Abstract
Background
Probiotic use to prevent gastrointestinal infections in critical care has shown great promise in recent clinical trials. Although well-documented benefits of probiotic use in intestinal disorders, the potential for probiotic treatment to ameliorate liver injury and hypoxic hepatitis following sepsis has not been well explored.
Methods
In order to evaluate, if Lactobacillus rhamnosus GG (LGG) treatment in septic rats will protect against liver injury, this study used 20–22-week-old Sprague–Dawley rats which were subjected to cecal ligation and puncture to establish sepsis model and examine mRNA and protein levels of IL-1β, NLRP3, IL-6, TNF-a, VEGF, MCP1, NF-kB and HIF-1α in the liver via real-time PCR, Elisa and Western blot.
Results
This study showed that LGG treatment significantly ameliorated liver injury following experimental infection and sepsis. Liver mRNA and protein levels of IL-1β, NLRP3, IL-6, TNF-a, VEGF, MCP1, NF-kB and HIF-1α were significantly reduced in rats receiving LGG.
Conclusions
Thus, our study demonstrated that LGG treatment can reduce liver injury following experimental infection and sepsis and is associated with improved hypoxic hepatitis. Probiotic therapy may be a promising intervention to ameliorate clinical liver injury and hypoxic hepatitis following systemic infection and sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, a syndrome of physiologic, pathologic and biochemical disorders induced by various infections, which is the leading cause of mortality and critical illness worldwide [1, 2]. With the increasing incidence [3, 4], the sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [5]. The occurrence of sepsis is often accompanied by the hepatocyte damage and other organ dysfunction [5]. It was reported that low cardiac output and septic shock could be the main cause of hypoxic hepatitis (HH) [6,7,8,9], which was an important manifestation of hypoxic liver injury. In intensive care unit (ICU), the incidence of HH was closer to 10% [10]. Pediatric patients diagnosed with sepsis are also susceptible to acute liver injury leading to hyperbilirubinemia [11].
From a pathophysiological standpoint, several damaged systems and organs participated in the pathogenesis and progress of sepsis. Previous study has shown that disturbance of balanced gut microbiota potentially leads to increased susceptibility of sepsis [12, 13]. Probiotics are living nonpathogenic bacteria colonizing intestine and providing benefit to the host with the potential to normalize the altered intestinal flora [14]. The benefit of probiotics in sepsis has been demonstrated in endotoxin and intra-abdominal infection sepsis models [15,16,17]. And a pioneering study has shown that the probiotics attenuate lung injury in septic mice [18]. However, the benefit of probiotics in gut bacterial translocation in a model where sepsis originated directly from gut leakage has not been tested. Due to the different characteristics of patients with sepsis of different etiologies, the probiotic treatment in patients with sepsis is still controversial [19]. Therefore, evaluation of probiotic-based therapeutic strategies in different animal models is needed.
The HH has attracted more and more attention of researchers; however, there was no standard and stable liver cell hypoxia damage in animal model for further study. Sepsis is one of the main causes of liver injury [6,7,8,9]. Because the cecal ligation and puncture (CLP) model was said to resemble closely to human sepsis, it was widely considered as the gold standard for animal research in sepsis [20]. This model fulfills the human condition that is clinically relevant [21]. Actually, the mechanism for the HH-associated sepsis has not been fully clarified, which is very critical for the prevention and therapy for the sepsis in clinical treatment. Although the benefits of probiotic use in intestinal disorders, the effects of probiotic treatment to protect against liver injury following sepsis are not well demonstrated. Previous study has shown the benefits of Lactobacillus rhamnosus GG (LGG) on improved survival and intestinal homeostasis in septic rats [16]. In this study, therefore, we hypothesized that LGG will also have a protective effect against liver injury and hypoxic hepatitis in septic rats.
Materials and Methods
Animals
A total of 60 male 20–22-week-old Sprague–Dawley rats, (weighing 380–420 g) obtained from Institute of Laboratory Animal Resources, Sun Yat-sen University, Guangzhou, China, were used in this study. The rats fasted for 12 h before the operation. All experiment procedures were designed based on international guidelines for animal studies and approved by the ethical committee of Institutional Animal Care and Use Committee of Sun Yat-sen University, Guangzhou, China.
CLP-Treated Procedure and LGG Treatment
Sixty male 20–22-week-old Sprague–Dawley rats were randomly divided into four groups: Sham (n = 8), Sham + LGG (n = 12), CLP (n = 20), CLP + LGG (n = 20). Except for the Sham and Sham + LGG groups, other rats were treated with CLP surgery as previously described [22]. In this experiment, the rats were orally gavaged with 200 ml of either LGG (1 × 109 CFU/ml) or sterile water (vehicle) immediately prior to initiation of the cecal ligation and puncture (CLP) procedure [23]. Briefly, the cecum was exposed through a middle way laparotomy and gently extruded to make sure a balanced distribution of the internal contents in rats. About 75% of the cecum were ligated with a 4–0 silk suture and punctured twice with an 18-gauge needle. A small amount of fecal material was squeezed out gently from the cecum to assure the cecal perforation. The abdominal incision was closed in two layers and each rat was resuscitated via warmed 0.9% normal saline. Meanwhile, sham mice were treated identically except the cecum was neither ligated nor punctured.
The murine sepsis score (MSS) was assessed at the corresponding time points of septic rats. And the general condition (appearance, level of consciousness, activity, response to stimulus, eyes and respiration) of rats was evaluated by it. The scoring process of all subjects was completed independently by two investigators. In this evaluation, the higher total score meant the worse general condition of subjects. The data were collected continuously for three times (interval of 15 s for each times) to obtain the average value.
Tissues Collection
0.2 ml blood samples respectively collected from the suprahepatic vena cava and abdominal aorta with 1-ml syringe as specimens of venous and arterial blood gas analysis. Then, drawing blood 5 ml blood from abdominal aorta with pro-coagulation tube until euthanasia. The liver tissues were dissected rapidly, and then some ones were fixed in 10% formalin solution and stored in 4 °C. The remaining liver tissues were settled in frozen tubes and stored in liquid nitrogen. Blood samples were placed for 2 h, and serum was removed centrifuging at 1000g for 15 min. After specific periods, serum was transferred into a cryogenic compatible tube and stored in liquid nitrogen.
RNA Extraction, RT-PCR and qPCR
The RNA was isolated from rat liver tissue using Trizol reagent (9108, Takara, Japan) according to manufacturers instructions. First-strand cDNA was synthesized using oligo (dT) primers and reverse transcriptase using a reverse transcriptase–polymerase chain reaction kit (RR036, Takara, Japan).
The 10-μl PCR mixture contained 50 ng template DNA, Sybr Green 5 μl (RR820, Takara, Japan) and primer mix 1 μl. Forty cycles of 95 °C for 15 s and 60 °C for 1 min were performed with an Mastercycler Epgradient S system (Eppendorf, Germany). All experiments were carried out three times, and from each of the three experiments, triplicate readings were taken and calculated for average. The data were analyzed using 2-∆∆Ct method.
Western Blot Analysis
A total of 20 μg of protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and blotted onto polyvinylidene difluoride membranes. Nonspecific binding was blocked by incubating the membranes in TBST with 5% nonfat milk on a shaker for 1 h. The membranes were incubated with primary rabbit HIF-1α, NF-kB, P-P65, P65 (1:2000; ABclonal, Boston, USA) and mouse β-actin (1:2000; ABclonal, Boston, USA), on a shaker at 4 °C overnight. All of the first antibodies were diluted by 5% fetal bovine serum (FBS). The membranes were then washed three times with 5% TBST at room temperature for 30 min. The anti-mouse IGg and anti-rabbit IGg diluted by 5% nonfat milk were used to conjugate the primary immunoglobulin and the baking continued for 1 h. After stripping three times in TBST for 30 min, the bands were detected by the Tanon 5200 Multi-system. The densitometric analysis was performed using automatic fluorescence and chemiluminescence imaging analysis system.
Immunohistochemistry Assay
Paraffin-embedded rat liver tissues were sectioned into slices. The tissues were heated to 70 °C for 1 h before dewaxing in xylene. The rehydration was done in decreasing concentrations of ethanol and 1 × phosphate buffer saline (PBS). Antigen repair was achieved by heating sodium citrate solution in a pressure cooker. Immunohistochemistry kit was provided by MaiXin, the concentrations of HIF-1α antibody was 1:100. In this study, DAB was the reagent for staining and the staining lasted for 10 min. The tissue was examined under a microscope and scored with the histochemistry score (H-Score) method by Image-Pro Plus system.
Serological Detection
Serum levels of alanine transaminase (ALT) and aspertate transaminase (AST) were measured in this study by using ELISA. The blood samples were rested for 2 h at room temperature and then centrifuged at 3000 r/min for 15 min. The serum was extracted and stored in liquid nitrogen before testing. The frozen serum was melting in room temperature. The reagent kit was provided by Cayman chemical for ALT testing and BioVision for AST testing. The interleukin-1β, NLRP3, interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) as inflammatory markers were measured by using ELISA kits. The optical density (OD) was detected by iMark Microplate Reader.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Inter-group comparisons were made with Student’s t test for two groups and by one-way analysis-of-variance test. (P < 0.05 was considered statically significant.) Statistical and graphing software used in this study was SPSS 20.0 and GraphPad Prism 7.0.
Results
General Conditions of CLP-Treated Animals
Although the rectal temperature in early sepsis (24 h) was higher compared to that in Sham group, it decreased significantly with the P value of 0.0197 at 72 h after CLP. The mean arterial pressure (MAP) in rats was changed with the increasing sepsis time. It remained stable within 24-h post-CLP, while MAP decreased significantly at 72 h (P < 0.0001). Blood lactate (Lac) was highly enriched in CLP-treated group. At 24 h, the increasing Lac was significant with the P value of 0.0014 (< 0.05). The lactate enrichment was more pronounced at 72 h with the P value of < 0.0001 compared with the sham group. The MSS reflected the general condition of rats and increased significantly in CLP-treated group, especially at late stage of sepsis. CLP-treated rats had a lower PaO2 and PaCO2 levels, which began to decrease obviously after 24 h of CLP and reached the lowest point at 72 h (PaO2, P < 0.05, PaCO2, < 0.0001). The AST level rise occurred after 72-h post-CLP compared to the Sham group (P < 0.05), while, the level of ALT did not change significantly during the whole study (Fig. 1). The results meant that a sepsis and subsequent liver dysfunction model was successfully established.
Blood gas analysis in both CLP and Sham group (n = 28). a Rectal temperature (°C); b Mean arterial pressure (mmHg); c Blood lactate (mmol/L); d Murine sepsis score (MSS); e Arterial PO2 (mmHg); f Arterial PCO2 (mmHg); g Aspartate transaminase (AST, U/L); h Alanine aminotransferase (ALT, U/L). *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.0001 versus sham group
LGG-Improved Liver Injury During Sepsis
Previous study has shown that probiotic treatment with LGG can improve survival following CLP [16]. Thus, we hypothesized that LGG can also attenuate liver injury in sepsis. In this study, sepsis led to marked histological injury 72 h after CLP treatment in septic rats. In addition, the liver injury was significantly improved in septic rats treated with LGG. As shown in Fig. 2 A1, liver cells were intact, their structures were clear, and they were uniformly distributed in the normal sections, but in the model sections (Fig. 2 A3), the section showed severe hepatocellular necrosis and inflammatory cell infiltration, and the liver cells had no regular arrangement around the central veins. After LGG treatment, pathologic damage degree was reduced compared with those of the model samples (Fig. 2 A4). The organ damage score (ODS) and level of endotoxin were used to evaluate the degree of liver injury.
LGG-Attenuated Pro-inflammatory Cytokine Release in the Liver After Sepsis
To evaluate the effect of LGG treatment on pro-inflammatory cytokine release in liver after CLP-induced sepsis, protein levels and mRNA expressions of IL-1β, IL-6, NLRP3 and TNF-a were analyzed by RT-PCR and ELISA, respectively. The mRNA expressions of IL-1β, IL-6, NLRP3 and TNF-a were significantly increased in liver of septic rats and decreased in LGG-treated rats (P < 0.05). In addition, the protein levels of IL-1β, IL-6, NLRP3 and TNF-a were also elevated in septic rats and ameliorated in LGG-treated rats (Fig. 3).
LGG-attenuated pro-inflammatory cytokine release in liver 72-h post-CLP (n = 60). a Relative IL-6 mRNA levels; b Relative TNF-α mRNA levels; c Elisa showing IL-6 levels; d Elisa showing TNF-α levels; e Relative IL-1β mRNA levels; f Relative NLRP3 mRNA levels; g Elisa showing IL-1β levels; h Elisa showing NLRP3 levels. *P < 0.05 versus all groups
LGG Protected Against CLP-Induced Liver Hypoxia
Hypoxia could decrease ATP synthesis and increase reactive oxygen species (ROS) [24]. In addition, hypoxia could decrease the activity of the cellular antioxidant system, which could lead to oxidative stress [25]. In this study, therefore, oxidative stress and lipid peroxidation in the liver were measured in order to elucidate the mechanism of LGG-induced protection against sepsis-induced liver hypoxia (Fig. 4). Levels of oxidative stress in the liver of CLP-treated rats were raised by 2.2-fold. Nonetheless, treatment of CLP-treated rats with LGG lowered levels of oxidative stress (Fig. 4a). Successively, administration of CLP to rats led to a significant 90% increase in hepatic lipid peroxidation (Fig. 2b). In comparison, exposure to LGG decreased CLP-induced lipid peroxidation in the liver by 75% (Fig. 4b).
LGG Decreased HIF-1α and Target Genes (VEGF and MCP1) Expressions by Regulating NF-kB Pathway in the Liver During Sepsis
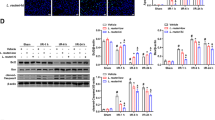
HIF-1α activity is closely regulated by oxygen-dependent control of the cell and elevated in response to infections. Gene expression and protein levels of HIF-1α were significantly increased in liver of septic rats (P < 0.05) and treatment with LGG significantly decreased (P < 0.05) HIF-1α levels in Sepsis + LGG group (Fig. 5a). In addition, a significantly higher number of HIF-1α positive cells in the septic animals compared to shams (P < 0.01). Treatment with LGG reduced the number of positive cells in the liver compared to shams (P < 0.01) (Fig. 5b). And HIF-1α target genes (VEGF and MCP1) were significantly increased in liver of septic rats and decreased in Sepsis + LGG group (Fig. 5c).
LGG decreased HIF-1α expression and regulates NF-kB pathway in the liver 72-h post-CLP (n = 60). a, c Western blot showing protein levels of HIF-1α and NF-kB. b IHC showing HIF1-α positive cells (magnification, × 200) (B1, Sham; B2, Sham + LGG; B3, Sepsis; B4, Sepsis + LGG); d Relative VEGF and MCP1 mRNA levels; e Western blot showing P-P65, P65, HIF-1α and β-Actin. *P < 0.05 versus all groups; **P < 0.01 BAY11-7085 versus control group
NF-kB transcriptional factor is a key activator of the cytokines involved in the innate immunity and oxidative stress response. There was a significant increase in NF-kB in the septic rats (P < 0.05) and significant decrease in the LGG-treated rats (P < 0.05) (Fig. 5d). Several studies showed that HIF-1α was upregulated by binding NF-kB to the hypoxia response element [26,27,28,29]. Activation of NF-κB was monitored from NF-κB p65 phosphorylation levels. Thus, we examined the existence of the NF-κB/HIF-1α pathway in septic rats. Western blot analysis revealed that treatment with NF-κB inhibitor (BAY 11-7085) caused a decrease in both NF-κB p65 phosphorylation and HIF-1α protein levels. qRTPCR analysis showed that HIF-1α mRNA level in the NF-κB inhibitor-treated septic rats was significantly reduced by 0.58-fold reduction compared with control (Fig. 5e).
The results suggested that NF-κB activation was necessary for hypoxia response. Additionally, NF-κB activation also modulated HIF-1α gene expression during septic rats with LGG treatment.
LGG Improved the Survival of Rats Subjected to CLP
As shown in Fig. 6, the mortality rates 72 h after operation were 0, 0, 41 and 25%, for the Sham, Sham + LGG, Sepsis, Sepsis + LGG groups, respectively. Compared to the Sepsis group, the mortality rate in Sepsis + LGG group markedly decreased (P < 0.05, Sepsis versus Sepsis + LGG) (Fig. 6).
Discussion
The present study is the first to investigative the relationship between Lactobacillus rhamnosus GG (LGG) and liver injury. First, we successfully established a sepsis and subsequent liver dysfunction model. Second, this study demonstrated that LGG can ameliorate liver injury and hypoxic hepatitis in rat model of CLP-induced sepsis.
Recent study has shown that the gut microbiota has been identified as origin and promoter of nosocomial sepsis [30]. And one review has reported that an extensive overview of the potential role of gut microbiota in the development of sepsis [31]. Critical illness and ICU-based therapies, such as proton pump inhibitors, opioids, vasopressors and antibiotics, alter the microflora favoring the growth of pathogens. This is due to the loss of key beneficial lactic acid bacteria that can provide health benefits and inhibit the overgrowth of pathogens by production of bacteriocins [32]. In addition, a number of bioactive factors secreted by LGG have been identified and their effects studied in intestinal injury as well as alcohol-induced liver injury models [33]. Polk et al. [34] demonstrated that Lactobacillus rhamnosus GG (LGG)-derived soluble protein, p40 ameliorated cytokine-induced apoptosis in intestinal epithelial cells through activation of the EGF receptor. And previous study showed that differential gene-regulatory networks and pathways regulate major basal mucosal processes and uncovered remarkable similarity to response profiles obtained for specific bioactive molecules and drugs [35]. Recently, a review showed that several probiotics can enhance nonspecific cellular immune response characterized by activation of macrophages, natural killer (NK) cells, antigen-specific cytotoxic T-lymphocytes and the release of various cytokines [36]. As a result, many studies support the potential use of microbial therapies in the treatment of oxidative stress and nonalcoholic fatty liver disease, but there are still many questions to be answered about their mechanisms of action [37,38,39]. In the sepsis, oxidative stress and pro-inflammatory cytokines cause tissue damage and multiple organ failure [40]. And sepsis can induces lipid peroxidation in the liver because of oxidative stress and inflammation [41]. Hence, we hypothesized protection by LGG against sepsis-induced liver injury and hypoxic hepatitis.
The pathophysiology of sepsis and subsequent liver dysfunction is characterized by hyperactive and dysregulated endogenous inflammatory mediators including IL-6, IL-1β, NLRP3 and TNF-α [42,43,44,45,46]. In this study, the rats were given LGG immediately before the surgery to better reflect the common clinical setting where a patient presenting with peritonitis could be treated at the time of surgery to attempt to prevent future hospital acquired infections and liver injury. Previous study has shown that early attenuation of transcription factor NF-kappaB activation and cytokine message expression correlates with improved outcome in polymicrobial sepsis [47]. Animal experiment data support an important role of the liver in the development of the multiple organ dysfunction syndrome (MODS) [48]. The release of pro-inflammatory mediators can lead to acute liver injury, and it has been reported that expressions of pro-inflammatory cytokines such as IL-1β, NLRP3, IL-6 and TNF-a are significantly elevated in the damaged liver [49, 50]. Similarly, in our rat model of CLP-induced sepsis, we found significantly increased mRNA and protein levels of pro-inflammatory cytokines IL-1β, NLRP3, TNF-a, IL-6 in the liver of septic rats, and levels of those decreased in LGG-treated group. In addition, the sepsis-induced lipid peroxidation, but LGG treatment could ameliorate this effect. Treatment at the time of onset of peritonitis with LGG normalized those cytokine expressions in the septic rats indicating the anti-inflammatory role of LGG possibly contributing to better overall outcome.
In general, HIF-1α is not expressed in healthy tissues under normal oxygen saturation but is rapidly induced in response to hypoxic condition and is elevated at sites of inflammation and injury [51] and is involved in pathogenesis of sepsis [27]. HIF-1α expression was previously shown to increase and inhibition of it successfully attenuated liver injury in the alcoholic fatty liver disease of rats [52]. As shown in several studies, pathogens induce HIF-1α expression via activated NF-kB signaling pathway [53, 54]. A large number of previous studies have confirmed that hypoxia can induces HIF1 target gene expressions (VEGF and MCP1) to participate in liver injury caused by poisoning through activation of HIF1 [55, 56]. In this study, elevated HIF1 directly regulated the target genes VEGF and MCP1 increase, which is consistent with previous literature reports. The NF-kB factor plays a central role in the initiation of innate immune responses and in the development of a subsequent pro-inflammatory response, which can lead to inflammation-induced organ injury. In this study, we demonstrated that HIF-1α and NF-kB were significantly higher in the liver of septic rats compared to healthy shams and lower in the liver of LGG group. We speculate that downregulation of HIF-1α through targeting NF-kB in the liver of LGG-treated rats may play a protective role in attenuating inflammation-induced liver injury following systemic sepsis and peritonitis.
In conclusion, probiotic therapy such as LGG can reduces liver injury following experimental sepsis and is associated with ameliorated hypoxic hepatitis. Probiotic therapy may be a promising intervention to improve clinical liver injury and hypoxic hepatitis.
References
Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386.
Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272.
Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810.
Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore). 2003;82:392–406.
Fuhrmann V, Madl C, Mueller C, et al. Hepatopulmonary syndrome in patients with hypoxic hepatitis. Gastroenterology. 2006;131:69–75.
Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45:129–133.
Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–1070.
Fuhrmann V, Kneidinger N, Herkner H, et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37:1302–1310.
Cui Y, Shan Y, Chen R, Wang C, Zhang Y. Elevated serum total bilirubin level is associated with poor outcomes in pediatric patients with sepsis-associated liver injury. Can J Infect Dis Med Microbiol. 2018;2018:4591729.
Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192:581–588.
Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2:135–143.
Hammerman C, Bin-Nun A, Kaplan M. Germ warfare: probiotics in defense of the premature gut. Clin Perinatol. 2004;31:489–500.
Liu DQ, Gao QY, Liu HB, Li DH, Wu SW. Probiotics improve survival of septic rats by suppressing conditioned pathogens in ascites. World J Gastroenterol. 2013;19:4053–4059.
Khailova L, Frank DN, Dominguez JA, Wischmeyer PE. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology. 2013;119:166–177.
Arribas B, Rodriguez-Cabezas ME, Camuesco D, et al. A probiotic strain of Escherichia coli, Nissle, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br J Pharmacol. 1917;157(2009):1024–1033.
Khailova L, Petrie B, Baird CH, Rieg JAD, Wischmeyer PE. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS ONE. 2014;9:e97861.
Boyle RJ, Robins-Browne RM, Tang MLK. Probiotic use in clinical practice: What are the risks? Am J Clin Nutr. 2006;83:1256–1264.
Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22–30.
Toscano MG, Ganea D, Gamero AM. Cecal ligation puncture procedure. J Vis Exp. 2011;51:e2860.
Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36.
Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality-rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335.
Kleszczynski K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (gamma-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J Pineal Res. 2016;61:187–197.
Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52.
Wu C, Li X, Zhang D, et al. IL-1beta-mediated up-regulation of WT1D via miR-144-3p and their synergistic effect with NF-kappaB/COX-2/HIF-1alpha pathway on cell proliferation in LUAD. Cell Physiol Biochem. 2018;48:2493–2502.
Zhan CY, Chen D, Luo JL, Shi YH, Zhang YP. Protective role of down-regulated microRNA-31 on intestinal barrier dysfunction through inhibition of NF-kappaB/HIF-1alpha pathway by binding to HMOX1 in rats with sepsis. Mol Med. 2018;24:55.
Taylor CT, Cummins EP. The role of NF-kappaB in hypoxia-induced gene expression. Ann N Y Acad Sci. 2009;1177:178–184.
Gorlach A, Bonello S. The cross-talk between NF-kappa B and HIF-1: further evidence for a significant liaison (vol 412, p e17. Biochem J. 2008;413(2008):571.
McDonald D, Ackermann G, Khailova L, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1:e00199-16.
Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4:59–72.
Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20.
Tian F, Chi F, Wang G, et al. Lactobacillus rhamnosus CCFM1107 treatment ameliorates alcohol-induced liver injury in a mouse model of chronic alcohol feeding. J Microbiol. 2015;53:856–863.
Yan F, Polk DB. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes. 2012;3:25–28.
van Baarlen P, Troost F, van der Meer C, et al. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc Natl Acad Sci USA. 2011;108:4562–4569.
Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54:938–956.
Ye HQ, Li Q, Zhang ZZ, Sun MC, Zhao CH, Zhang TH. Effect of a novel potential probiotic Lactobacillus paracasei Jlus66 isolated from fermented milk on nonalcoholic fatty liver in rats. Food Funct. 2017;8:4539–4546.
Aller R, de Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–1095.
Bouhafs L, Moudilou EN, Exbrayat JM, Lahouel M, Idoui T. Protective effects of probiotic Lactobacillus plantarum BJ0021 on liver and kidney oxidative stress and apoptosis induced by endosulfan in pregnant rats. Renal Fail. 2015;37:1370–1378.
Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG. The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth. 1992;68:613–618.
AbdEl-Latif AAE, Sayed AA, Soliman AM, Fahmy SR. Exploration of the therapeutic potential effect of Sepia officinalis in animal model of sepsis induced by cecal ligation and puncture. Injury. 2016;47:2709–2717.
Li CYC, Munitic I, Mittelstadt PR, Castro E, Ashwell JD. Suppression of dendritic cell-derived IL-12 by endogenous glucocorticoids is protective in LPS-induced sepsis. PLoS Biol. 2015;13:e10022696.
Hou TY, Huang DH, Zeng R, Ye ZM, Zhang Y. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:15238–15245.
Montoya-Ruiz C, Jaimes FA, Rugeles MT, Lopez JA, Bedoya G, Velilla PA. Variants in LTA, TNF, IL1B and IL10 genes associated with the clinical course of sepsis. Immunol Res. 2016;64:1168–1178.
Ozen BD, Uyanoglu M. Effect of carvacrol on IL-6/STAT3 pathway after partial hepatectomy in rat liver. Bratisl Med J Bratisl Lek Listy. 2018;119:593–601.
Gil-Farina I, Di Scala M, Vanrell L, et al. IL12-mediated liver inflammation reduces the formation of AAV transcriptionally active forms but has no effect over preexisting AAV transgene expression. PLoS ONE. 2013;8:e67748.
Williams DL, Ha T, Li C, Kalbfleisch JH, Laffan JJ, Ferguson DA. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1 → 3)-beta-d-glucan increases long-term survival in polymicrobial sepsis. Surgery. 1999;126:54–65.
Hou YX, Liu SW, Wang LW, Wu SH. Physiopathology of multiple organ dysfunctions in severely monocrotophos-poisoned rabbits. Chem Biol Interact. 2017;278:9–14.
Campana L, Lewis PJS, Pellicoro A, et al. The STAT3-IL-10-IL-6 pathway is a novel regulator of macrophage efferocytosis and phenotypic conversion in sterile liver injury. J Immunol. 2018;200:1169–1187.
Lu H, Zhang L, Gu LL, Hou BY, Du GH. Oxymatrine induces liver injury through JNK signalling pathway mediated by TNF- in vivo. Basic Clin Pharmacol Toxicol. 2016;119:405–411.
Chen W, Jadhav V, Tang J, Zhang JH. HIF-1 alpha inhibition ameliorates neonatal brain damage after hypoxic-ischemic injury. Acta Neurochir Suppl. 2008;102:395–399.
Ma Z, Zhang Y, Li Q, Xu M, Bai J, Wu S. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1alpha expression and mitochondrial ROS production. PLoS ONE. 2017;12:e0183426.
Kim Y, Kim BH, Lee H, et al. Regulation of skin inflammation and angiogenesis by EC-SOD via HIF-1alpha and NF-kappa B pathways. Free Radic Biol Med. 2011;51:1985–1995.
Lau TY, Xiao J, Liong EC, et al. Hepatic response to chronic hypoxia in experimental rat model through HIF-1 alpha, activator protein-1 and NF-kappa B. Histol Histopathol. 2013;28:463–471.
Shneor D, Folberg R, Pe’er J, Honigman A, Frenkel S. Stable knockdown of CREB, HIF-1 and HIF-2 by replication-competent retroviruses abrogates the responses to hypoxia in hepatocellular carcinoma. Cancer Gene Ther. 2017;24:64–74.
Zamara E, Galastri S, Aleffi S, et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46:230–238.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Co-first author: Yihang Gong.
Rights and permissions
About this article
Cite this article
Ding, L., Gong, Y., Yang, Z. et al. Lactobacillus rhamnosus GG Ameliorates Liver Injury and Hypoxic Hepatitis in Rat Model of CLP-Induced Sepsis. Dig Dis Sci 64, 2867–2877 (2019). https://doi.org/10.1007/s10620-019-05628-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05628-0