666.
Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 2003;3:521–533.
Dionne S, Hiscott J, D’agata I, Duhaime A, Seidman E. Quantitative PCR analysis of TNF-α and IL-1β mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci 1997;42:1557–1566. https://doi.org/10.1023/A:1018895500721.
Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–646.
Neurath MF, Pettersson S, Zum Büschenfelde K-HM, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF–κB abrogates established experimental colitis in mice. Nat Med 1996;2:998–1004.
Liu F, Zhang Y-Y, Song N, Lin J, Liu M-k, Huang C-L, Zhou C, Wang H, Wang M, Shen J-F. GABAB receptor activation attenuates inflammatory orofacial pain by modulating interleukin-1β in satellite glial cells: Role of NF-κB and MAPK signaling pathways. Brain Res Bull 2019;149:240–250.
Ardite E, Panes J, Miranda M, Salas A, Elizalde J, Sans M, Arce Y, Bordas J, Fernández-Checa J, Pique J. Effects of steroid treatment on activation of nuclear factor κB in patients with inflammatory bowel disease. Br J Pharmacol 1998;124:431–433.
Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010;29:781–788. https://doi.org/10.1038/onc.2009.421.
Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology 2004;113:427–437. https://doi.org/10.1111/j.1365-2567.2004.01984.x.
Dudhgaonkar SP, Tandan SK, Kumar D, Raviprakash V, Kataria M. Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology 2007;15:188–195. https://doi.org/10.1007/s10787-007-1603-3.
Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, Papalois A, Pikoulis E. The TNBS-induced colitis animal model: an overview. Ann Med Surg 2016;11:9–15.
Yamada T, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology 1992;102:1524–1534.
Acknowledgment
This study was supported by a grant from the Experimental Medicine Research Center, Tehran University of Medical Sciences (Grant No. 99-1-209-48299). The authors also want to thank the Iran National Science Foundation (INSF) for their kind support.
Funding
This study was supported by a grant from the Experimental Medicine Research Center, Tehran University of Medical Sciences (Grant No. 99-1-209-48299).
Author information
Authors and Affiliations
Contributions
Study conception and design: AD; acquisition of data: AA, MT, NN, SMT; analysis and interpretation of data: AA, MT, AR, RM; drafting of the manuscript: AA, MT, BM, AD, SI; critical revision: AD, AR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8523, revised in 2011). All the study protocols were also approved by the Ethics in Medical Research Committee of Tehran University of Medical Sciences (No. IR.TUMS.MEDICINE.REC.1399.135) and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aryannejad, A., Tabary, M., Noroozi, N. et al. Anti-inflammatory Effects of Ivermectin in the Treatment of Acetic Acid-Induced Colitis in Rats: Involvement of GABAB Receptors. Dig Dis Sci 67, 3672–3682 (2022). https://doi.org/10.1007/s10620-021-07258-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07258-x