Abstract
Background
Pyoderma gangrenosum (PG) is an uncommon but severe extra-intestinal manifestation (EIM) of inflammatory bowel disease (IBD). The incidence and risk factors for PG are disputed.
Aims
To assess the incidence of PG and identify factors associated with PG in IBD patients.
Methods
A search of electronic databases (Ovid and PubMed) was conducted between 1966 and 2019. Studies that calculated the incidence of PG in IBD patient cohorts were included. Patient demographics, IBD subtype, and EIM presence were recorded. A review of our institutional database of 1057 IBD patients was conducted. A multivariate regression model and meta-analysis were conducted to identify risk factors for PG. A random effects model was used to combine the data of included studies.
Results
Fourteen studies were included in addition to 1057 IBD patients and 26 PG cases from the Louisville cohort. In total, there were 379 cases of PG in the cumulative cohort of 61,695 IBD patients. The PG incidence in individual studies ranged from 0.4 to 2.6%. In the institutional cohort, ocular EIMs and a permanent stoma were significant risk factors for PG. In the meta-analysis, PG was associated with female gender (RR = 1.328, 95% CI 1.161–1.520), Crohn’s disease (RR = 1.193, 95% CI 1.001–1.422), erythema nodosum (RR = 9.281, 95% CI 6.081–14.164), and ocular EIM (RR = 4.55, 95% CI 3.04–6.81). There was study heterogeneity when assessing IBD subtype, ocular, and joint EIMs.
Conclusions
There are conflicting data on the incidence and risk factors for PG. This meta-analysis confirms an association between PG and female gender, Crohn’s disease, erythema nodosum, and ocular EIM that have been described in smaller studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract and is classically subdivided into Crohn’s disease and ulcerative colitis. In the USA, an estimated 1.3 million individuals suffer from IBD [1]. The effects of IBD are not limited to the digestive system. Almost every organ system, including musculoskeletal, ocular, integumentary, and hepatobiliary systems, can be affected. Approximately 21–54% of IBD patients have at least one “extra-intestinal manifestation” (EIM). Of these patients, about one-third are diagnosed with a cutaneous manifestation. Most studies report an overall higher incidence of EIMs in patients with Crohn’s disease than in patients with ulcerative colitis [2,3,4,5].
The most common cutaneous manifestations are erythema nodosum and pyoderma gangrenosum (PG). It has been reported that erythema nodosum occurs in 3–8% of IBD patients, whereas PG occurs in 0.75–1.5% of IBD patients [5, 6]. IBD can also be associated with other skin disorders including hidradenitis suppurativa [7], psoriasis [8], and Sweet’s syndrome [9].
PG is a relatively uncommon disorder associated with autoimmune and inflammatory conditions including IBD and rheumatoid arthritis. A recent meta-analysis indicated that systemic disease is associated with 57% of PG, and that IBD is the most common systemic disease associated with PG (18%) [10]. The legs are the most commonly affected site in classic ulcerative lesions of PG. In addition, PG can occur in patients postoperatively including peristomal sites and other site of surgical incisions (Fig. 1), most often following breast or abdominal surgery [11, 12].
While some individual studies suggest that cigarette smoking, the presence of intestinal stomas, a history of other EIMs, and demographic factors such as race and family history of IBD are associated with PG, there are limited and conflicting data concerning the incidence of PG in this population and risk factors for development of PG in IBD patients overall. We hypothesized that combining studies in the literature with data from our large institutional IBD database and through a systematic review and meta-analysis approach we would more clearly delineate the incidence and risk factors associated with PG in patients with IBD.
Methods
Systematic Review
Study Selection
This study was conducted using the “Preferred Reporting Items for Systematic Reviews and Meta-analysis Guidelines” [13, 14]. The PubMed (NCBI, Bethesda, MD) and Ovid (Wolters Kluwer Health, Netherlands) databases were searched from January 1966 to June 2019 using the following search terms: “pyoderma gangrenosum,” “inflammatory bowel disease,” “Crohn’s disease,” “ulcerative colitis,” and “extraintestinal manifestations.” A search of the gray literature was also performed. The Boolean operators (and/or/not) were used to expand the search. No language restrictions were applied.
Inclusion and Exclusion Criteria
Eligible studies included prospective or retrospective cohort studies with at least 5 years of follow-up. Variables of interest were PG incidence, gender, IBD type, EIMs, ethnicity, family history, previous IBD-related operations, the presence of an intestinal stoma, and cigarette smoking status. Only IBD populations were included. Studies were required to report data both upon PG patients and upon the larger IBD cohort of which they were a part. In the case of duplicate patient cohorts, the most recent publication was used unless variables of interest were only included in the older manuscript. Case series, case reports, and review articles were excluded. Studies that did not report the number of patients in both PG and total IBD cohorts were excluded. Full-text articles meeting these criteria were selected for review.
Data Extraction and Outcomes
The following information for each study was recorded: names of authors, year of publication, study type, gender, total number of cases of IBD, IBD subtype, duration of follow-up, number of cases of PG, presence of other EIMs, and presence of a stoma. Two of the authors performed the literature search and extracted data from the papers (VS, SO’B). A third author verified the extracted data (MP). When the data were unclear, the senior author (SG) was contacted to clarify the data extraction. Study quality was assessed using the Newcastle–Ottawa scale [15].
University of Louisville Cohort
A retrospective review of a prospectively maintained IBD database from a tertiary referral center was performed. This database is derived of patients from a geographic area comprising Kentucky and Southern Indiana and accrued during the period from November 1997 through November 2016. All patients signed written informed consent, and University of Louisville Institutional Review Board approval was obtained. The patients in this cohort were followed by their gastroenterologists, surgeons, and enterostomal therapists. The diagnosis of IBD was verified by conventional radiologic, endoscopic, and/or histopathologic findings. Patients with PG were assessed by a dermatologist, and the diagnosis of PG was made through a combination of clinical and histological findings. Patients with an ocular manifestation were followed by an ophthalmologist. Family history was defined as the presence of IBD in the first- or second-degree relatives.
The frequency of the following EIMs was examined: (1) cutaneous EIMs (PG, erythema nodosum, cutaneous Crohn’s disease, Sweet’s syndrome); (2) arthritis (peripheral arthritis/arthralgia and ankylosing spondylitis and excluding osteoarthritis); (3) ocular EIMs (iritis/uveitis/episcleritis/scleritis). The location of PG was defined by anatomical location. Surgical incision PG was defined as PG at the midline incision from a previous laparotomy.
Smoking data were obtained through a modified Behavioral Risk Factor Surveillance System survey [16]. Current smokers were defined as patients who smoked at least 100 cigarettes in their lifetime and reported smoking “some days to everyday” over the past 6 months. Former smokers reported smoking at least 100 cigarettes in their lifetime and had not smoked in the past 6 months.
A total of 579 patients had complete documentation concerning cutaneous manifestations, while the remainder had partial or missing data. Patients with partial or missing data were not included for further multivariable analysis regarding predisposing variables for PG. Data on patient age, age of diagnosis, duration of disease, disease location, gender, IBD type (Crohn’s disease or ulcerative colitis), ethnicity, family history, EIMs, previous IBD-related operations, presence of an intestinal stoma, and smoking status were recorded and analyzed.
Statistical Analysis
A univariate logistic regression model was used to identify risk factors for the development of PG in the institutional cohort. Significant variables in the univariate analysis were included in the multivariate logistic regression model. Results were declared significant at a level of 0.05. SAS software (v9.4, SAS Institute Inc. Cary, NC, USA) was used for the univariate and multivariate regression model. Random effects models were used for all meta-analyses. Risk ratios were calculated for each study, and the pooled relative risk was calculated using a random effects model, as described by DerSimonian and Laird [17]. Publication bias across studies was assessed using a funnel plot. Forrest plots for the meta-analysis were created using Stata software (v14.2, Stata corporation, College Station, TX, USA). The studies were determined to be heterogeneous when the I2 statistic was > 50% and the Cochran’s Q statistic was p < 0.01 [18, 19].
Results
Systematic Review
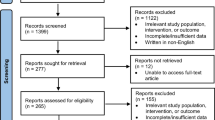
A total of 990 titles and abstracts were screened. Of the examined studies, 14 included demographic data regarding PG patients within IBD cohorts. These 14 studies were included for analysis (Supplementary Figure 1). They included both prospective and retrospective cohort studies that examined the incidence of cutaneous EIMs of IBD. A total of 60,638 IBD patients and 353 PG patients were identified from the included studies [61,695 IBD patients and 379 cases of PG including the Louisville cohort (1057 cases of IBD with 26 cases of PG)]. Study characteristics are summarized in Table 1 [2, 20,21,22,23,24,25,26,27,28,29,30,31,32].
The average incidence of PG across the studies was 0.6% and ranged from 0.1 to 2.6%. Of the studies, only six provided statistical analysis of data concerning PG. The other studies recorded demographic data regarding PG patients but did not conduct statistical analysis. Six studies reported on the difference in PG incidence between genders. Although five studies demonstrated that ≥ 50% of the patients were female, no study reported a significant gender association with PG (Supplementary Table 1a). Twelve studies described the distribution of PG between Crohn’s disease and ulcerative colitis patients, but no study reported a significant association between PG and Crohn’s disease or ulcerative colitis (Supplementary Table 1b). Studies that examined the relationship between PG and other EIMs are shown in Supplementary Table 1c. Three studies found erythema nodosum to be significantly associated with PG [20, 22, 24]. Three studies also found ocular manifestations to be significantly associated with PG [21, 22, 24]. In contrast, there were differing results regarding the association between joint manifestations and PG. Yuskel et al. and Vavricka et al. both found joint manifestations to be associated with PG; however, Farhi et al. did not [21, 22, 24]. Only one study reported on the association between PG and intestinal stomas, the authors identifying a significant association between a permanent intestinal stoma and PG (p < 0.001) (Supplementary Table 1d) [22]. Additionally, several studies analyzed associations not described in the tables. Vavricka et al. found an association between aphthous ulcers and PG (p < 0.05) [24]. Similarly, Farhi et al. found perianal Crohn’s disease (p = 0.001) and aphthous ulcers (p < 0.0001) to be significantly associated with PG [22]. In a multivariate analysis, Farhi et al. reported that PG also was independently associated with (black) African origin, a family history of ulcerative colitis, and the presence of pancolitis; however, tobacco use was not found to be related to the presence of PG [22].
University of Louisville Cohort
There were 1057 patients with IBD in the institutional colorectal surgery IBD database, with 26 confirmed cases of PG, yielding an incidence of 2.5% (26/1057). All patients with PG are followed by the senior author (SG). Complete data regarding the presence of other EIMs existed for 579 patients, and these data were included for further statistical analysis. The demographics of all PG patients and the patients with complete EIM data are shown in Table 2. The median disease duration was 26 years. Three hundred and forty-one patients (59%) were female. Three hundred and sixty-five patients (63%) had Crohn’s disease, and 214 (37%) had ulcerative colitis. Fifty-one patients had confirmed cutaneous EIMs, of which 26 were diagnosed with PG. All of the PG cases were of the ulcerative subtype. Of these patients, 21 (81%) were female and 5 (19%) were male. The mean patient age was 58 ± 18 years, while the mean age of diagnosis was 32 ± 18 years. Twenty-five patients (96%) underwent IBD-related surgery, and seventeen (65%) had either a temporary or permanent stoma. For patients with a permanent stoma, the PG developed within 6–12 months after stoma creation. The distribution of PG is described in Supplementary Figure 2. Seventeen patients (50%) had peristomal PG, six (23%) had PG involving a surgical incision, six (23%) had PG involving the extremities, two (8%) had PG of the breast, and two (8%) had PG of the perineum. All of the cases of surgical incision PG were at the location of a previous laparotomy for intestinal resection for Crohn’s disease. Other cutaneous manifestations included 17 patients (17/579, 3.0%) with erythema nodosum and 8 with cutaneous Crohn’s disease (8/579, 1.4%). No patients were diagnosed with both PG and erythema nodosum, concomitantly.
A univariate and multivariate regression analyses were conducted to identify risk factors associated with the development of PG (Table 3). In univariate regression analysis, female gender (OR 3.06, 95% CI 1.1–8.23, p = 0.03), an IBD subtype of Crohn’s disease (OR 4.73, 95% CI 1.4–15.95, p = 0.01), the presence of ocular EIMs (OR 2.83 95% CI 1.19–6.75, p = 0.02), and the presence of a permanent stoma (OR 9.6 95% CI 3.6–25.58, p < 0.001) were risk factors for PG. In multivariate regression analysis, only the presence of ocular EIMs (OR 2.77 95% CI 1.09–7.07, p = 0.03) and the presence of a permanent stoma (OR 6.77 95% CI 3.6–25.58, p < 0.001) were risk factors for PG.
Meta-Analysis
Meta-analysis was conducted to calculate the pooled risk estimates of a number of variables associated with PG (Fig. 2a–e). Using the calculated relative risk from data in each study, female gender was associated with an increased relative risk of PG compared to males (RR = 1.328 95% CI 1.161–1.520, p < 0.001). Crohn’s disease was associated with an increased pooled relative risk of PG compared to ulcerative colitis (RR = 1.193, 95% CI 1.001–1.422 p = 0.048); however, there was heterogeneity seen between studies. Each of the individual studies which examined for the presence of EIMs demonstrated an association between erythema nodosum and PG. The meta-analysis showed a strong positive pooled relative risk between erythema nodosum and PG (RR = 9.281, 95% CI 6.081–14.164, p < 0.001). There were heterogeneous individual and pooled results with regard to the presence of joint EIM, and there was no significant association between PG and joint EIM (RR = 1.981 95% CI 0.835–4.701, p = 0.121). All of the studies which examined for the presence of ocular EIMs showed a significant individual risk associated with PG, and the pooled relative demonstrated a significant positive association as well (RR = 5.248 95% CI 2.396–11.497, p < 0.001), but there was heterogeneity seen in these studies. Finally, analysis of the funnel plot revealed a slight publication bias across the included studies (Fig. 3).
a Meta-analysis of the studies examining the difference in the incidence of PG between IBD subtype. The individual studies and the pooled relative risk show heterogeneous results, but there is a significant positive pooled relative risk with Crohn’s disease. However, more studies are needed to calculate a more accurate pooled risk (RR = 1.193, 95% CI 1.001–1.422, p = 0.048, Cochran’s Q = 16.75 (d.f. = 12) I2 = 55.1%, p = 0.008). b Meta-analysis of the studies examining the difference in the incidence of PG between male and female genders. The majority of studies showed an individual trend toward an association with the female gender. The overall pooled estimate showed a significant increased relative risk trend (RR = 1.328, 95% CI 1.161–1.520, p < 0.001, Cochran’s Q = 1.89 (d.f. = 6) I2 = 0%, p = 0.929). c Meta-analysis of the studies examining the association between erythema nodosum and PG. The individual studies showed a positive association between erythema nodosum and PG, and the pooled relative risk showed a statistically significant positive trend (RR = 9.281, 95% CI 6.081–14.164, p < 0.001, Cochran’s Q = 0.07 (d.f. = 2), I2 = 0%, p = 0.964). d Meta-analysis of the studies examining the association between joint EIMs and PG. The individual studies showed variable results, but there was no difference in the pooled relative risk between joint EIMs and PG. However, more studies are needed to calculate a more accurate pooled risk (RR = 1.981 95% CI 0.835–4.701, p = 0.121, Cochran’s Q = 39.45 (d.f. = 3) I2 = 92.4%, p = < 0.001). e Meta-analysis of the studies examining the association between ocular EIMs and PG. All of the individual studies and the pooled relative risk showed a positive association between ocular EIMs and PG. However, more studies are needed to calculate a more accurate pooled risk (RR = 5.248, 95% CI 2.396–11.497, p < 0.001, Cochran’s Q = 9.36 (d.f. = 3) I2 = 67.9%, p = 0.025)
Discussion
The reported incidence of PG in the literature ranges from 0.6 to 2.6% which is comparable to our institutional incidence of 2.5% [22, 25, 26, 33, 34]. The association of PG with specific risk factors has been debated in the literature, but in this meta-analysis, female gender, Crohn’s disease, erythema nodosum, and ocular manifestations were all significantly associated with PG.
Patients with one existing EIM have a 70% risk of developing a second during the next 10 years [18]. In our cohort, 86% of patients underwent surgery for their IBD and 64.3% of patients had ≥ 1 EIM. This patient cohort may therefore not be representative of the other reported series and may represent a population with a more severe disease phenotype. This relatively high incidence of EIMs may in turn suggest that EIMs are associated with a more severe disease phenotype. Cutaneous EIMs commonly present within 2 years of IBD diagnosis [35]. Multiple studies report an increased incidence of PG in IBD patients with coexisting EIMs. Associations between arthritis, ocular manifestations, erythema nodosum, and the development of PG have been previously reported [21,22,, 22, 24, 36]. Skin manifestations often overlap, and this may explain the association that was observed between PG and erythema nodosum in this study. Ocular PG is a rare manifestation but is recognized to have major consequences for patients. A systematic review identified 34 cases, the majority of whom were treated with corticosteroids and a systemic immunomodulatory and surgery for reconstruction as required [37, 38].
The incidence of PG in Crohn’s disease compared to ulcerative colitis has been disputed. Several publications cite an association with either Crohn’s disease or with ulcerative colitis [21,22,, 39,40,41]. In our cohort, Crohn’s disease patients were not more likely to have PG in the multivariate analysis (p = 0.13). Although other groups have found no correlation between PG and IBD type [22, 25], our meta-analysis results would indicate a small increased relative risk in patients with Crohn’s disease.
Peristomal PG occurs in approximately 0.6% of all patients with intestinal stomas [42]. This may be as a result of the repeated skin “trauma” observed with intestinal stomas during removal and application of stomas appliances, but further study is required to identify a temporal association. In a case–control study of patients with peristomal PG, Wu et al. [43] determined that female gender, autoimmune disease, and BMI were risk factors. Farhi et al. [22] conducted a prospective study of 2402 IBD patients and found a statistically significant association between intestinal stomas and PG in both univariate and multivariate analyses. Peristomal PG accounted for 65% (17/26) of our PG cohort. This large discrepancy is likely due to the senior authors’ patient cohort consisting of complex surgical patients. This may be a limitation as other patients with PG that do not require surgical intervention for IBD would not be captured in this dataset. A recent systematic review of peristomal PG described that the majority of patients had a multimodal approach for treatment, but there is no consensus on the type of intervention [44]. In both univariate and multivariate analyses, the presence of a permanent stoma was a risk factor for the development of both PG and peristomal PG in our cohort.
Farhi et al. reported that IBD patients of African race were associated with a higher risk of developing PG. In contrast, Nguyen et al. did not find any associations between race and the development of cutaneous EIMs [45]. We did not examine race in our cohort due to a significant majority of Caucasian patients (93%).
Smoking has been correlated with an increased incidence in the total number of EIMs in IBD patients, most notably joint and ocular manifestations. A number of studies suggest that smoking is not a risk factor for the development of cutaneous EIM (when including both PG and EN) in IBD patients [3, 28, 46]. However, in one prospective cohort study of ulcerative colitis patients, there was a significant relationship between (current) smoking and the development of PG [47]. In our IBD cohort, a history of tobacco use (former or current cigarette smokers) was not correlated with an increase in PG incidence.
While family history of IBD was not a significant variable in our study, it has been correlated with an increased risk of EIMs in Crohn’s disease patients [24]. In a multivariate analysis, Farhi et al. [22] found that a family history of ulcerative colitis was independently associated with PG. This may suggest that there is a genetic predisposition to the development of certain EIMs [48].
While this study presents a large cohort of patients with long follow-up, it is limited by missing data with respect to certain variables including BMI and medical management of IBD and EIMs. In addition, the Louisville cohort included a disproportionately large number of female, Caucasian, and surgical patients. Thus, the results of the meta-analyses should be carefully interpreted. The limited number of studies included in the separate analyses, taken together with the relative rarity of PG, impacts the power of the conclusions derived from the data. All patients with an ocular EIM had a diagnosis prior to referral to the colorectal surgery practice, but the exact timing was not available from the database or the electronic medical record.
PG has typically been defined as a diagnosis of exclusion, which represents a major challenge in studying this condition. Delphi consensus guidelines for the diagnosis of PG have recently been published [49]. Therefore, the results of this study should be carefully considered as the majority of the included studies were published prior to the consensus guidelines for PG diagnosis.
Literature concerning risk factors for PG is limited and conflicting. This systematic review and meta-analysis has reviewed a large number of studies reporting this incidence of PG and has identified that female gender, Crohn’s disease, erythema nodosum, and ocular EIM are associated with PG.
References
Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2007;5:1424–1429.
Lakatos L, Pandur T, David G, et al. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300–2307.
Roberts H, Rai SN, Pan J, et al. Extraintestinal manifestations of inflammatory bowel disease and the influence of smoking. Digestion. 2014;90:122–129.
Ricart E, Panaccione R, Loftus EV Jr, et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2004;10:207–214.
Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34.
Christodoulou DK, Katsanos KH, Kitsanou M, Stergiopoulou C, Hatzis J, Tsianos EV. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in Northwest Greece and review of the literature. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2002;34:781–786.
Garg A, Hundal J, Strunk A. Overall and subgroup prevalence of Crohn disease among patients with hidradenitis suppurativa: a population-based analysis in the United States. JAMA Dermatol. 2018;154:814–818.
Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377–390.
Huang BL, Chandra S, Shih DQ. Skin manifestations of inflammatory bowel disease. Front Physiol. 2012;3:13.
Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19:479–487.
Tuffaha SH, Sarhane KA, Mundinger GS, et al. Pyoderma gangrenosum after breast surgery: diagnostic pearls and treatment recommendations based on a systematic literature review. Ann Plast Surg. 2016;77:e39–e44.
Xia FD, Liu K, Lockwood S, et al. Risk of developing pyoderma gangrenosum after procedures in patients with a known history of pyoderma gangrenosum—a retrospective analysis. J Am Acad Dermatol. 2018;78:e311.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Mahid SS, Hornung CA, Minor KS, Turina M, Galandiuk S. Systematic reviews and meta-analysis for the surgeon scientist. Br J Surg. 2006;93:1315–1324.
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826.
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Accessed February 24, 2018; 2018.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed). 2003;327:557–560.
Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008.
Polcz M, Gu J, Florin T. Pyoderma gangrenosum in inflammatory bowel disease: the experience at Mater Health Services’ Adult Hospital 1998–2009. J Crohn’s Colitis. 2011;5:148–151.
Yuksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:546–550.
Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine. 2008;87:281–293.
Moravvej H, Razavi GM, Farshchian M, Malekzadeh R. Cutaneous manifestations in 404 Iranian patients with inflammatory bowel disease: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:607–610.
Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119.
Mebazaa A, Aounallah A, Naija N, et al. Dermatologic manifestations in inflammatory bowel disease in Tunisia. La Tunisie Med. 2012;90:252–257.
Isene R, Bernklev T, Hoie O, et al. Extraintestinal manifestations in Crohn’s disease and ulcerative colitis: results from a prospective, population-based European inception cohort. Scand J Gastroenterol. 2015;50:300–305.
Ampuero J, Rojas-Feria M, Castro-Fernandez M, Cano C, Romero-Gomez M. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291–295.
Karmiris K, Avgerinos A, Tavernaraki A, et al. Prevalence and characteristics of extra-intestinal manifestations in a large cohort of Greek patients with inflammatory bowel disease. J Crohn’s Colitis. 2016;10:429–436.
Cardoneanu A, Cijevschi Prelipcean C, Danciu M, et al. Looking beyond gut inflammation in inflammatory bowel disease. Rom J Morphol Embryol. 2018;59:1097–1105.
Roth N, Biedermann L, Fournier N, et al. Occurrence of skin manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. PLoS One. 2019;14:e0210436.
Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137–6146.
Vide J, Osório F, Costa-Silva M, et al. Cutaneous morbidity among inflammatory bowel disease patients: a cohort study. J Crohns Colitis. 2018;12:442–451.
Freeman HJ. Erythema nodosum and pyoderma gangrenosum in 50 patients with Crohn’s disease. Can J Gastroenterol. 2005;19:603–606.
Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine. 1976;55:401–412.
Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome? World J Gastroenterol. 2011;17:2702–2707.
Mir-Madjlessi SH, Taylor JS, Farmer RG. Clinical course and evolution of erythema nodosum and pyoderma gangrenosum in chronic ulcerative colitis: a study of 42 patients. Am J Gastroenterol. 1985;80:615–620.
McElnea E, Stephenson K, Fulcher T. Pyoderma gangrenosum affecting the eye, orbit, and adnexa. A review. Orbit (Amsterdam, Netherlands).. 2018;37:26–31.
Gupta AS, Ortega-Loayza AG. Ocular pyoderma gangrenosum: a systematic review. J Am Acad Dermatol. 2017;76:512–518.
Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122.
Menachem Y, Gotsman I. Clinical manifestations of pyoderma gangrenosum associated with inflammatory bowel disease. Israel Med Assoc J IMAJ. 2004;6:88–90.
Trost LB, McDonnell JK. Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J. 2005;81:580–585.
Lyon CC, Smith AJ, Griffiths CE, Beck MH. The spectrum of skin disorders in abdominal stoma patients. Br J Dermatol. 2000;143:1248–1260.
Wu XR, Mukewar S, Kiran RP, Remzi FH, Hammel J, Shen B. Risk factors for peristomal pyoderma gangrenosum complicating inflammatory bowel disease. J Crohn’s Colitis. 2013;7:e171–e177.
Afifi L, Sanchez IM, Wallace MM, Braswell SF, Ortega-Loayza AG, Shinkai K. Diagnosis and management of peristomal pyoderma gangrenosum: A systematic review. J Am Acad Dermatol. 2018;78:e1191.
Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–1023.
Ott C, Takses A, Obermeier F, Schnoy E, Muller M. Smoking increases the risk of extraintestinal manifestations in Crohn’s disease. World J Gastroenterol. 2014;20:12269–12276.
Manguso F, Sanges M, Staiano T, et al. Cigarette smoking and appendectomy are risk factors for extraintestinal manifestations in ulcerative colitis. Am J Gastroenterol. 2004;99:327–334.
Satsangi J, Grootscholten C, Holt H, Jewell DP. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741.
Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461–466.
Acknowledgments
The statistical methods for this paper were reviewed by Dr. Shesh Rai, Wendell Cherry Chair in Clinical Trial Research, Professor of Biostatistics, University of Louisville, Louisville, Kentucky, USA.
Funding
This work was supported by the John W. Price and Barbara Thruston Atwood Price Trust and a grant from the Mary K. Oxley Foundation.
Author information
Authors and Affiliations
Contributions
VS, SG helped in original study design; VS, SOB, HR, MP, KF helped in data collection ; JR, EP, RB, KF helped in data analysis; VS, SOB, SG helped in data interpretation; VS, SOB, HR, JR, KF helped in drafting of article; VS, SOB, MP helped in figures; SOB, MP, EP, KF, RB SG helped in revision of manuscript. All authors had access to all the data and approved final draft of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
EP is an employee and shareholder in Eli Lily & Company, Indianapolis, Indiana, USA. Eli Lily & Company had no role in this manuscript. This work was completed while she was a graduate student at University of Louisville. There are no other conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure
1 PRISMA flow diagram of search process, inclusion, and exclusion criteria for studies investigating PG patient cohorts. (TIFF 11683 kb)
Supplementary Figure
2 Venn diagram representation of the distribution of PG among total Louisville patient cohort (TIFF 56 kb)
Rights and permissions
About this article
Cite this article
States, V., O’Brien, S., Rai, J.P. et al. Pyoderma Gangrenosum in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci 65, 2675–2685 (2020). https://doi.org/10.1007/s10620-019-05999-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05999-4