Abstract
Background
Endoplasmic reticulum stress (ERS) has been studied as critical factor during occurrence and development of ulcerative colitis (UC). However, the role of ERS in inflamed UC remains unclear.
Aims
The purpose of this study was to analyze the role of inositol-requiring kinase 1 (IRE-1), a major regulator of ER, in regulating ERS and cell viability.
Methods
In UC mucosa tissue, IRE-1, BiP, XBP-1s, CHOP caspase-12 and GADD34 mRNA were assayed by qRT-PCR. Then, human normal colon epithelial cell line (NCM-460) and colon fibroblast cell line (CCD-33Co) were cultured, and downregulated or upregulated IRE-1 expression. ERS was induced with 100 ng/mL of Interleukin 6 (IL-6). CCK8 assay was performed to analyze cell proliferation. Flow cytometry analysis was conducted to detect the apoptosis. Western blot assay was used to examine ERS markers.
Results
IRE-1, BiP, XBP-1s, caspase-12 and CHOP mRNA were highly expressed in UC mucosa tissue, and the expression of GADD34 mRNA significantly decreased. These results show that ERS-induced unfolded protein response was enhanced in UC mucosa tissue. In cells, silencing the expression of IRE-1 could suppress cell proliferation and promote apoptosis through activating unfolded protein response, while the over-expression of IRE-1 had the opposite effect. IL-6 could induce ERS and cells apoptosis. Furthermore, we demonstrated that shRNA IRE-1 could enhance the inhibition of IL-6 on cells viability.
Conclusions
Inhibition of IRE-1 enhanced unfolded protein response and cells apoptosis and IL-6-induced ERS and suggested that IRE-1 might be a potential target of UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) and Crohn disease are the most common forms of inflammatory bowel disease (IBD). UC is a common, recurrent, and nonspecific chronic inflammation of the colon and is confined to the mucosa and submucosa [1]. Most lesions start with the sigmoid colon and rectum but can also spread to the descending colon. UC often involves the entire colon, with the characteristics of a long course and often relapses. The characteristic of UC is inflammation activation and the loss of intestinal barrier integrity in the colon. The intestinal barrier is a dynamic protein network established by intestinal epithelial cells (IECs) [2]. In UC patients, the proliferation apoptosis of IECs is disorder, especially in the areas of inflammatory activation, IECs accelerate apoptosis, which leading to the destruction of protein network and intestinal barrier function [3]. This is an essential player in the development and persistence of UC intestinal inflammation. As the course of disease progresses, intestinal fibrosis is a tricky complication of IBD, which correlate with high numbers of activated fibroblasts that resulted in fibrosis and tissue remodelling [4]. The mucosal matrix remodeling is related to the integrity of the IECs homeostasis [5]. IECs and fibroblasts are essential for establishing an intestinal epithelial barrier to maintain normal intestinal homeostasis and prevent inflammation. Although some specific factors have been directly related to IBD, including genetic factors, abnormal intestinal immunity, and/or gut microbiota modifications, the etiology is still unknown [6]. The endoplasmic reticulum (ER) is the site for synthesis, folding, and quality control of secreted and metabolism proteins. ER stress (ERS) induces IECs apoptosis and intestinal epithelial homeostasis destruction, which is the basis of the pathogenesis of many intestinal diseases [3, 7, 8]. Patients with IBD exhibit signs of ERS in their ileal and/or colonic epithelia with active disease [9, 10]. However, the role of ERS in inflamed UC remains unclear.
ER is an important organelle in eukaryotic cells. It is very sensitive to change in intracellular and extracellular pressure because of the dynamic synthesis undertaken. Intestinal mucosa often subjected to severe, persistent stress stimuli, such as those induced by pathogenic microorganisms, oxidative stress, and physical and chemical factors, which affected the process of protein synthesis in the ER. Excessive unfolded or misfolded proteins accumulate in ER, which would cause ERS [11]. The ER through activating the unfolded protein response (UPR) and restoring cellular function to respond ERS-induced cellular damage [9]. The abnormal accumulation of unfolded or misfolded proteins in ER will damage the maintenance of ER function and restoration of cells homeostasis [12]. UPR maintains the normal function of the cells by reducing unfolded or misfolded proteins in ER. Mild to moderate ERS-induced UPR is seen as a compensatory mechanism to restore the homeostasis of ER, whereas persists ERS damages cellular functions and switches to an adaptation mechanism of apoptosis to remove irreversibly injured cells [13]. Excessive apoptosis can lead to the inability to repair IECs, which damages the intestinal epithelial integrity and homeostasis. ERS is significantly increased in intestinal epithelial and act as a major contributor to IBD [10]. ERS can cause IECs and Paneth cells deficiency and overactive responses to IBD-inducers [9]. Intestinal inflammation can originate solely from ERS abnormalities in IECs, thus cell-specific ERS link to organ-specific inflammation.
ERS trigger a cascade of pro-inflammatory factors to induce UC inflammatory. For self-protection and reduction ERS, the UPR is activated to degrade unfolded or misfolded proteins by inositol-requiring kinase 1 (IRE-1), protein kinase R-like endoplasmic reticulum kinase (PERK), and activated transcription factor 6 (ATF-6) [14]. IRE-1 is the most conservative branch of UPR. Once the misfolded or damaged proteins accumulate in ER, IRE-1 is automatically phosphorylated to form a dimer, which activates its endonuclease region and splices X-box binding protein 1 (XBP-1) mRNA to XBP-1s. XBP-1s acts as a transactivator and major effector of the UPR and induces a large number of chaperone proteins to repair unfolded and misfolded proteins, consequently alleviating ER stress [15]. At the same time, chaperone protein immunoglobulin binding protein (Bip) is dissociated from the ER stressors of IRE-1 and PERK, and activation of PERK-ATF4-C/EBP homologous protein 10 (CHOP) and protein phosphatase 1 regulatory subunit 15A (GADD34) to induce cells apoptosis [16]. Persistent ERS-UPR contributes to intestinal mucosal injury and inflammation, which is related to the dysregulation of phosphatidylinositol, NF-κB and pro-inflammatory interleukin signal [17]. The pro-inflammatory Interleukin 6 (IL-6) and TNF-α can induce IECs apoptosis by upregulating ERS via increasing the expression of p-IRE-1 and Bip to activate p-JNK, Bax/Bcl-2 ratio, and cleaved caspase-3 [18]. IL-6 is the strongest correlations with clinical features of IBD, such as positive correlation with the production of triglycerides VLDL, MCP-1, IL-15, and IL-17, and act as the predictor of the biologics-treated IBD [19, 20]. But, the interplay of ERS and IL-6 is complex. IL-6 not only induces ERS, ERS can in turn increase IL-6 expression. That is, IRE-1-XBP1 and PERK-ATF4-CHOP axes upregulate of IL-6 expression that exacerbates JAK/STAT1/STAT3 signaling, thus inducing chronic inflammation [16]. Meanwhile, IRE-1 regulated XBP-1s and IL-6 expression that mediates inflammation-induced fibrosis [21]. Therefore, IL-6 is an important regulator of ERS-UPR.
ERS can influence the occurrence and development of UC by regulating the IECs apoptosis and inflammatory response signaling pathways [3, 8,9,10]. With increasingly in-depth research into molecular pathways, scholars have studied UC in recent years and started paying more attention to the role of ERS in the pathogenesis of UC. Further understanding of ERS-UPR in IECs may help fill the gap in current IBD pathomechanism. This study briefly explored the role of IRE-1 in ERS-induced UC.
Materials and Methods
Patients and Biopsy Specimens
Twenty patients with UC at the Department of gastroenterology, Second Hospital Affiliated to Nanchang University, were included in the study. The UC’s diagnosis was based on Lennard-Jones criteria, and clinical disease activity was assessed according to the Colitis Disease Activity Index. Patients’ age ranged from 24 to 54. All the UC patients had digestive system manifestations. The main clinical manifestations were diarrhea, mucous pus and blood stool, and abdominal pain of varying degrees. The patients were mainly chronic recurrent and mild to moderate lesions. Colonoscopy revealed changes in hyperemia, edema, and erosion of the colonic mucosa. Among them, four (25%) patients had wasting, 9 (45%) had fever, and 7 (35%) had fatigue. Colonic biopsy samples were obtained via endoscopic investigations in 20 patients with UC, include inflamed and noninflammed tissue. One biopsy samples were set apart from each patient for pathological examination, and the other samples were collected from mucosal tissue and stored at − 80 °C until analysis. The Ethics Committee of Second Hospital Affiliated to Nanchang University approved the study protocol, and informed consent was obtained from all patients before enrollment. The investigation adhered to the principles outlined in the Declaration of Helsinki.
Histopathological Analysis
Biopsy specimens were routinely stained with H & E. All sections were blindly examined by a pathologist experienced in digestive diseases. The indicators of observation and the criteria for judgment are mainly referred to “the standard for the diagnosis of inflammatory bowel disease biopsy developed by the British Gastroenterology Society”. Specific indicators include mucosal surface, crypt structure, number of crypts, lamina propria cells formation, cryptitis, crypt abscess, distribution of polymorphonuclear cells, epithelial changes, goblet cells reduction, related epithelial pathological changes, Subcutaneous collagen increases, granuloma, submucosa, and other tissue reactions such as giant cells. The histopathological features of each section were analyzed according to Theodossi scoring system to determine the pathological diagnosis of UC, and the clinical and endoscopic UC pathological diagnosis was analyzed.
Reverse Transcription: Polymerase Chain Reaction
The colon tissue of inflamed and noninflamed was ground in liquid nitrogen, and total RNA was extracted from the 50 mg tissue powder using the NucleoZOL® (Gene, Co., Ltd, Shanghai, China). Primers were resuspended by adding RNase free water. Master mix was prepared for each single real-time polymerase chain reaction (PCR) reaction, including 10 µL qPCR SYBR® Green Master Mix Universal, 10 µL primer, 3 µL RNase-free water, and 2 µL of RT product.
Reverse transcription (RT) was performed using the Reverse Transcription System Kit (Takara, Dalian, China). The synthesized cDNA was amplified by quantitative PCR using the HEAL FORCE (Xianggang, China). The reaction conditions were 42 °C for 60 min, followed by cooling to 4 °C. The resultant cDNA was used as a template for subsequent PCR. Forty cycles of PCR amplification were performed, with initial incubation at 95 °C for 10 min and final extension at 72 °C for 5 min. Each cycle comprised denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. The mRNA expression level of IRE-1, BiP, XBP-1s, caspase-12, CHOP and GADD34 were normalized to GAPDH. Relative expression of genes were calculated by the formula ∆∆Ct = (Ct.Target − Ct.internal reference)X − (Ct.Target − Ct.internal reference)Control, and the estimated expression ratio is equal to 2−ΔΔCt. The primer sequences: IRE-1: FORWARD is GTTCTTCCAGGACGTGAGCG, REVERSE is CAGTCCATCTTCACCACGGC; Bip: FORWARD is ACGCTGGAACTATTGCTG GC, REVERSE is ACCCAGGTCAAACACCAGGA; caspase-12: FORWARD is AGCACTGGGATCAAGAGCCA, REVERSE is CAATCCCAGCACCATTGCCT; XBP-1s: FORWARD is AAACAGAGTAGCAGCTCAGACTGC, REVERSE is TCCTTCTGGGTAGACCTGGAG; CHOP: FORWARD is CTGCTTCTCTGGCTT GGCTGAC, REVERSE is TTGGTCTTCCTCC TCTTCCTCCTG; GADD34: FORWARD is CAGGAGAGGACACAGAGGAAGAGG, REVERSE is AGCAGGA GTGGAAGAGGAAGCC; GAPDH: FORWARD is GTCATCCCTGAGCTGAACG G, and REVERSE is CCACCTGGTGCTCAGTGTAG.
Cell Culture and Transfection
Human normal colon epithelial cell line (NCM-460) was purchased from INCELL (San Antonio, Texas, USA). The NCM-460 cells cultured in M3D medium (INCELL) and add 10% fetal bovine serum (FBS, HyClone, USA). The culture temperature was 37 °C and the CO2 concentration was 5%.
Human normal colon fibroblast cell line (CCD-33Co) was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). The CCD-33Co cells cultured in RPMI-1640 medium and add 10% fetal calf serum (FBS, HyClone, USA). The culture temperature was 37 °C and the CO2 concentration was 5%. Prior to the experiment, cells were seeded at appropriate densities in cell culture plates.
Cells were firstly inoculated into 24-well plates before transfection. Lipofectamine 2000 Transfection Reagent (Invitrogen, USA) was utilized for cell transfection. For the gene knockdown and overexpression experiment, cells were transfected with an appropriate amount of sh-IRE-1 and OE-IRE-1. The sense sequences are as follows: negative control shRNA was 5′-GATCCCAGGGCGCTTATTGCATCAGCTACATT CAAGAGATGTAGCTGATGCAATAAGCGCCCTGTTTTTG-3′; shRNA-IRE-1 was: 5′-GATCCCAGCGGGCTTATTGCATCAGCTACATTCAAGAGATGTAGCTGATG CAATAAGCCCGCTGTTTTTG-3′ (Life Technologies, Carlsbad, CA, USA). For IRE-1 overexpression, the cells were transfected with the IRE-1 overexpression construct (pcDNA3.1-IRE-1-1). After 36 h of transfection, relevant tests were used to compare the differences.
Cell Proliferation Ability by CCK-8 Assay
The cells were trypsinized and seeded in 96-well plates at a concentration of 2 × 103 cells/mL, 3 replicate wells for each concentration. Incubate for 24 h in a 37 °C incubator and stimulate the cells with IL-6 (100 ng/mL). After stimulation for 24 h, 10% CCK-8 was added, and the OD value was measured at 450 nm after further incubation for 2 h in a 37 °C incubator.
Apoptosis Detection
Cells had been inoculated in a six-well plate and cultured overnight. Cells were collected when had confluency of 80–90%. The cells were washed twice with PBS and centrifuged at 1000 rpm for 3 min at 4 °C. Then the cells were incubated in Annexin V-FITC and PI binding buffer for 5 min in the dark, according to the manufacturer’s protocol (BD Biosciences, USA). Flow cytometry (Becton–Dickinson, USA) assay was used to analyze the cells death and apoptosis in each group. Three independent experiments were performed.
Western Blot
The cells were gently scraped and harvested. RIPA buffer (Takara Bio Inc., Tokyo Japan), and protease inhibitor (Roche Diagnostics) were used for cell lysis. The protein concentration was measured by bicinchoninic acid protein assay Kit (epizyme, china). Equal amounts of protein samples were separated on sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then transferred to apolyvinylidene difluoride membranes. Membranes were incubated with blocking buffer (5% skim milk in 20 mM Tris–HCl, 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature, followed by the incubation with primary antibodies (1:1000) at 4 °C overnight. The membranes were blocked with 1 × blocking buffer for 30 min. The following primary antibodies were used: IRE-1, BiP, XBP-1s, CHOP, caspase-12, cleaved-caspase-12, GADD34 and β-actin (Cell Signaling Technology, USA). The secondary-HRP-antibodies (Protein-Tech, USA) incubated for 2 h at room temperature, and applied ECL Western blotting reagents (GE Healthcare, USA) on the membranes to detect the protein levels. The pictures of protein bands were taken by a gel imaging system Versa Doc™ imaging system and the results were analyzed with ImageJ software.
Statistical Analysis
The statistical analysis was executed by GraphPad Prism. The Student’s t test was performed to compare groups’ differences. And significance of difference between groups was further determined with ANOVA. p < 0.05 was considered statistically significant differences. Data are presented as the mean ± SD of the three experiments.
Results
UC Mucosal Tissue Damage Initiated ERS-Induced UPR
The characteristics of participants are presented in Table 1. A total of 20 patients with UC were recruited into the study, aged 38.3 ± 9.9 years, of which 13 (65%) were 40–60 years and 7 (35%) were > 40 years. UC patients consisted of 55% male and 45% female, and their disease duration was 3.9 ± 2.1 years. Left-sided UC was the most common location of disease, with 11 patients (55%), followed by pancolitis (35%) and ulcerative proctitis (10%). The most common symptoms of UC patients were diarrhea (80%), and followed by mucous pus and blood (70%), and abdominal pain (70%). Colonoscopy results show that 90% of UC patients had hyperemia, 65% had edema, and 55% had erosion. The pathological examination shown that the histopathological features of UC was abnormal crypt structure, such as crypt branching, distortion and atrophy, abnormal epithelial morphology, such as goblet cells, intracellular mucus reduction and IECs decreased, and inflammatory infiltration such as crypt abscess, lymphocyte aggregation, and fibroblast infiltration (Fig. 1a–f).
Pathological changes in ulcerative colitis. Crypt structure abnormalities: crypt branching, distortion, and atrophy. Abnormal epithelial morphology: reduction of goblet cells or intracellular mucus. Inflammatory infiltration: crypt abscess and lymphocyte aggregation. Green arrow: crypt branch and distortion; orange arrow: crypt abscess and cryptitis; blue arrow: reduced mucus in goblet cells; black arrow: crypt atrophy; red arrow: epithelial cells decreased; yellow arrow: fibroblast infiltration; red star: lymphocyte infiltration. Bar = 100 μm
To observe the difference in ER stress-related protein expression in normal and UC mucosal tissues obtained by biopsy, RT-PCR was used to detect the IRE-1, BiP, XBP-1s, caspase-12, CHOP and GADD34 genes. The mRNA expression was analyzed to determine the effect of inflammatory injury on ERS. The results showed that the mRNA expression of IRE-1, BiP, XBP-1s, caspase-12 and CHOP in the ulcerative colitis mucosal were remarkably higher than those in the normal tissues (Fig. 2a–e), while GADD34 expression was significantly lower than that of the normal tissue (Fig. 2f). ERS-induced UPR was enhancement in UC mucosa.
ERS-induced UPR in ulcerative colitis mucosal. RT-PCR detected the relative expression IRE-1, BiP, XBP-1s, caspase-12, CHOP and GADD34 mRNA in normal and ulcerative colitis biopsy tissues. In addition to GADD34, the mRNA expression of IRE-1, BiP, XBP-1s, caspase-12 and CHOP in the ulcerative colitis mucosal were remarkably increased than those in the normal mucosal tissues *p < 0.05; **p < 0.01 and *** p < 0.001 versus NC
IRE-1 Affected Cell Proliferation and Apoptosis
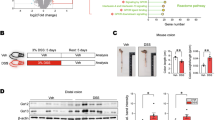
The optimal stimulation conditions were obtained by stimulating the cells with a concentration of 100 ng/mL of IL-6 for 24 h. IL-6 stimulation induced cells apoptosis and inhibited proliferation, which show that IL-6 could induce inflammatory damage of colon epithelial cells. The IRE-1 in the NCM-460 cells was knocked down or over-expressed to analyze the effect of IRE-1 on cells proliferation and apoptosis. Cells viability was significantly reduced upon IRE-1 RNA interference or by stimulation with IL-6. The cells viability of the OE-IRE-1 and OE-IRE-1 + IL-6 groups increased significantly (p < 0.001) (Fig. 3a). In addition, whether by IRE-1 interference or by stimulation with IL-6, the ability of the NCM-460 cells to undergo apoptosis increased significantly. After stimulation with both sh-IRE-1 and IL-6, the apoptotic ability increased more significantly (p < 0.001). However, over-expression of IRE-1 decreased cells apoptosis rate and rescued IL-6-induced cells apoptosis (Fig. 3b). In a word, sh-IRE-1 and IL-6 could induce apoptosis of colon epithelial cells.
IRE-1-induced UPR regulated proliferation and apoptosis of NCM-460 cells. a CCK-8 method was used to detect cells proliferation. IRE-1 increased cell proliferation and improved IL-6-induced proliferation slower. b Flow cytometry for detection of cells apoptosis. Over-expression of IRE-1 decreased cell apoptosis rate and rescued IL-6-induced cell apoptosis. c Western blot was used to detect the expression of XBP-1s and BIP. Sh-IRE-1 and IL-6 could facilitate ERS-induced UPR via increasing XBP-1s and BIP expression. d Western blot was used to detect the expression of CHOP, caspase-12, cleaved-caspase-12, and GADD34. Sh-IRE-1 and IL-6 could promote ERS-induced UPR via increasing CHOP, caspase-12, cleaved-caspase-12 and GADD34 expression. **p < 0.01 and ***p < 0.001 versus NC
Role of IRE-1 in the ERS-Induced UPR
The ER activates the UPR to counteract cell damage induced by ERS, to restore cell function caused by damage to the cell and to restore overall cell function [22, 23]. The UPR is a protective stress response mediated by an ER chaperone, glucose-regulated protein 78/binding immunoglobulin protein (GRP78/BIP), and three ER stressor proteins [24]. In one response, the IRE-1-CHOP induced apoptosis pathway. To explore the role of IRE-1 in the ERS regulatory pathway, we examined the expression level of IRE-1, BiP, and XBP-1s. IL-6 stimulation induced the expression of IRE-1 and XBP-1s in NCM-460 cells, indicating that IL-6 could induce UPR. After knocking down of IRE-1 or stimulated with IL-6, the expression of Bip and XBP-1s protein was significantly increased (p < 0.05). On the contrary, overexpression of IRE-1 decreased Bip and XBP-1s protein expression and inhibited IL-6-induced Bip and XBP-1s expression (Fig. 3C). In short, sh-IRE-1 and IL-6 could facilitate ERS-induced UPR in colon epithelial cell.
Moreover, to explore the role of IRE-1 in ERS-regulated apoptosis, we examined the expression of the ERS pro-apoptotic proteins CHOP, GADD34, caspase-12, and cleaved-caspase-12. The results showed that IL-6 stimulation significantly increased the expression of CHOP, GADD34, caspase-12, and cleaved-caspase-12 in NCM-460 cells (p < 0.05). IRE-1 knockdown significantly increased the expression of CHOP, GADD34, caspase-12, and cleaved-caspase-12 proteins, especially in the sh-IRE-1 + IL-6 group (p < 0.05). The opposite results were observed in the over-expression group. Treatment with OE-IRE-1 decreased the expression of CHOP, GADD34, caspase-12 and cleaved-caspase-12 proteins and reduced IL-6-induced proteins expression (Fig. 3d). In brief, sh-IRE-1 and IL-6 could promote ERS-induced UPR to increase apoptosis of colon epithelial cell.
In addition, similar results were observed in normal colon fibroblast CCD-33Co cells. Silenced IRE-1 expression and IL-6 stimulation suppressed cells proliferation and promoted apoptosis significantly (p < 0.001). IRE-1 knockdown enhanced IL-6-induced inflammation damage in CCD-33Co cells (Fig. 4A, B). Furthermore, we demonstrated that sh-IRE-1 or IL-6 stimulation could promote XBP-1s, BiP, CHOP and caspase-12 expression. IRE-1 knockdown enhanced the action of IL-6-induced XBP-1s and GADD34 expression (Fig. 4C, D). Collectively, IRE-1 could inhibit ERS-induced UPR to protect colon fibroblast cell.
IRE-1-induced UPR regulated proliferation and apoptosis of CCD-33Co cells. a CCK-8 method was used to detect cells proliferation. Sh-IRE-1 inhibited cell proliferation and promoted IL-6-induced proliferation slower. b Flow cytometry for detection of cells apoptosis. Sh-IRE-1 increased cell apoptosis and promoted IL-6-induced cell apoptosis. c RT-PCR was be used to detect XBP-1s mRNA expression. Sh-IRE-1 induced XBP-1s expression and increased IL-6-induced XBP-1s expression. d Western blot was used to detect the expression of BIP, CHOP, caspase-12, and GADD34. Sh-IRE-1-induced BIP, CHOP, and caspase-12 expression and enhanced IL-6-induced BIP, CHOP, caspase-12 and GADD34 expression. *p < 0.05; **p < 0.01 and ***p < 0.001 versus NC
Discussion
UC is a continuous inflammation of the colonic mucosa and submucosa. In this study, we observed the abnormal of crypt structure and epithelial morphology, and inflammatory infiltration in UC mucosa (Fig. 1). These were the histopathological features of UC. Besides, our results showed that ERS-induced UPR were enhanced in mucosa tissue of UC patients (Fig. 2). Among them, this study proved that IRE-1 could inhibit ERS-induced UPR to protect colon mucosa and fibroblast cells (Figs. 3, 4).
In the ER, the accumulation of misfolded and unfolded proteins will lead to ERS. ERS can trigger the UPR to restore homeostasis and correlate with the degree of colonic inflammation. In this study, the ERS-induced UPR-related genes were upregulated in the UC mucosa tissue [10]. A deregulation of the genes involved in the ERS and UPR pathways may be a key component of the inflammatory response in IBD [23]. The colon epithelial cells are exposed to bacteria, toxins and metabolic factors et al., which can adversely affect cell vitality, thus inducing stress on the protein folded machinery. When UC inflammation-induced cell damage leading to the protein folding ability of the ER does not meet the need, the cell will enter an ERS state and start UPR by activating IRE-1, PERK and ATF-6 pathways [23,24,25]. The IRE-1 plays an important role in sensing and responding to ERS signals. We found that IRE-1 upregulated in UC mucosa. IRE-1 impacted early in the inflammatory process of mucosal. When exposed to dextran sodium sulfate to induce IBD, IRE-1b−/− mice developed colitis earlier than wild-type or IRE-1b+/− mice significantly [26]. IRE-1 activated can reduce ER dysfunction and participate in the development of colitis. The activated IRE-1 accelerates XBP-1s expression [15]. XBP-1s is a key component of ERS response and is required for the development and maintenance of secretory cells. The significant increase in XBP-1s mRNA promoted BIP expression, indicating the hyperactivation of the IRE-1/XBP-1s axis in UC mucosa. The activated IRE-1/XBP-1s blocked the eIF2 pathway, which mediated protein translation and stress response leading to the reduction of stress granules and colonic epithelial barrier function [27]. However, XBP-1 deletion could induce spontaneous enteritis, result in Paneth cells apoptosis, epithelial cells hyperproliferation, antimicrobial function impaired, and inflammatory increased [9]. This may be due to the absence of XBP-1 hindering its beneficial effect on ER homeostasis, thereby increasing ER burden and UC risk. BIP activated by XBP-1s can promote CHOP and GADD34 expression [16]. Activation of CHOP is a direct result of the ERS response, and elevated CHOP expression is a hallmark of ERS. CHOP is involved in the ERS response, especially ERS-induced cell apoptosis. Upregulation of CHOP expression exacerbates the development of colitis by various stimulatory mechanisms. In CHOP-null mice, the effect of Mac-1-induced macrophage infiltration, Ero-1α-induced ROS production, and caspase-11-induced IL-1β production and mucosal cell apoptosis were suppressed, thereby ameliorating colitis [28]. ERS and cell apoptosis play an important role in the pathogenesis of UC. ERS markers CHOP, BiP, and caspase-12 were significantly highly expressed in IBD mice induced by 2,4, 6-trinitrobenzene sulfonic acid, and the co-localization of CHOP and cleaved caspase-3 in the colon promoted apoptotic cell death [29]. The reduction in GADD34 expression implies that IRE-1 and ATF-6 pathway were activated [10]. GADD34 has the effect of returning to homeostasis of the ER. Downregulation of GADD34 in UC mucosal tissues, promotes ERS and cell apoptosis. Taken together, in ERS-induced UPR, the IRE-1/XBP-1s signal was significantly enhanced in UC mucosal tissues.
As a core regulator of ERS, IRE-1 plays an essential role in maintaining ER and cell function. Our results showed that knockdown of IRE-1 significantly increased the expression of BiP and XBP-1s, and increased the expression of the apoptotic proteins CHOP, caspase-12, cleaved-caspase-12, and GADD34, thereby causing colonic epithelial cells to proliferate slower and undergo apoptosis. But, the results in IRE-1 over-expression group were opposite. IRE-1 is a major sensor of ERS and directly involved in IBD processes. Cell damage caused by IRE-1 silencing leads to decreased ER protein folding ability and triggers UPR. IRE-1α deficiency-induced CHOP expression led to spontaneous colitis in mice, which was related to the loss of goblet cells and intestinal epithelial cells, increased the sensitivity of bacterial endotoxin, and failure of intestinal epithelial barrier function [8]. Besides, IRE-1α knockdown also repressed β-catenin expression via activating eIF2α signaling, which suppressed the proliferation of colon cells and prevented the colitis-associated colonic tumorigenesis [30]. It can be seen that IRE-1 mediates the ERS-induced UPR and regulate of the colonic epithelial cells proliferation and apoptosis. IRE-1 also acted as a inductor of IL-6 [16, 21]. In this study, we found IL-6 could induce cell apoptosis by upregulating UPR-related IRE-1, XBP-1s, CHOP, caspase-12, and GADD34 expression. IL-6 stimulated ERS-induced UPR, which was similar to the pro-inflammatory cytokine IFN-γ/TNF-α/tunicamycin-induced ERS and apoptosis of intestinal epithelial cells, both by promoting ERS molecular markers of BIP, XBP-1s and caspase-12 expression [31, 32]. Knocking out IRE-1 significantly enhanced the inhibitory effect of IL-6 on the proliferation of NCM-460 cells, which suggested that IRE-1 could inhibit ERS-induced UPR to protect colon epithelial cell. These effects are the same as XBP-1-silenced inhibited proliferation of colon cells and increased the sensitivity of dextran sulfate sodium-induced apoptosis [33]. IRE-1XBP-1 signal plays a vital role in maintaining ER homeostasis and cell vitality. Lastly, we found that sh-IRE-1 could facilitate IL-6-induced ERS and apoptosis in colon fibroblast cell [34]. Colon fibroblast cell play the role in injury healing in the early stage of inflammation, but their excessive proliferation and activation promote intestinal fibrosis, thereby leading to IBD complication. These results indicate that IRE-1 plays an essential role in maintaining ER homeostasis and colon epithelial cell function. IRE-1 may provide a new potential target for the treatment of UC.
In conclusion, our study demonstrated that IRE-1 is a critical regulator of ERS signal in UC. IRE-1 over-expression decreased ERS-induced UPR and improved the sensitivity of IL-6-induced apoptosis. Understanding the exact role of IRE-1 in UC can increase our knowledge of the biological basis of inflammation and may also facilitate to develop new therapeutic strategies. While IRE-1is great direction to study, however, the moderate expression of IRE-1 in normal tissues is beneficial. So the level of IRE-1 that causes excessive ERS needs to be identified, and we will investigate in the future.
References
Yu YR, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349–355.
Soroosh A, Rankin CR, Polytarchou C, Lokhandwala ZA, Patel A, et al. miR-24 Is elevated in ulcerative colitis patients and regulates intestinal epithelial barrier function. Am J Pathol. 2019;189:1763–1774.
Cao SS, Epithelial ER. Stress in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:984–993.
Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–1097.
Mortensen JH, Lindholm M, Langholm LL, Kjeldsen J, Bay-Jensen AC, et al. The intestinal tissue homeostasis—the role of extracellular matrix remodeling in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2019;13:977–993.
Rodríguez C, Romero E, Garrido-Sanchez L, Alcaín-Martínez G, Andrade RJ, et al. Microbiota insights in Clostridium difficile infection and inflammatory bowel disease. Gut Microbes. 2020. https://doi.org/10.1080/19490976.2020.1725220.
Guinet-Charpentier C, Champigneulle J, Williet N, Peyrin-Biroulet L, Morali A. The association of autoimmune diseases with pediatric ulcerative colitis does not influence its disease course. Scand J Gastroenterol. 2016;51:33–40.
Zhang HS, Chen Y, Fan L, Xi QL, et al. The endoplasmic reticulum stress sensor IRE1α in intestinal epithelial cells is essential for protecting against colitis. J Biol Chem. 2015;290:15327–15336.
Kaser A, Lee AH, Franke A, Glickman JN, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756.
Bogaert S, De Vos M, Olievier K, Peeters H, Elewaut D, et al. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS ONE. 2011;6:e25589.
Lee JH, Kwon EJ, Kim DH. Calumenin has a role in the alleviation of ER stress in neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2013;439:327–332.
Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007;125:411–424.
Rashid HO, Yadav RK, Kim HR, Chae HJ. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–1977.
Calfon M, Zeng H, Urano F, Till JH, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96.
Huang HW, Zeng X, Rhim T, Ron D, Ryoo HD. The requirement of IRE1 and XBP1 in resolving physiological stress during Drosophila development. J Cell Sci. 2017;130:3040–3049.
Studencka-Turski M, Çetin G, Junker H, Ebstein F, Krüger E. Molecular insight into the IRE1α-mediated type I interferon response induced by proteasome impairment in myeloid cells of the brain. Front Immunol. 2019;10:2900.
Thakur PC, Davison JM, Stuckenholz C, Lu L, Bahary N. Dysregulated phosphatidylinositol signaling promotes endoplasmic-reticulum-stress- mediated intestinal mucosal injury and inflammation in zebrafish. Dis Model Mech. 2014;7:93–106.
Wang X, Cui X, Zhu C, Li M, Zhao J, et al. FKBP11 protects intestinal epithelial cells against inflammation-induced apoptosis via the JNK-caspase pathway in Crohn’s disease. Mol Med Rep. 2018;18:4428–4438.
Martinez-Fierro ML, Garza-Veloz I, Rocha-Pizaña MR, Cardenas-Vargas E, Cid-Baez MA, et al. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. Medicine (Baltimore). 2019;98:e17208.
Caviglia GP, Rosso C, Stalla F, Rizzo M, Massano A, et al. On-treatment decrease of serum interleukin-6 as a predictor of clinical response to biologic therapy in patients with inflammatory bowel diseases. J Clin Med. 2020. https://doi.org/10.3390/jcm9030800.
Liu A, Song Q, Zheng Y, Xu G, Huang C, et al. Expression of XBP1s in peritoneal mesothelial cells is critical for inflammation-induced peritoneal fibrosis. Sci Rep. 2019;9:19043.
Martin-Jiménez CA, García-Vega Á, Cabezas R, Aliev G, et al. Astrocytes and endoplasmic reticulum stress: a bridge between obesity and neurodegenerative diseases. Prog Neurobiol. 2017;158:45–68.
Negroni A, Prete E, Vitali R, Cesi V, Aloi M, et al. Endoplasmic reticulum stress and unfolded protein response are involved in paediatric inflammatory bowel disease. Dig Liver Dis. 2014;46:788–794.
Hu YB, Wu X, Qin XF, Wang L, Pan PH. Role of endoplasmic reticulum stress in silica-induced apoptosis in RAW2647 cells. Biomed Environ Sci. 2017;30:591–600.
Hiss DC, Gabriels GA. Implications of endoplasmic reticulum stress, the unfolded protein response and apoptosis for molecular cancer therapy. Part I: targeting p53, Mdm2, GADD153/CHOP, GRP78/BiP and heat shock proteins. Expert Opin Drug Discov. 2009;4:799–821.
Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593.
Pratt HL, Carroll RC, McClendon S, Smathers EC, Souza LM, Lothrop CD Jr. Effects of recombinant granulocyte colony-stimulating factor treatment on hematopoietic cycles and cellular defects associated with canine cyclic hematopoiesis. Exp Hematol. 1990;18:1199–1203.
Namba T, Tanaka K, Ito Y, Ishihara T, et al. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol. 2009;174:1786–1798.
Crespo I, San-Miguel B, Prause C, Marroni N, Cuevas MJ, et al. Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS ONE. 2012;7:e50407.
Li XX, Zhang HS, Xu YM, Zhang RJ, et al. Knockdown of IRE1α inhibits colonic tumorigenesis through decreasing β-catenin and IRE1α targeting suppresses colon cancer cells. Oncogene. 2017;36:6738–6746.
Karna KK, Choi BR, You JH, Shin YS, Cui WS, et al. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complem Altern Med. 2019;19:333.
Hao X, Yao A, Gong J, Zhu W, Li N, Li J. Berberine ameliorates pro-inflammatory cytokine-induced endoplasmic reticulum stress in human intestinal epithelial cells in vitro. Inflammation. 2012;35:841–849.
Li M, Zhang S, Qiu Y, He Y, Chen B, et al. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017;8:e2699.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666.
Funding
The study was supported by Jiangxi Provincial Health and Family Planning Commission Science and Technology Plan (Project No: 20172246).
Author information
Authors and Affiliations
Contributions
Bei Zhang and ZhiLi Wen made substantial contributions to the conception and design of the work. Bei Zhang, XiaoYan Su, ZhengYuan Xie, Hao Ding, Ting Wang, RuYi Xie and ZhiLi Wen made substantial contributions to the acquisition, analysis and interpretation of data for the work. Bei Zhang participated in drafting the work,ZhiLi Wen and XiaoYan Su revising it critically for important intellectual content. Each author approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Second Hospital Affiliated to Nanchang University. We obtained informed consent from the patients and all subjects signed their informed consent in accordance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, B., Su, X., Xie, Z. et al. Inositol-Requiring Kinase 1 Regulates Apoptosis via Inducing Endoplasmic Reticulum Stress in Colitis Epithelial Cells. Dig Dis Sci 66, 3015–3025 (2021). https://doi.org/10.1007/s10620-020-06622-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06622-7