Abstract
Inflammatory bowel disease (IBD) is considered a chronic inflammatory gastrointestinal disease with treatment options which exhibit low efficacies and lead to considerable side effects. Hence, the challenge to alleviate IBD complications is remained to be resolved. The purpose of this study is evaluating anti-inflammatory impacts of gabapentin on acetic acid-induced colitis in rats. Colitis was induced by the instillation of 2 mL of 3% acetic acid solution into rat’s colons. Rats were randomly allocated into six groups including normal group, colitis control group, gabapentin-treated groups (25, 50, and 100 mg/kg; i.p.), and dexamethasone-treated group (1 mg/kg; i.p.). Based on the macroscopic assessment besides histological and biochemical findings [myeloperoxidase (MPO), pro-inflammatory cytokines], the efficacy of gabapentin was investigated. Gabapentin (50 and 100 mg/kg), and dexamethasone considerably reduced macroscopic and microscopic colonic lesions induced by acetic acid in rats in comparison with colitis control group. These results were confirmed by reduced levels of MPO activity and colonic concentrations of interleukin-6, interleukin-1 beta, and tumor necrosis factor-alpha, in inflamed colon tissue. Our data demonstrated that gabapentin exerts profitable impacts in experimental colitis that might be ascribed to its anti-inflammatory features and thus can be a potential therapeutic agent for IBD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory Bowel Disease (IBD) is considered a group of chronic relapsing inflammatory conditions of gastrointestinal (GI) tract which is categorized into two distinct disorders; Ulcerative Colitis (UC) and Crohn’s disease [1]. Despite extensive research, IBD etiology has not yet to be well elucidated [2]. It seems that microbial pathogens besides genetic, environmental, and immunological factors play crucial roles in the development and persistence of the disease [3, 4]. IBD is characterized by extensive cellular infiltration, disturbance in autophagy regulation, disruption in the balance of both generation and liberation of pro-inflammatory cytokines that bring about inflammation and ulceration of GI, dysenteriae, hyperthermia, abdominal pain, anemia and weight loss [5, 6].
Administration of salicylates (e.g., sulfasalazine) and corticosteroids is regarded the most common therapy for UC. Furthermore, immune suppressants and biological medicines can be administered as alternative choices [3]. Unfortunately, the low efficacies and undesirable side effects of these drugs remain a main clinical issue of IBD therapy [3]. Thus, there is a signified requirement for study to find new therapeutic approaches for the treatment of IBD.
Gamma Amino Butyric Acid (GABA), highly known as an inhibitory neurotransmitter of central nervous system, has a significant role in regulating motility, secretion, and immunity of GI system [7]. Recent investigations proposed potential key role of GABA in neuroimmune dialogue between enteric nervous system and intestinal mucosal immune system through inflammatory conditions like IBD [8, 9]. More to the point, the contribution of GABA and GABAergic signaling to IBD pathogenesis and lower serum concentrations of GABA in the colonic mucosa of patients diagnosed with UC have been reported [10]. Thus, targeting the neuroimmune dialogue between GABA and GI immunological system can be a new strategy to attenuate inflammation in UC [8].
Gabapentin, was initially utilized as an anticonvulsant highly to control partial seizures and has become a potential analgesic agent for the treatment of postsurgical and neuropathic pain in most of chronic pain syndromes including diabetic neuropathy [11], postherpetic neuralgia [12], trigeminal neuralgia [13], and fibromyalgia [14]. Although gabapentin has structural similarities to GABA, there’s no definite evidence that it inhibits GABA metabolism or uptake nor it binds to GABA receptors [15]. It has been shown that gabapentin selectively blocks α2δ-1 subunit-containing calcium channels and regulates calcium influx in the nerve terminals [16]. Consequently, the release of multiple neurotransmitters such as glutamate, serotonin, noradrenaline, GABA, and substance P (SP) is modulated [17, 18]. Furthermore, this drug can inhibit the levels of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α) in several rat model of neuropathic pain and carrageenan-induced paw edema [19, 20]. Gabapentin promoted the expression of δ subunit-containing GABAA receptor [21]. In addition, it has been demonstrated gabapentin increases GABA synthesis through the stimulation of enzyme glutamic acid decarboxylase, in vitro [22].
Regarding anti-inflammatory potential of GBP, the present study set out to assess the potential profitable impact of gabapentin via its anti-inflammatory features upon colonic inflammation markers in acetic acid-induced colitis in rats.
Materials and methods
Animals
The present research was carried out on male Wistar rats (250 ± 20 g, 12-week-old), bred in Animal House of Guilan University of Medical Sciences. The animals were divided into groups of six per cage with access to food and water ad libitum, under standard housing conditions with a 12/12 h light–dark cycle at 22 ± 2 °C. All experimental protocols utilized in this research was approved by Ethics Committee of Guilan University of Medical Sciences in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (Ethics No: IR.GUMS.REC.1398.020).
Chemicals
Dexamethasone and gabapentin were purchased from Iran Hormone Pharmaceutical Co. (Iran) and Sobhan Daru Co. (Iran), respectively. Hexadecyltrimethyl-ammonium bromide (HTAB), O-dianisidine dihydrochloride, aprotinin A, bovine serum albumin, phenylmethyl-sulfonyl fluoride, benzethonium chloride, ethylene diamine tetra acetic acid (EDTA), and Tween 20 were all purchased from Sigma Chemical Company (St. Louis, MO, USA). Diethyl ether oxide, glacial acetic acid, and formalin solution (35%) were purchased from Merck (Darmstadt, Germany). The levels of colonic rat IL-1β, IL-6 and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) kits (Boster Co., Pleasanton, CA, USA).
Treatment schedule
Animals were divided into six groups (n = 6). The colitis control group received intraperitoneal (i.p.) injection of normal saline, 24 h before colitis induction, and then daily afterward. Dexamethasone (1 mg/kg) and gabapentin (25, 50, and 100 mg/kg) were administered to dexamethasone and gabapentin groups, respectively. All treatments were intraperitoneally done, 24 h before induction of colitis by acetic acid, and continued daily until animals were sacrificed on day 4 (Fig. 1). Dosage selection was specified based on previous studies [23,24,25].
Induction of colitis
Before colitis induction, all animals were fasted for 36 h with free access to water. Colitis Induction was done using a method described by Karmeli et al. After light ether anesthesia, 2 mL of acetic acid 3% in normal saline (v/v) was intrarectally administered employing a polyethylene catheter inserted 8 cm proximal to anus. The animals were positioned head-down for 2–3 min to cease anal leakage of instillate and were then returned to their cages with free access to food and water. The enema of 0.25 ml normal saline was administered in normal group instead of acetic acid [26].
Macroscopic assessment
During the period of the experiment, all animals were daily weighed and their body weight loss percentage was calculated. The animals were sacrificed 72 h after colitis induction applying diethyl ether inhalation. The distal colon was excised, gently washed with 0.9% saline, and thereafter, wet weight (mg) (8 cm from the anus) and weight/length ratio of each specimen was calculated. The severity of macroscopically colon damage was scored according to our previously described method [27]. To determine ulcer area and percent of necrosis, the colon tissue was located on a nonabsorbent surface and a digital photo was taken by a Canon camera (Canon IXUS 130.Tokyo, Japan) and analyzed by using image processing software (ImageJ) [28]. The colon segments were then divided longitudinally into 3–4 pieces and fixed in 10% buffered formalin for histopathological assessment or instantly frozen in liquid nitrogen for biochemical assay.
Microscopic assessment
For the microscopic assessment, one of the four sections of colon tissue was fixed in 10% formalin solution, and after dehydration was embedded in paraffin, sliced into 4 μm-thick sections, and stained with hematoxylin/eosin (H&E). Total colitis index was calculated using the modified validated scoring system according to our previously described approach [27], and measured by summing three scores of inflammation severity, inflammation extent, and crypt damage. The histological and microscopic assessment proceeded by a pathologist blinded to experimental groups.
Determination of colonic myeloperoxidase activity
Myeloperoxidase (MPO) activity, an indicator of neutrophil infiltration into the tissue of the intestine through acetic acid-induced colon lesion was measured utilizing modified approach explained by Bradley et al. [29].
Pro-inflammatory cytokines measurements
By means of ELISA kits, the levels of IL-1β, IL-6 and TNF-α in colon tissue were measured as described earlier [25].
Statistical analysis
Statistical analysis was carried out by SPSS statistical package (version 17). All data were expressed as the mean ± standard error of mean (SEM). Comparison between groups was made using one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Non-parametric data were analyzed by Mann–Whitney U test. A P- value < 0.05 was considered as statistically significant.
Results
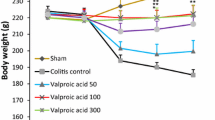
Effect of gabapentin on rats’ body weight and macroscopic features
As depicted in Table 1, Acetic acid-treated animals showed significant weight loss after 3 days, compared with the normal group (P < 0.001). Although the animals treated with dexamethasone and gabapentin (50 mg/kg and 100 mg/kg) showed significant weight loss comparing to the normal group, their body weight loss percentage was significantly lower than acetic acid-treated group (P < 0.01).
In spite of the normal group which macroscopic characteristics of colon remained intact, distal colon of colitis control group showed severe hemorrhage, ulceration, inflammation, necrosis and an increase in the thickness of the colon wall (P < 0.001). The animals treated with gabapentin (50 and 100 mg/kg) experienced a considerable reduction in ulcer severity, weight/length ratio, ulcer area and percentage of necrosis compared to the acetic acid-treated group. Furthermore, in dexamethasone group, aforementioned parameters were considerably reduced in comparison with colitis control group.
Effect of gabapentin on histopathological characteristics
As shown in Fig. 2, the colon walls of normal group maintained the consistency of its architecture with intact epithelium opposing to acetic acid control group which exhibited severe and intense transmural inflammation and/or diffuse necrosis, inflammatory granulomas, and submucosal neutrophils infiltration.
Microscopic presentation of colon in acetic acid-induced colitis in rats. a Normal group: Mucus layer and crypts are normal; b Acetic acid control group: Epithelial destruction, architectural deformity of crypts and inflammatory cell infiltrates; c Dexamethasone (1 mg/kg): Mild to moderate mucosal and submucosal inflammation and mucosal inflammatory cell infiltrates; d: Gabapentin (25 mg/kg): Destruction of mucosal architecture and infiltration of neutrophils; e and f Gabapentin (50 and 100 mg/kg): Regeneration of epithelium and reduced number of inflammatory cells in lamina propria
Total Colitis index of damaged colons in gabapentin (50 and 100 mg/kg)-and dexamethasone-treated groups was significantly lower than that of the colitis control group (Table 1). Moreover, these groups showed the regeneration of epithelium and a decrease of inflammatory cell infiltration in lamina propria.
Effect of gabapentin on myeloperoxidase activity
As shown in Table 2, MPO activity had a dramatic increase in acetic acid-treated rats in comparison with normal group. Although dexamethasone (1 mg/kg)-and gabapentin (50 and 100 mg/kg)-treated groups also exhibited elevated levels of MPO activity, we observed a significant reduction of this parameter in those groups compared to colitis control group (P < 0.001, P < 0.05 and P < 0.01, respectively).
Effect of gabapentin on cytokines profile
As can be noted in Table 2, TNF-α level raised dramatically in the colitis control group, compared to that of normal group (P < 0.001). The mentioned parameter was significantly diminished in dexamethasone- and gabapentin (50 and 100 mg /kg)-treated rats compared to acetic acid control group (P < 0.001, P < 0.05 and P < 0.01, respectively).
As shown in Table 2, colonic IL-6 level was raised in the colitis control group in comparison with that of normal group (P < 0.001). Animals treated with gabapentin (50 and 100 mg) and dexamethasone showed a significant reduction in colonic IL-6 level compared to colitis control group (P < 0.01, P < 0.001 and P < 0.001, respectively).
The colitis control group showed a considerable increase in the colonic IL-1β content, in comparison with that of normal group. This parameter was significantly decreased in rats treated with either dexamethasone (P < 0.001) or gabapentin (50 and 100 mg/kg) (P < 0.05 and P < 0.01, respectively) in comparison with colitis control group (Table 2).
Discussion
In the present study, the anti-inflammatory effects of different doses of gabapentin were evaluated in acetic acid-induced colitis in rats. As shown by improved macroscopic damages, lower levels of MPO activity, and inflammatory cytokines (TNF-α, IL-6, and IL-1β) along with dramatic attenuation of histopathological damages in colon tissues, we manifested treatment with gabapentin exerts a protective effect on colon inflammation in experimental colitis.
Recently, the urgency of understanding about the pathophysiology of IBD has been increasing by using several experimental animal models of UC. Intrarectal administration of acetic acid in the rat, as a well-recognized experimental model of induction of colitis, is like the human UC in terms of pathophysiological outlook and pro-inflammatory mediators involved [30]. Acetic acid causes increased non-transmural inflammation, mucosal permeability, and colon weight. It contributes to the influx of neutrophils into injured colon, colonic mucosa dysfunction, liberation of inflammatory biomarkers such as cytokines, and excess reactive oxygen species (ROS) due to MPO activity [30,31,32]. TNF-α and IL-1β play crucial roles in IBD pathogenesis and are responsible for neutrophil activation and mobilization, cytotoxic initiation, and proliferation of fibroblasts [33]. The results of our experiment are in line with the findings of previous studies showing the increasing level of pro-inflammatory cytokines subsequent to intracolonic acetic acid administration [31, 32].
Gabapentin as an antiepileptic agent was approved for use in partial seizures [34]. New potential indications have also been revealed for this drug including treatment of neuropathic pain syndromes [35]. The anti-inflammatory characteristics of gabapentin has been demonstrated through inhibiting the action of various inflammatory biomarkers, neutrophil infiltration, and oxidative stress in vivo [20]. Furthermore, preliminary studies elucidated the gastric protective impact of gabapentin through pleiotropic effects in acute gastric mucosal damages induced by indomethacin and ethanol [23].
Yamaghuchi et al. showed the anti‑inflammatory effect of gabapentin by the regulation of SP‑mediated neurokinin‑1 receptor (NK1R) response, in U373 MG human glioblastoma astrocytoma cells. These researchers reported that gabapentin might prevent the SP-induced IL‑6 and IL‑8 production via the inhibition of p38 mitogen-activated protein kinase (p38 MAPK) and nuclear factor kappa B (NFκB) [36]. It is worth mentioning that the expression of SP receptor and bradykinin receptors, NFκB activation, and release of pro-inflammatory cytokines are considerably increased in the colonic mucosa of UC patients [37,38,39]. Therefore, it is conceivable that some beneficial effects of gabapentin on an experimental model of colitis can be at least mediated by its proclivity to downregulate kinin receptors in inflammation site via NF-kB pathway suppression [40]. Subsequently, restriction of vascular permeability mediators such as bradykinin leads to the neutrophil diapedesis prohibition through the vessels and mitigates the inflammation [37].
Furthermore, the study carried out by de Brito et al. showed gabapentin reduces intestinal inflammation in rats via the activation of Peroxisome Proliferator-activated Receptor gamma (PPAR-gamma) which is the major regulator of NFκB [41]. Our results corroborate the study of de Brito et al. showing the protective effect of gabapentin on the inflamed colon. Although these researchers mentioned oral treatment with gabapentin at a dose of 15 mg/kg inhibits intestinal inflammation, our result showed the anti-inflammatory effect of drug following i.p. injection was produced with doses of 50 and 100 mg/kg and dose of 25 mg/kg of gabapentin didn’t considerably reduce macroscopic, microscopic, and biochemical markers in the experimental model of colitis. Based on these studies, it seems that several mechanisms are involved in the anti-inflammatory activity of gabapentin; however, further studies are required to explain the precise pathways.
MPO is an oxidant-generating enzyme and its activity increases in both human and experimental models of IBD, as a result of neutrophil infiltration into the inflamed colon [26, 42]. According to our results following biochemical evaluation, gabapentin considerably reduced MPO activity and the level of pro-inflammatory cytokines. These data are consistent with the alleviation of macroscopic and microscopic lesion scores. Thus, we can conclude gabapentin might decline neutrophil infiltration to the inflamed colon and prevent the synthesis and/or liberation of pro-inflammatory cytokines.
It must be noticed that the present study was designed to investigate the effect of i.p. injection of gabapentin on an acute model of induction of colitis. Although acetic acid is a reliable model for colitis induction which mimics the pathological characteristics of human IBD, it might exhibit some limitations like the involvement of non-specific immunity [43]. Thus, we propose further research to evaluate the effects of this drug following a longer period of experiment and other routes of administration in both acute and chronic animal models of colitis.
To conclude, we found that gabapentin has profitable effects in experimental rat colitis at least partly via decreasing pro-inflammatory cytokines and MPO activity. The anti-inflammatory activity of gabapentin in an experimental model of UC is in accord with the clinical efficacy of drug as an analgesic agent in patients with acute inflammatory pain [44]. Regarding the low incidence of adverse effects of gabapentin, it is contemplated that gabapentin may be beneficial in the therapy of the patients suffering from UC. However, further research such as clinical trials is required to assess the efficacy of gabapentin as add-on therapy in the patients with IBD.
Data availability
The datasets used and analyzed in the current study are available.
References
Zhang YZ, Li YY (2014) Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20(1):91. https://doi.org/10.3748/wjg.v20.i1.91
Podolsky DK (1991) Inflammatory bowel disease. N Engl J Med 325(13):928–937
Braus NA, Elliott DE (2009) Advances in the pathogenesis and treatment of IBD. Clin Immunol 132(1):1–9
Cho JH, Abraham C (2007) Inflammatory bowel disease genetics: Nod2. Annu Rev Med 58:401–416
Warner W, Sanchez R, Dawoodian A, Li E, Momand J (2013) Pro-inflammatory cytokines in the pathogenesis of IBD. NIH Public Access 80(4):631–637
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369(9573):1641–1657
Ma X, Sun Q, Sun X, Chen D, Wei C, Yu X, Liu C, Li Y, Li J (2018) Activation of GABAA receptors in colon epithelium exacerbates acute colitis. Front Immunol 9:987
Zizzo MG, Serio R (2017) Therapeutic potential of the gabaergic system in ulcerative colitis: current status and perspectives. Dig Dis Sci 62(10):2780
Barragan A, Weidner JM, Jin Z, Korpi E, Birnir B (2015) GABA ergic signalling in the immune system. Acta Physiol 213(4):819–827
Aggarwal S, Ahuja V, Paul J (2017) Attenuated GABAergic signaling in intestinal epithelium contributes to pathogenesis of ulcerative colitis. Dig Dis Sci 62(10):2768–2779
Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E, Gabapentin Diabetic Neuropathy Study Group (1998) Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 280(21):1831–1836
Singh D, Kennedy DH (2003) The use of gabapentin for the treatment of postherpetic neuralgia. Clin Ther 25(3):852–889
Poellmann W, Feneberg W (2008) Current management of pain associated with multiple sclerosis. CNS Drugs 22(4):291–324
Gur A, Oktayoglu P (2009) Advances in diagnostic and treatment options in patients with fibromyalgia syndrome. Open Access Rheumatol 1:193
Jensen AA, Mosbacher J, Elg S, Lingenhoehl K, Lohmann T, Johansen TN, Abrahamsen B, Mattsson JP, Lehmann A, Bettler B, Bräuner-Osborne H (2002) The anticonvulsant gabapentin (Neurontin) does not act through γ-aminobutyric acid-B receptors. Mol Pharmacol 61(6):1377–1384
Maneuf YP, Luo ZD, Lee K (2006) alpha2delta and the mechanism of action of gabapentin in the treatment of pain. Semin Cell Dev Biol 17(5):565–570
Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L (1998) A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 29(3):233–249
Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN (2012) The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1 H-MRS study. Neuropsychopharmacol Rep 37(13):2764–2771
Lee B-S, Jun I-G, Kim S-H, Park JY (2013) Intrathecal gabapentin increases interleukin-10 expression and inhibits pro-inflammatory cytokine in a rat model of neuropathic pain. J Korean Med Sci 28(2):308–314
Dias JM, de Brito TV, de Aguiar MD, da Silva Santos PW, Batista JA, do Nascimento Dias EG, Barros Fernandes H, Damasceno SR, Silva RO, Aragão KS, Souza MH (2014) Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation 37(5):1826–1836
Yu J, Wang DS, Bonin RP, Penna A, Alavian-Ghavanini A, Zurek AA, Rauw G, Baker GB, Orser BA (2019) Gabapentin increases expression of δ subunit-containing GABA(A) receptors. EBioMedicine 42:203–213
Taylor CP (1997) Mechanisms of action of gabapentin. Revue Neurologique 153:S39-45
Abdel-Salam OM, Sleem AA (2009) Study of the analgesic, anti-inflammatory, and gastric effects of gabapentin. Drug Discov Ther 3(1):18–26
Badawy GM, Atallah MN, Sakr SA (2019) Effect of gabapentin on fetal rat brain and its amelioration by ginger. Heliyon 5(9):e02387
Motavallian A, Minaiyan M, Rabbani M, Andalib S, Mahzouni P (2013) Involvement of 5HT3 receptors in anti-inflammatory effects of tropisetron on experimental TNBS-induced colitis in rat. BioImpacts 3(4):169
Karmeli F, Cohen P, Rachmilewitz D (2000) Cyclo-oxygenase-2 inhibitors ameliorate the severity of experimental colitis in rats. Eur J Gastroenterol Hepatol 12(2):223–231
Motavallian-Naeini A, Minaiyan M, Rabbani M (2012) Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS-induced colitis in rat. EXCLI J 11:30–44
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11(7):36–42
Bradley P, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Sanei MH, Hadizadeh F, Adibi P, Alavi SA (2014) Inflammatory cells’ role in acetic acid-induced colitis. Adv Biomed Res 3(1):193
Osafo N, Obiri DD, Danquah KO, Essel LB, Antwi AO (2019) Potential effects of xylopic acid on acetic acid-induced ulcerative colitis in rats. Turk J Gastroenterol 30(8):732
Owusu G, Obiri DD, Ainooson GK, Osafo N, Antwi AO, Duduyemi BM, Ansah C (2020) Acetic acid-induced ulcerative colitis in sprague dawley rats is suppressed by hydroethanolic extract of cordia vignei leaves through reduced serum levels of TNF-α and IL-6. Int J Chronic Dis. https://doi.org/10.1155/2020/8785497
Yang XL, Guo TK, Wang YH, Huang YH, Liu X, Wang XX, Li W, Zhao X, Wang LP, Yan S, Wu D (2012) Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int Immunopharmacol 12(2):408–414
Satzinger G (1994) Antiepileptics from gamma-aminobutyric acid. Arzneimittelforschung 44(3):261–266
Rose M, Kam P (2002) Gabapentin: pharmacology and its use in pain management. Anaesthesia 57(5):451–462
Yamaguchi K, Kumakura S, Someya A, Iseki M, Inada E, Nagaoka I (2017) Anti-inflammatory actions of gabapentin and pregabalin on the substance P-induced mitogen-activated protein kinase activation in U373 MG human glioblastoma astrocytoma cells. Mol Med Rep 16(5):6109–6115
Stadnicki A, Pastucha E, Nowaczyk G, Mazurek U, Plewka D, Machnik G, Wilczok T, Colman RW (2005) Immunolocalization and expression of kinin B1R and B2R receptors in human inflammatory bowel disease. Am J Physiol Gastrointest 289(2):G361–G366
Ter Beek W, Biemond I, Muller E, Van den Berg M, Lamers C (2007) Substance P receptor expression in patients with inflammatory bowel disease: determination by three different techniques, ie, storage phosphor autoradiography, RT-PCR and immunohistochemistry. Neuropeptides 41(5):301–306
Hegazy SK, El-Bedewy MM (2010) Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol 16(33):4145
Zhang Y, Cardell LO, Edvinsson L, Xu CB (2013) MAPK/NF-κB-dependent upregulation of kinin receptors mediates airway hyperreactivity: a new perspective for the treatment. Pharmacol Res 71:9–18
de Brito TV, Júnior GJ, da Cruz Júnior JS, Silva RO, da Silva Monteiro CE, Franco AX, Vasconcelos DF, de Oliveira JS, da Silva Costa DV, Carneiro TB, Duarte AS (2020) Gabapentin attenuates intestinal inflammation: role of PPAR-gamma receptor. Eur J Pharmacol 873:172974. https://doi.org/10.1016/j.ejphar.2020.172974
Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, Yasa N, Khademi Y, Abdollahi M (2009) On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Open Life Sci 4(2):204–213
Elson CO, Sartor RB, Tennyson GS, Riddell RH (1995) Experimental models of inflammatory bowel disease. Gastroenterology 109(4):1344–1367
Chang CY, Challa CK, Shah J, Eloy JD (2014) Gabapentin in acute postoperative pain management. Biomed Res Int. https://doi.org/10.1155/2014/631756
Acknowledgements
The research was supported by the Vice Chancellery of Research of Guilan University of Medical Sciences, Rasht, Iran.
Funding
The research was financially supported by the Vice Chancellery of Research of Guilan University of Medical Sciences, Rasht, Iran (Contract Number: 546-41).
Author information
Authors and Affiliations
Contributions
AM contributed to data collection, data analyses, and drafting the manuscript; SB and FAT contributed to data collection and drafting the manuscript; EZ and SD contributed to statistical analysis and drafting the manuscript; PK and MM contributed to data collection and drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Research involving human and animal rights
This study involved animals and was approved by Ethics Committee of Guilan University of Medical Sciences in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (Ethics No: IR.GUMS.REC.1398.020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motavallian, A., Bouzari, S., Zamani, E. et al. An investigation of the anti-inflammatory effects of gabapentin on acetic acid-induced colitis in rats. Mol Biol Rep 48, 3423–3430 (2021). https://doi.org/10.1007/s11033-021-06357-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06357-2