Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related deaths in the USA. Although management strategies have evolved, there are continued controversies about the use of neoadjuvant chemotherapy (NAC) and pretreatment biliary drainage (PBD) in patients with resectable and potentially resectable disease.
Aims
We aimed to characterize the practice trends and outcomes for NAC and PBD.
Methods
A single-center cohort study was performed. Electronic medical records were reviewed between 2011 and 2019, and 140 patients who had pancreaticoduodenectomy for PDAC were included. Diagnosis, treatment, and outcome data were captured.
Results
There were no statistically significant temporal trends relating to the use of chemotherapy and PBD. Overall, 41% of patients received NAC and had improved survival, independent of other factors. Of the 71% who received PBD, only 40% had appropriate indications; 30% experienced postprocedure complications, and 34% required reintervention. Factors associated with the application of PBD included preoperative jaundice (OR 70.5, 95% CI 21.4–306.6) and evaluation by non-tertiary therapeutic endoscopists (OR 3.9, 95% CI 1.3–13.6). PBD was associated with a 12-day delay in surgery among those who did not receive NAC (p = 0.005), but there were no differences in surgical complications or mortality.
Conclusions
Our findings suggest that (1) NAC may confer a survival benefit and (2) PBD should be reserved for individuals with jaundice requiring NAC. Implementation of guidelines by North American gastroenterology societies, multidisciplinary treatment models, and delivery of care at high-volume tertiary centers may help optimize management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common subtype of pancreatic exocrine neoplasms and represents an aggressive cancer that impacts 50,000 patients in the USA annually [1]. Since nearly 80% have unresectable disease at the time of diagnosis, the five-year survival for PDAC is less than 10%, making it the third leading cause of cancer-related deaths in the nation [2]. Patients who have resectable or potentially resectable cancers have the possibility of achieving remission with the timely initiation of treatment. However, the landscape of management for PDAC has evolved over the past decade, leading to new controversies.
Classically, the treatment approach among individuals with neoplasms of the head of the pancreas and adequate performance status has been pancreaticoduodenectomy (Whipple procedure) followed by chemotherapy. Over the past 40 years, medical oncology research for resectable PDAC has been predominantly dedicated toward improving outcomes with adjuvant chemotherapy with recent evidence supporting the use of fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) as first-line therapy [3,4,5,6,7,8]. However, in the last 10 years, the use of preoperative or neoadjuvant chemotherapy (NAC) has also emerged as a possible strategy, and although it has become increasingly popular among some oncologists, there remains disagreement about its future role, especially in the context of conflicting recommendations from major oncology societies [9,10,11,12,13,14].
Many patients with resectable or potentially resectable PDAC undergo pretreatment biliary drainage (PBD) prior to chemotherapy or surgery. Among patients who receive NAC, jaundice is a common contraindication to treatment due to concerns about the increased toxicity of chemotherapeutic agents in this setting. Endoscopic retrograde cholangiopancreatography (ERCP) with biliary stenting is routinely performed prior to the initiation of chemotherapy in individuals with even minor elevations in total bilirubin (TB) levels, though there is a paucity of the literature supporting this practice. Historically, patients destined to have immediate surgery with subsequent adjuvant chemotherapy also typically received preoperative biliary drainage to manage or prevent complications of cholestasis. However, over the last decade, retrospective studies, meta-analyses, and one randomized controlled trial indicate that drainage prior to immediate surgery does not improve outcomes, may delay surgery, and likely increases the risk of pre- and postsurgical complications [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Although North American gastroenterology societies have not yet provided recommendations for PBD, the European Society of Gastrointestinal Endoscopy (ESGE) suggests that this practice should be reserved for patients with jaundice who will be receiving NAC or those who have cholangitis or intense pruritus with an anticipated delay in surgery [39]. Nevertheless, in many centers, PBD is still routinely performed in most patients for unclear reasons [40].
In addition to describing the temporal trends relating to NAC and PBD, we aimed to (1) characterize the impact of NAC on mortality and (2) understand the factors associated with the application of PBD and its effect on morbidity and mortality. Our goal was to provide a framework for optimizing care for patients with resectable or potentially resectable PDAC.
Methods
Patient Selection
Our study was approved by the Institutional Review Board at Yale-New Haven Hospital, a National Cancer Institute-designated Comprehensive Cancer Center. Electronic medical records were queried for patients who underwent pancreaticoduodenectomy between 2011 and 2019. The initial search did not include the indication for surgery as a qualifier. Of the 310 patients identified, 170 individuals were subsequently excluded either because of their primary neoplasm (duodenal carcinoma, cholangiocarcinoma, or pancreatic cyst) or due to incomplete treatment records, most commonly missing laboratory data. Patients who were excluded for the latter reason were more likely to have received one or more aspects of their care outside of our tertiary care center. The study ultimately included 140 patients with resectable or potentially resectable PDAC of the head of the pancreas with complete treatment records. For our purposes, the term potentially resectable was used in an analogous fashion to borderline-resectable and included non-metastatic neoplasms that partially involve the splanchnic vasculature but may be amenable to resection after chemotherapy and/or with subsequent surgical reconstruction [41]. Patients who became unresectable and did not proceed to surgery after receiving PBD or after the administration of NAC were not included in this study based on the screening criteria utilized. Jaundice was defined by TB levels of greater than 3 mg/dL because this threshold is commonly used by hepatologists and medical oncologists and may impact treatment considerations relating to the use of chemotherapy [42].
Data Collection and Statistical Analysis
Demographic, procedural, laboratory, and outcome data were collected for all patients (Tables 1 and 2). The diagnosis date represents the date of definitive biopsy with the exception of one case where biopsy could not be obtained due to gastric bypass anatomy. Follow-up occurred until January 1, 2020. TB values represent those just prior to surgery and biliary drainage. Pearson’s chi-squared tests were performed to assess for changes in temporal trends relating to the use of chemotherapy and biliary drainage. Kaplan–Meier survival plots and univariate and multivariate Cox proportional hazards were calculated to determine factors that may impact survival. Logistic regression analyses were performed to determine factors associated with the use of PBD and the presence of perioperative infections. An unpaired t test with Welch’s correction was used to determine whether biliary drainage impacted time to surgery. Analyses were performed using the survival, survminer, and ggplot2 packages in R (R Core Team, 2019) [43,44,45].
Results
Temporal Trends Relating to Chemotherapy and Pretreatment Biliary Drainage
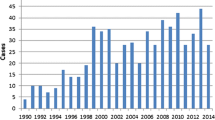
Of the 140 patients included, half were diagnosed from 2012 to 2016; the remaining half were diagnosed between 2016 and 2019. In total, 71 received adjuvant chemotherapy, 58 received NAC, and 11 did not receive chemotherapy; 99 patients received PBD (Table 1). NAC was administered to patients with either potentially resectable or resectable disease, whereas patients who received upfront surgery generally had resectable disease at the time of diagnosis. Annual trends from 2013 through 2018 are depicted in Table 3. When comparing practices between the 2012–2016 and 2016–2019 periods, there were increases in the proportions of patients receiving NAC (26/70 versus 32/70; p value = 0.39), first-line FOLFIRINOX (28/70 versus 39/70; p value = 0.09), and PBD (47/70 versus 52/70, p value = 0.46), though these trends were not statistically significant.
Impact of Neoadjuvant Chemotherapy
Kaplan–Meier survival analysis (Fig. 1) and Cox proportional hazard models (Table 4) suggest a survival benefit for patients who received NAC followed by surgical resection, although our study did not include patients who were unable to undergo surgery due to unexpected progression of disease or other complications attributed to NAC. Staging at the time of surgery revealed that, among patients who received NAC, 24% had 0, IA, or IB disease, 57% had IIA or IIB disease, and 19% had III or IV disease, in comparison with 6%, 80%, and 13%, respectively, among patients who received upfront surgery. The choice of initial chemotherapy regimen (FOLFIRINOX versus alternative regimens), the presence of jaundice, and the use of PBD via either endoscopic or percutaneous techniques did not impact survival (Table 4). Our analysis included 8 patients who were deemed to have locally advanced and unresectable disease during their operation with premature termination of the procedure, five of whom had received NAC. The mean time from diagnosis to initiation of chemotherapy for the NAC group was identical to the mean time from diagnosis to surgery for the adjuvant chemotherapy group (33 days).
Characteristics of the Patients Who Received Pretreatment Biliary Drainage
Biliary drainage occurred in the context of the clinical features noted in Fig. 2. Among those who received drainage, 80 had their procedures performed at the time of their initial presentation, most often concurrently with diagnostic endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA), and 19 had their procedures after the initial diagnosis but before chemotherapy or surgery. Twelve patients who had either (1) no jaundice or (2) subclinical jaundice with upfront resection received biliary drainage. Of the 86 patients with clinically relevant jaundice who received PBD, only 39 (45%) received NAC. Of all patients who received PBD, 59 were initially evaluated and had one or more endoscopic procedures performed by non-tertiary gastroenterologists and 53 had their drainage procedures performed by non-tertiary therapeutic endoscopists or interventional radiologists. Only 14 total patients were evaluated by either medical or surgical oncology prior to biliary drainage.
Factors Associated with the Application of Pretreatment Biliary Drainage
In univariate logistic regression analysis, only the presence of jaundice was associated with the use of PBD. However, in multivariate logistic regression analysis, the presence of jaundice and initial evaluation by non-tertiary gastroenterologists were both associated with the use of PBD (Table 5). The use of NAC was not included as a factor in this analysis because chemotherapy strategies were rarely delineated prior to biliary drainage. Disagreement between the borderline-significant p value and 95% confidence interval for the impact of early evaluation by surgical or medical oncology occurred as a result of sample size limitations.
Impact of Pretreatment Biliary Drainage
Endoscopic drainage was successful in relieving jaundice in most cases, but initial failure, complication, and re-intervention rates were relatively high (Table 2). Twenty patients had at least one failed attempt at endoscopic biliary drainage, of which 7 ultimately had successful endoscopic drainage, 11 required percutaneous drainage, and two patients proceeded to surgery without biliary drainage. Thirty patients experienced postprocedural complications, including pancreatitis (8), cholangitis (9), and stent or drain dysfunction without cholangitis (13), of which 23 required reintervention. There was no association between postprocedural complications and the type of stent deployed, either metal or plastic, or the manner of drainage, either endoscopic or percutaneous, in univariate and multivariate analyses (Table 5). An additional 11 patients required preoperative reintervention for routine stent exchanges in the absence of complications. Furthermore, there were likely patients who experienced complications due to PBD such as severe necrotizing pancreatitis and were unable to proceed to surgery and thus not included in our study.
The use of PBD did not impact survival in univariate or multivariate Cox proportional hazard models (Table 4) and Kaplan–Meier analysis (Fig. 3). However, the mean time between diagnosis and resection or attempted resection for those who received up-front surgery was longer for patients who underwent biliary drainage (37 versus 25 days; p value = 0.005). In some cases, the delay was to allow for the resolution of jaundice or PBD-related complications, whereas in others, delays occurred as a result of unrelated medical comorbidities. Among all patients included in the study, 14 patients (10%) experienced surgical infections with or without additional complications and four patients died in the postoperative period (Table 2). There was no association between either biliary drainage or jaundice at time of resection and surgical infections among all patients (Table 5) and also among the subset of patients who only received surgery followed by either adjuvant chemotherapy or no chemotherapy (analysis not shown).
Discussion
Over the past decade, the management of resectable and potentially resectable PDAC has evolved. Although there is still a need for ongoing studies to identify the optimal chemotherapy strategies, the data relating to PBD are exhaustive and robust, including more than 20 comparative studies over the past decade with a majority suggesting increased morbidity among patients who receive PBD and do not receive NAC [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Our study includes data from our tertiary center over a period of approximately 8 years and focuses on characterizing trends in practice patterns and clinical outcomes at our institution, making comparisons to evidence from recent studies and identifying possible factors for the differences.
NAC was increasingly used with improved survival among patients who went on to have resection or attempted resection, and additional factors, including the application of biliary drainage, did not impact this outcome. These findings are generally consistent with a recent multicenter randomized phase III trial, which demonstrated improved survival among the subset of patients who underwent resection [9]. Furthermore, although a higher proportion of patients in the NAC group received FOLFIRINOX as first-line chemotherapy, no differences in outcomes were observed among those who received FOLFIRINOX versus other regimens since most received multi-agent treatments, which is consistent with the most recent evidence available from the SWOG S1505 trial [11].
The use of PBD remained fairly common over the study period. Nearly 60% of patients who received PBD either had no jaundice or had surgery prior to chemotherapy, generally precluding the need for drainage. Most received biliary drainage at time of their initial presentation, simultaneously with a diagnostic EUS and FNA, presumably in an effort to expedite care and minimize the need for additional procedures. However, while there was no increase in operative complications or mortality associated with drainage, the rates of postdrainage complications (30%) and reintervention (34%) were high, illustrating that PBD did not ultimately streamline care. Furthermore, as expected, biliary drainage was associated with a short delay in the time to surgical resection, for which the long-term impact is not entirely clear [46]. Although prior studies have demonstrated inconsistent findings relating to outcomes associated with metal versus plastic stenting, our study did not detect differences in complication rates based on stent type [47, 48]. Likewise, no differences were observed between endoscopic and percutaneous approaches. The use of uncovered metal stents was exceedingly rare in our study, and thus, no analysis was performed to compare covered and uncovered metal stents.
Aside from the presence of jaundice and the use of NAC, there are likely other factors that contribute to the increased use of PBD in patients with PDAC. In our study, patients treated by non-tertiary gastroenterologists were more likely to undergo drainage procedures. It has been theorized that the management of patients with potentially resectable PDAC at high-volume tertiary care centers may help optimize the delivery of care and investigators have previously addressed this particular question with varying results [49, 50]. However, those studies did not assess how the venue of care and physician expertise impact the use of biliary drainage in the manner posed in our study. Although the underlying reasons for this finding are not entirely clear, some community providers may be less familiar with the management of PDAC and with multidisciplinary practice patterns. Furthermore, we also postulated that preprocedure evaluation by a medical or surgical oncologist may prevent unnecessary drainage procedures. However, we observed that only a minority of patients were evaluated by oncologists prior to drainage, thereby prohibiting meaningful conclusions.
Although our study has limitations, including a small sample size from one tertiary center and use of retrospective methodology, it highlights important principles pertaining to the management of PDAC. Our findings support the use of NAC and suggest that PBD is being over-utilized in the absence of strong indications. Over the coming decade, the management of PDAC will continue to change and the role of NAC will likely become clearer. While this may result in a continued need for PBD, clinicians should aim to incorporate this modality only when appropriate indications exist. It should be reserved for jaundiced patients destined to receive NAC or for those with life-threatening complications of cholestasis and avoided in stable patients with or without jaundice who are awaiting immediate surgery.
To minimize the unnecessary of use biliary drainage, patients who have radiographic findings consistent with resectable or potentially resectable pancreatic head ductal adenocarcinoma should be referred to high-volume centers and have multidisciplinary case reviews. Because gastroenterologists often serve as the initial point-of-contact for patients with newly diagnosed PDAC, they maintain a critical role in the early management of this condition. Therefore, the use of evidence-based practices relating to PBD should be supported by guidelines proposed by North American gastroenterology societies as this may encourage practitioners to apply more objective criteria for biliary drainage, thereby reducing the rates of unnecessary interventions in both tertiary centers and community practices.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10–27.
Neoptolemos JP, Dunn JA, Stocken DD et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576.
Oettle H, Post S, Neuhaus P et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267.
Neoptolemos JP, Stocken DD, Bassi C et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073.
Regine WF, Winter KA, Abrams RA et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019.
Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol 2019; 37S: ASCO #4000.
Conroy T, Hammel P, Hebbar M et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395.
Versteijne E, Suker M, Groothuis K et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763–1773.
Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190–194.
Sohal D, Duong MT, Ahmad SA, et al: SWOG S1505: Results of perioperative chemotherapy with mFOLFIRINOX versus gemcitabine/nab-paclitaxel for resectable pancreatic ductal adenocarcinoma. ASCO20 Virtual Scientific Program. Abstract 4504.
Chawla A, Ferrone CR. Neoadjuvant Therapy for Resectable Pancreatic Cancer: An Evolving Paradigm Shift. Front Oncol. 2019;9:1085.
Khorana AA, Mangu PB, Berlin J et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541–2556.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–1061.
Shen Z, Zhang J, Zhao S, Zhou Y, Wang W, Shen B. Preoperative biliary drainage of severely obstructive jaundiced patients decreases overall postoperative complications after pancreaticoduodenectomy: A retrospective and propensity score-matched analysis. Pancreatology. 2020;20:529–536.
Darnell EP, Wang TJ, Lumish MA, et al. Preoperative cholangitis is an independent risk factor for mortality in patients after pancreatoduodenectomy for pancreatic cancer [published online ahead of print, 2020 Aug 11]. Am J Surg. 2020;S0002–9610(20)30479–7.
Pamecha V, Sadashiv Patil N, Kumar S et al. Upfront pancreaticoduodenectomy in severely jaundiced patients: is it safe? J Hepatobiliary Pancreat Sci. 2019;26:524–533.
De Pastena M, Marchegiani G, Paiella S et al. Impact of preoperative biliary drainage on postoperative outcome after pancreaticoduodenectomy: An analysis of 1500 consecutive cases. Dig Endosc. 2018;30:777–784.
Lee PJ, Podugu A, Wu D, Lee AC, Stevens T, Windsor JA. Preoperative biliary drainage in resectable pancreatic cancer: a systematic review and network meta-analysis. HPB (Oxford). 2018;20:477–486.
El Nakeeb A, Salem A, Mahdy Y et al. Value of preoperative biliary drainage on postoperative outcome after pancreaticoduodenectomy: A case-control study. Asian J Surg. 2018;41:155–162.
Cazauran JB, Perinel J, Kepenekian V et al. Unnecessary preoperative biliary drainage: impact on perioperative outcomes of resectable periampullary tumors. Langenbecks Arch Surg. 2017;402:1187–1196.
Scheufele F, Schorn S, Demir IE et al. Preoperative biliary stenting versus operation first in jaundiced patients due to malignant lesions in the pancreatic head: A meta-analysis of current literature. Surgery. 2017;161:939–950.
Moole H, Bechtold M, Puli SR. Efficacy of preoperative biliary drainage in malignant obstructive jaundice: a meta-analysis and systematic review. World J Surg Oncol. 2016;14:182.
Sahora K, Morales-Oyarvide V, Ferrone C et al. Preoperative biliary drainage does not increase major complications in pancreaticoduodenectomy: a large single center experience from the Massachusetts General Hospital. J Hepatobiliary Pancreat Sci. 2016;23:181–187.
Furukawa K, Shiba H, Shirai Y et al. Negative Impact of Preoperative Endoscopic Biliary Drainage on Prognosis of Pancreatic Ductal Adenocarcinoma After Pancreaticoduodenectomy. Anticancer Res. 2015;35:5079–5083.
Chen Y, Ou G, Lian G, Luo H, Huang K, Huang Y. Effect of Preoperative Biliary Drainage on Complications Following Pancreatoduodenectomy: A Meta-Analysis. Medicine (Baltimore). 2015;94:e1199.
Uemura K, Murakami Y, Satoi S et al. Impact of Preoperative Biliary Drainage on Long-Term Survival in Resected Pancreatic Ductal Adenocarcinoma: A Multicenter Observational Study. Ann Surg Oncol. 2015;22:S1238–S1246.
Liu C, Lu JW, Du ZQ, Liu XM, Lv Y, Zhang XF. Association of Preoperative Biliary Drainage with Postoperative Morbidity after Pancreaticoduodenectomy. Gastroenterol Res Pract. 2015;2015:796893.
Sun C, Yan G, Li Z, Tzeng CM. A meta-analysis of the effect of preoperative biliary stenting on patients with obstructive jaundice. Medicine (Baltimore). 2014;93:e189.
Arkadopoulos N, Kyriazi MA, Papanikolaou IS, et al. Preoperative biliary drainage of severely jaundiced patients increases morbidity of pancreaticoduodenectomy: results of a case-control study [published correction appears in World J Surg. 2015 Feb;39:554]. World J Surg. 2014;38:2967–2972.
Kitahata Y, Kawai M, Tani M et al. Preoperative cholangitis during biliary drainage increases the incidence of postoperative severe complications after pancreaticoduodenectomy. Am J Surg. 2014;208:1–10.
di Mola FF, Tavano F, Rago RR et al. Influence of preoperative biliary drainage on surgical outcome after pancreaticoduodenectomy: single centre experience. Langenbecks Arch Surg. 2014;399:649–657.
Wang S, Wang X, Li L, Dai H, Han J. Association of preoperative obstructive jaundice with postoperative infectious complications following pancreaticoduodenectomy. Hepatogastroenterology. 2013;60:1274–1279.
Ngu W, Jones M, Neal CP, Dennison AR, Metcalfe MS, Garcea G. Preoperative biliary drainage for distal biliary obstruction and post-operative infectious complications. ANZ J Surg. 2013;83:280–286.
Singhirunnusorn J, Roger L, Chopin-Laly X, Lepilliez V, Ponchon T, Adham M. Value of preoperative biliary drainage in a consecutive series of resectable periampullary lesions. From randomized studies to real medical practice. Langenbecks Arch Surg. 2013;398:295–302.
Feng J, Huang ZQ, Chen YL et al. Zhonghua Wai Ke Za Zhi. 2012;50:294–298.
van der Gaag NA, Rauws EA, van Eijck CH et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137.
Mezhir JJ, Brennan MF, Baser RE et al. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13:2163–2169.
Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910–930.
Bakens M, van Rijssen B, van Woerden V. Evaluation of Preoperative Biliary Drainage in Patients Undergoing Pancreatoduodenectomy For Suspected Pancreatic or Periampullary Cancer. J Pancreas. 2018;19:24–28.
Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20:10740–10751.
Álvarez R, Carrato A, Adeva J et al. Management of hyperbilirubinaemia in pancreatic cancer patients. Eur J Cancer. 2018;94:26–36.
Therneau T. A Package for Survival Analysis in S. R package version 2.38, 2015.
Kassambara A, Kosinski M, Biecek P. survminer: Drawing Survival Curves using 'ggplot2'. R package version 0.4.8, 2020.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2016.
Eshuis WJ, van der Gaag NA, Rauws EA et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840–849.
Song TJ, Lee JH, Lee SS et al. Metal versus plastic stents for drainage of malignant biliary obstruction before primary surgical resection. Gastrointest Endosc. 2016;84:814–821.
Tol JA, van Hooft JE, Timmer R et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut. 2016;65:1981–1987.
Bateni SB, Gingrich AA, Hoch JS, Canter RJ, Bold RJ. Defining Value for Pancreatic Surgery in Early-Stage Pancreatic Cancer. JAMA Surg. 2019;154:e193019.
Shannon AB, Mo J, Song Y, et al. Does multicenter care impact the outcomes of surgical patients with gastrointestinal malignancies requiring complex multimodality therapy? [published online ahead of print, 2020 Jun 20]. J Surg Oncol. 2020;https://doi.org/10.1002/jso.26075.
Funding
NIH T32 2T32DK007356-41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saffo, S., Peng, C., Salem, R. et al. Impact of Neoadjuvant Chemotherapy and Pretreatment Biliary Drainage for Pancreatic Head Ductal Adenocarcinoma. Dig Dis Sci 67, 1409–1416 (2022). https://doi.org/10.1007/s10620-021-06967-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06967-7