Abstract
Purpose
In this study, we sought to find the effects and mechanisms of probiotic Lactobacillus casei Zhang (L. casei Zhang) on the pro-inflammatory cytokine production and hepatic inflammatory response in a rat model of acute liver failure induced by lipopolysaccharide (LPS) and d-galactosamine (GalN).
Methods
Male Wistar rats were orally administrated with or without L. casei Zhang for 30 days prior to challenge with LPS and GalN. Dexamethasone administrated group serving as a positive anti-inflammation control. Serum, intestinal and liver samples were collected 8 h after LPS/GalN challenge for histological, molecular and biochemical analysis.
Results
LPS/GalN challenge alone resulted in significantly increased production of endotoxin, tumor necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β) and nitric oxide as compared to the normal control rats. Pretreatment with L. casei Zhang not only reduced these changes, but also attenuated hepatic inflammation as shown by improved histological assessment, decreased myeloperoxidase activity and reduced expression of IL-1β and inducible nitric oxide synthase in the liver. L. casei Zhang supplementation significantly inhibited LPS/GalN-triggered phosphorylation of ERK, JNK and p-38 MAPK, but increased the expression of TLR2, TLR9 and PPAR-γ. Moreover, L. casei Zhang treatment prevented intestinal injury and modulated the intestinal ecology by increasing the fecal Lactobacillus and Bifidobacterium levels.
Conclusions
Probiotic L. casei Zhang reduces LPS/GalN-induced pro-inflammatory cytokine and hepatic inflammation through modulating the TLR-MAPK-PPAR-γ signaling pathways and intestinal microbiota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute liver failure (ALF) is a life-threatening clinical syndrome resulting from massive apoptosis of hepatocytes. Viral infections are the predominant causes of ALF for patients living in the developing world. Other causes of ALF encompass drug and toxin exposure, alcohol, ischemia, metabolic disorders, septic shock, massive malignant infiltration and chronic autoimmune hepatitis [1, 2].
Although liver transplantation is an effective therapy for specific causes of ALF, this kind of treatment is not universally available due to the shortage of liver donors and the rapid progression of ALF [3]. In particular, the development of sepsis and subsequent multiple organ failure markedly reduce the efficacy of transplantation. Therefore, preventive therapies to control the onset of sepsis are crucial to ALF patients who are awaiting a liver transplant.
A frequently used animal model of ALF is the combined injection of a subtoxic dose of d-galactosamine (GalN) and lipopolysaccharide (LPS) [4, 5]. LPS challenge can induce pro-inflammatory cytokine production by hepatic kupffer cells via pattern recognition through Toll-like receptors (TLRs). Among the identified TLRs, TLR4 is a well-defined receptor for recognition of gram-negative bacteria which produce LPS. TLR2 mainly recognizes the cell wall component of gram-positive bacteria such as peptidoglycan and lipopeptides, while TLR9 binds to either bacterial or viral DNA. Upon ligand recognition, TLRs trigger signal transduction involving in the mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) pathways, resulting in the production of pro-inflammatory cytokines such as TNF-α, an early and important mediator of liver injury [6]. Peroxisome proliferator-activated receptor gamma (PPAR-γ) is a nuclear hormone receptor and transcription factor that has been demonstrated to inhibit NF-κB activity [7]. Since there are high levels of PPAR-γ expression in liver, activation of PPAR-γ may attenuate hepatic inflammation and the onset of septic shock.
There is accumulating evidence that the gut plays a vital role in the pathogenesis of liver diseases [8–10]. Gram-negative bacteria-derived endotoxaemia, in conjunction with impaired gut barrier function and translocation of bacteria to the liver, is the proposed mechanism of septic liver injury. Thus, a therapy protecting against alterations to gut microbiota could be useful in preventing hepatic inflammation. Previous studies have shown that chronic liver diseases resulted from alcohol consumption, viral infection and metabolic disorders are attenuated when treated with probiotics [8–10]. In addition, supplementation of probiotic Lactobacillus or Bifidobacterium has been found to reduce endotoxin-induced acute liver failure [11, 12]. Our previous study showed that probiotic L. casei Zhang possessed antioxidative capacities and exhibited hepatoprotective effects against LPS/GalN-induced liver injury [13, 14]. However, the detailed molecular mechanisms underlying the hepatoprotective effects of L. casei Zhang against ALF are unknown. The aim of the present study was to further explore the effects and mechanisms of L. casei Zhang on the pro-inflammatory cytokine production and hepatic inflammation in a rat model of ALF.
Materials and methods
Animals and diets
Male Wistar rats were purchased from Vital River Laboratories Animal Co. Ltd. (Beijing, China). Rats received a standard laboratory diet and water ad libitum as described previously [14]. Experiments on rats began after 1 week’s adaptation. All protocols for animal experiments were approved by the Animal Care and Use Committee at Inner Mongolia Agricultural University.
Bacteria strain and culture conditions
The Lactobacillus strain used in this study, Lactobacillus casei Zhang (L. casei Zhang), is a novel lactic acid bacteria isolated from the Mongolian beverage koumiss and has a set of favorable probiotic properties [15]. The strain was cultured anaerobically in de Man, Rogosa and Sharpe (MRS) broth (Hopebio Co., Qingdao, China) at 37 °C for 36 h. The bacterial pellets were harvested by centrifugation, washed twice with PBS, and lyophilized. The lyophilized powder was suspended in physiological saline and adjusted to 1 × 109 CFU/ml before use.
Experimental design
Forty 5- to 6-week old male Wistar rats were randomly divided into normal control (NC), LPS and GalN-induced ALF control (L/G), L. casei Zhang pretreatment (L/G + LCZ) and dexamethasone group (L/G + DXM), which served as a positive anti-inflammation control. The NC, L/G and L/G + DXM group received saline by oral gavage, and the L/G + LCZ group was gavaged with L. casei Zhang (109 CFU/rat/day) for 30 days. Except for the NC group, ALF in all rats was induced by intraperitoneal injection of 50 μg/kg LPS and 300 mg/kg GalN (Sigma-Aldrich, Missouri, USA). The rats in the L/G + DXM group were given an intraperitoneal injection of dexamethasone (10 mg/kg) at the same time as the LPS/GalN challenge. Eight hours after the LPS/GalN challenge, all the rats were anesthetized with Avertin (250 mg/kg), before blood and tissue samples were collected. The rats were subsequently killed by cervical dislocation. Hepatic samples were taken from the right liver lobe, and intestinal samples were taken from the ileum. Parts of the samples were fixed in 10 % formalin for histological observation. The remainder of the samples was stored in liquid nitrogen until use.
Biochemical analysis of serum
Serum was obtained by centrifuging blood at 3,000×g for 15 min, which was then stored at 4 °C. Endotoxin in serum was quantitatively determined by the limulus amoebocyte lysate test according to the manufacturer’s instruction (Xiamen Horseshoe Crab Reagent Manufactory Co., Ltd., Fujian, China). Serum levels of TNF-α and IL-1β were measured by an enzyme-linked immunosorbent assay (ELISA) using the commercially available ELISA kit (R&D Systems, Minneapolis, USA). The concentration of NO in serum was detected with Griess reagent (Sigma-Aldrich, Missouri, USA) as described previously [16].
Hepatic myeloperoxidase (MPO) activity assay
Liver samples (100 mg) were homogenized in 50 mM of potassium phosphate buffer solution (pH 6.0) with 0.5 % hexadecyltrimethylammonium bromide at 4 °C and then centrifuged at 15,000×g at 4 °C for 30 min. Supernatants were sonicated and then mixed with hydrogen peroxide-sodium acetate and tetramethylbenzidine solutions. MPO activity was measured spectrophotometrically at A650 in a spectrophotometer (Shanghai Precision & Scientific Instrument Co. Ltd, China). Results were showed as units of MPO activity per gram of liver tissue.
Real-time PCR assay
Relative expression of TNF-α, IL-1β, iNOS, TLR2, TLR4, TLR9 and PPAR-γ mRNA was detected by real-time PCR with the SYBR Real-Time PCR kit (Takara, Dalian, China). Primers used to amplify the above genes are listed in Supplemental Table 1. Real-time PCRs were initiated by the denaturation step of 5 min at 95 °C, followed by 40 cycles of amplification, which were performed according to the following thermal cycling protocol, denaturation for 5 s at 95 °C, and annealing and extension for 1 min at 60 °C (or 56 °C for PPAR-γ and GAPDH). GAPDH was used as an endogenous control to normalize the expression of target transcripts. Relative mRNA expression was calculated by the 2−ΔΔCt method.
Histological evaluation and immunohistochemical staining
Hepatic and small intestinal samples were fixed, embedded in paraplast, and cut into 3- to 4-μm sections. Sectioned tissues were stained with hematoxylin-eosin (HE) and assessed blindly by a pathologist. The degree of intestinal mucosal injury was performed using the Chiu Scoring System [17]. Briefly, the degree of intestinal injury was recorded and classified as grades 0 (normal mucosa), 1 (development of subepithelial spaces at villus tips), 2 (extension of the subepithelial space with moderate lifting of the epithelial layer), 3 (massive epithelial lifting with a few denuded villi), 4 (denuded villi with exposed capillaries) and 5 (disintegration of the lamina propria, ulceration and hemorrhage). Immunohistochemistry staining was performed according to the procedures which were described in our previous experiment [14].
Western blotting analysis
The isolation of the liver proteins and Western blotting analysis were performed as previously described [18]. Membranes were probed using antibodies against phospho-JNK, total-JNK, phospho-p38 MAPK, total-p38 MAPK and PPAR-γ (Cell Signaling, Danvers, MA, USA). Beta-actin served as a loading control. The membranes were washed 5 times with the washing buffer and then incubated with IRDye 800CW Goat Anti-rabbit IgG(H + L) (LI-COR, Nebraska, USA) at a concentration of 1:15000 for 1 h. Antibody-bound proteins were detected with the LI-COR Odyssey Infrared Imaging System.
Determination of fecal microbiota composition
Intestinal microbiota was analyzed using specific primers to amplify the predominant bacteria of Lactobacillus, Bifidobacterium, Bacteroides fragilis and Clostridium in the feces of rats. DNA was extracted from the feces of rats and used as the templates of real-time PCR. The specific primers used for real-time PCR and the PCR were described previously [19].
Statistical analysis
All data are expressed as mean ± SEM. Statistical analysis was performed by one-way factorial analysis of variance (ANOVA) and Scheffe formula for post hoc multiple comparisons. P values <0.05 were considered to be statistically significant.
Results
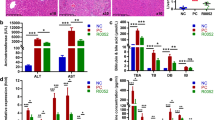
The LPS/GalN challenge resulted in significantly increased serum endotoxin as compared to the NC group (P < 0.01) (Fig. 1a). However, pretreatment with L. casei Zhang remarkably reduced the levels of endotoxin (P < 0.01). Significant attenuation in the levels of endotoxin was also found in the L/G + DXM group (P < 0.01). There was a smaller difference between the L/G + LCZ group and the L/G + DXM groups in the production of endotoxin (P > 0.05).
Administration of LPS/GalN also markedly induced elevation of serum TNF-α and NO levels (Fig. 1b, d, P < 0.01). Likewise, L. casei Zhang supplementation and dexamethasone treatment had a significant effect on lowering the production of serum TNF-α and NO, and the effect was more evident in the latter group (P < 0.01). L. casei Zhang supplementation had a similar lowering effect on LPS/GaLN-induced IL-1β production (Fig. 1c). Little difference was found between the L/G + LCZ and L/G + DXM groups in the production of IL-1β (P > 0.05).
Histological evaluation of liver sections is presented in Fig. 2. The NC group exhibited an integral hepatic lobular architecture and normal hepatocytes (Fig. 2a). Massive inflammatory accumulation and loss of lobular architecture were observed in the L/G group (Fig. 2b). Compared with the L/G group, liver histology was significantly improved in both the L/G + LCZ and L/G + DXM groups, indicating a marked decrease in inflammatory cell infiltration and well-arranged hepatic lobular architecture (Fig. 2c, d).
Representative photomicrographs of hematoxylin-eosin-stained liver sections. a Histology of the NC group with normal liver structures. b L/G group exhibited multiple inflammatory cell infiltration and loss of lobular architecture. c L. casei Zhang pretreatment attenuated LPS/GalN-induced hepatic inflammation. d L/G + DXM group exhibited recovered liver structures and reduced inflammatory cell infiltration. Magnification 200×
The hepatic myeloperoxidase (MPO) activity was significantly higher in the L/G group, indicating an increased liver inflammation (Fig. 3a). Pretreatment with L. casei Zhang prevented LPS/GalN-increased MPO activity (P < 0.01). Dexamethasone treatment also significantly attenuated the activity of MPO (P < 0.01). Likewise, the mRNA levels of IL-1β and iNOS were significantly increased in the L/G group, but this increase was attenuated by L. casei Zhang supplementation and dexamethasone administration (Fig. 3b, c). We found the same trend of decreased expression of TNF-α mRNA in both the L/G + LCZ and L/G + DXM groups as seen in our previous results [14] (Data not shown).
The mRNA expression of TLR2 and TLR9 in the livers of different groups was quantified. LPS/GalN challenge resulted in reduced expression of TLR2 mRNA (P < 0.01) and no significant change in TLR9 mRNA expression as compared to the NC group (P = 0.45). Compared with the L/G group, pretreatment with L. casei Zhang increased the expression of TLR2 and TLR9 mRNA (P < 0.01). However, there is a significant difference in the expression of TLR2 mRNA between the L/G + LCZ and L/G + DXM groups (P < 0.01). A robust increase in TLR9 mRNA expression was found after dexamethasone treatment (Fig. 4).
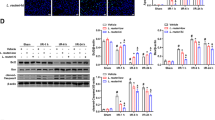
There was a MAPK signal transduction pathway change in different group of rats (Fig. 5). Compared with the NC group, LPS/GalN administration resulted in increased phosphorylation of ERK. Pretreatment of L. casei Zhang and dexamethasone treatment decreased LPS/GalN-induced ERK phosphorylation (Fig. 5a). Similarly, elevated expression of phosphorylated-JNK (p-JNK) and phosphorylated-p38 (p-p38) MAPK was found in the L/G group, and a lower level of p-JNK and p-p38 MAPK was observed in the L/G + LCZ and L/G + DXM groups (Fig. 5b).
There was a markedly reduced expression of hepatic PPAR-γ both at the mRNA and protein level in the L/G group as compared to the NC group (Fig. 6a, b). In contrast, L. casei Zhang supplementation reserved hepatic expression of PPAR-γ. There is no significant difference in relative PPAR-γ mRNA expression between the L/G group and the L/G + DXM group. However, there was a significant difference in PPAR-γ expression between the L/G + LCZ and L/G + DXM groups (P < 0.01).
HE staining of intestinal sections showed normal intestinal mucosa with integral villi in the control rats. However, the challenge with LPS/GalN resulted in the highest disorder in the structure of intestinal mucosa with necrosis of epithelial cells, extension of the subepithelial space and structural damage of villi. Intestinal histology was significantly improved by the L. casei Zhang pretreatment and dexamethasone administration, showing alleviated swelling of intestinal mucosa, less expansion of the subepithelial space and well-arranged structure of villi (Fig. 7a). The pathological grade of the intestinal injury in the L/G group was significantly higher than that in the NC group (P < 0.01). In contrast, L. casei Zhang supplementation and dexamethasone treatment significantly reduced the intestinal pathological grades (P < 0.01, Fig. 7b).
LPS/GalN challenge induced a lower level of Lactobacillus, Bifidobacterium and Bacteroides fragilis (Fig. 8a–c, P < 0.01) and a higher level of Clostridium as compared to those in the NC group (Fig. 8d, P < 0.01). Compared with the L/G group, pretreatment with L. casei Zhang remarkably increased the numbers of Lactobacillus and Bifidobacterium, while decreased the numbers of Clostridium in the feces (P < 0.01). Such change was not found in the rats of the L/G + DXM group. There was no significant change in the numbers of Bacteroides fragilis in the L/G + LCZ group and the L/G + DXM group as compared to the L/G group (Fig. 8c).
Discussion
Our previous results showed that probiotic L. Casei Zhang exerted immuno-modulatory effects both in vitro and in vivo [15, 20] and L. Casei Zhang supplementation protected against LPS/GalN-induced ALF [14]. Although ALF can be caused by a variety of insults including viral infection and drug effects, the resulting clinical manifestation is quite similar across the different etiologies, reflecting common patterns of inflammatory responses. This study aimed to further explore the effects and underlying mechanisms of L. casei Zhang on the production of pro-inflammatory cytokine and hepatic inflammation in a rat model of ALF. Our results indicate that L. casei Zhang supplementation inhibits the production of pro-inflammatory cytokine and hepatic inflammation through modulation of the TLR-MAPK-PPAR-γ signaling pathways and the composition of intestinal microbiota.
A major finding of this study was that 30 days of L. casei Zhang supplementation reduced pro-inflammatory cytokine production and attenuated LPS/GalN-induced hepatic inflammation. A recent study demonstrated that Lactobacillus rhamnosus GG reduced hepatic TNF-α production and inflammation in a mouse model of chronic alcohol-induced liver injury [21]. In this study, pretreatment with L. casei Zhang not only reduced hepatic inflammation, but also attenuated systematic inflammatory mediators such as TNF-α, IL-1β and NO. It was notable that L. casei Zhang supplementation significantly reduced LPS-GalN-induced endotoxin production (Fig. 1). It seems that the decreased inflammatory responses resulted from L. Casei Zhang supplementation are related to decreased endotoxemia.
We also found that L. casei Zhang supplementation reduced the infiltration of inflammatory cells and decreased myeloperoxidase (MPO) activity in liver (Figs. 2, 3). MPO is abundant in neutrophils and can attract neutrophil’s infiltration [22]. It is used as an early and reliable marker for the detection of neutrophil infiltration in an inflammatory setting. Our results suggest that L. casei Zhang can modulate the early inflammatory response in vivo.
Although endotoxin-TLR4 is the major and well-studied mechanism in ALF [5, 23], we speculate that other TLRs may be responsible for some pathological changes. Our previous study had shown that L. casei Zhang supplementation and dexamethasone treatment significantly suppressed LPS/GalN-induced TLR4 mRNA expression [14]. In this study, it was remarkable that TLR2 mRNA expression decreased upon LPS/GalN challenge and normalized by L. casei Zhang supplementation and dexamethasone administration (Fig. 5b). Data from patients indicated that TLR2 mRNA was increased in hepatitis and decreased in hepatocarcinoma [24]. Recent studies demonstrated that bacterial lipoprotein (BLP) and surfactant protein (SP)-A inhibited inflammation via TLR2 [25, 26]. Our previous study showed that L. Casei Zhang increased TLR2 expression in RAW264.7 macrophages [19]. We consider TLR2 play a negative role in the process of LPS/GalN-induced ALF, and the inhibitory effect of L. Casei Zhang may be carried out via TLR2. This point needs to be confirmed with further experimentation. In this study, we found that L. Casei Zhang supplementation and dexamethasone administration significantly increased TLR9 mRNA expression. A recent study has shown that TLR9 was required for Lactobacillus rhamnosus-mediated protection against necrotizing enterocolitis in mice [27]. It seems that TLR9 is involved in the anti-inflammatory effects of L. Casei Zhang.
MAPK signaling cascade is involved in many cellular processes, such as growth, apoptosis and inflammation [28]. Upon ligand recognition, TLRs are activated and initiate a pro-inflammatory response, which depends on the activation of MAPK and NF-κB. Previous studies have shown that LPS/GalN challenge resulted in increased activation of p-ERK and p–JNK [29, 30]. Our results are in agreement with the experimental results from Jin et al. [31] that LPS/GalN treatment increased the expression of p-ERK, P-JNK and p38 MAPK. However, L. casei Zhang pretreatment and dexamethasone administration markedly inhibited LPS/GalN-induced activation of ERK, JNK and p38 MAPK. It implies that the anti-inflammatory effects of L. casei Zhang are associated with a down-regulation of the MAPK signaling pathway.
Oral administration of probiotics modulates intestinal and hepatic expression of PPAR-γ, which provides counter-regulatory signal to inflammation-induced colitis and hepatitis [32, 33]. Data have already demonstrated that PPAR-γ activation may inhibit the activation of TLR4 signaling cascade [34]. Moreover, activation of PPAR-γ has been shown to attenuate LPS-induced acute lung injury and inflammation by suppressing the activation of NF-κB [35]. Our previous study and other reports have shown that probiotics could modulate NF-κB activity [14, 36]. In this study, we provided further evidence that LPS/GalN challenge resulted in the down-regulation of hepatic PPAR-γ protein and that L. casei Zhang supplementation recovered the expression of PPAR-γ (Fig. 6). It suggests that the recovered hepatic expression of PPAR-γ may contribute as a counter-regulatory mechanism to down-regulate the LPS/GalN-induced hepatic inflammatory responses. Such phenomena were not found in the dexamethasone-treated group, indicating that modulation of PPAR-γ expression is not a regulatory mechanism undertaken by dexamethasone.
Previous study has shown that acute liver injury disturbs the intestinal microbiota and impairs the intestinal mucosal barrier function [37]. In this study, it was found that LPS/GalN challenge significantly decreased the numbers of Lactobacillus and Bifidobacterium, while increasing the numbers of Clostridium in the intestine. However, pretreatment with L. casei Zhang reversed the effects of LPS/GalN by increasing the numbers of Lactobacillus and Bifidobacterium and decreasing the numbers of Clostridium. Our previous study also demonstrated a similar modulation of intestinal microbial by L. casei Zhang in hyperinsulinemia rats [16]. We found that the increase in the numbers of Lactobacillus and Bifidobacterium was correlated with intact intestinal mucosa and decreased plasma endotoxin. However, dexamethasone treatment had no significant alteration of the disturbed intestinal microbiota induced by LPS/GalN. Indeed, many studies have demonstrated that dexamethasone itself can perturb the intestinal microbiota [38]. However, the structure of intestinal histology was improved, and serum endotoxin was declined after dexamethasone treatment. Based on this point, disturbances in the intestinal microbiota do not necessarily lead to impaired barrier function. Modulation of intestinal microbiota is just one of the mechanisms of the anti-inflammatory effects of L. casei Zhang.
In conclusion, this study shows that L. casei Zhang supplementation reduces LPS/GalN-induced pro-inflammatory cytokine production and attenuates liver inflammation in a rat model. The molecular mechanisms include inhibition of ERK, JNK and p-38 MAPK phosphorylation, induction of TLR2, TLR9 and PPAR-γ expression and modulation of intestinal microbiota.
Abbreviations
- ALF:
-
Acute liver failure
- GalN:
-
Galactosamine
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MPO:
-
Myeloperoxidase
- NF-κB:
-
Nuclear factor κB
- PPAR-γ:
-
Peroxisome proliferator-activated receptor gamma
- TLRs:
-
Toll-like receptors
- TNF-α :
-
Tumor necrosis factor-α
References
Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369:2525–2534
Schiødt FV, Lee WM (2003) Fulminant liver disease. Clin Liver Dis 7:331–349
Mukherjee S, Mahmoudi TM, Mukherjee U (2009) Liver transplant for viral hepatitis and fulminant hepatic failure. Minerva Gastroenterol Dietol 55:83–100
Xu Y, Wang H, Bao S, Tabassam F, Cai W, Xiang X, Zhao G, Wu H, Gao T, Li H, Xie Q (2013) Amelioration of liver injury by continuously targeted intervention against TNFRp55 in rats with acute-on-chronic liver failure. PLoS ONE 8:e68757
Ben Ari Z, Avlas O, Pappo O, Zilbermints V, Cheporko Y, Bachmetov L, Zemel R, Shainberg A, Sharon E, Grief F, Hochhauser E (2012) Reduced hepatic injury in Toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem 29:41–50
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:461
Genolet R, Wahli W, Michalik L (2004) PPARs as drug targets to modulate inflammatory responses? Curr Drug Targets Inflamm Allergy 3:361–375
Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C et al (2005) Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 39:540–543
Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, Del Vecchio Blanco C, Loguercio C (2011) Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis 43:431–438
Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W (2011) Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol 179:2866–2875
Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G (2007) Endotoxin- and D-galactosamine induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis 39:849–856
Rishi P, Bharrhan S, Singh G, Kaur IP (2011) Effect of Lactobacillus plantarum and l-arginine against endotoxin-induced liver injury in a rat model. Life Sci 89:847–853
Zhang Y, Du RT, Wang LF, Zhang HP (2010) The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur Food Res Technol 231:151–158
Wang Y, Li Y, Xie J, Zhang Y, Wang J, Sun X, Zhang H (2013) Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol 15:30–37
Ya T, Zhang Q, Chu F, Merritt J, Bilige M, Sun T, Du R, Zhang H (2008) Immunological evaluation of Lactobacillus casei Zhang: a newly isolated strain from koumiss in Inner Mongolia, China. BMC Immunol 9:68–76
Xu H, An H, Yu Y, Zhang M, Qi R, Cao X (2003) Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J Biol Chem 278:334–340
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN (1970) Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101:478–483
Wang Y, Chen T, Han C, He D, Liu H, An H, Cai Z, Cao X (2007) Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 110:962–971
Zhang Y, Wang L, Zhang J, Li Y, He Q, Li H, Guo X, Guo J, Zhang H (2014) Probiotic Lactobacillus casei Zhang ameliorates high-fructose-induced impaired glucose tolerance in hyperinsulinemia rats. Eur J Nutr 53:221–232
Wang Y, Xie J, Wang N, Li Y, Sun X, Zhang Y, Zhang H (2013) Lactobacillus casei Zhang modulate cytokine and toll-like receptor expression and beneficially regulate poly I:C-induced immune responses in RAW264.7 macrophages. Microbiol Immunol 57:54–62
Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W (2013) Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem 24:1609–1615
Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V et al (2011) Myeloperoxidase attracts neutrophils by physical forces. Blood 117:1350–1358
Wang H, Li Y (2006) Protective effect of bicyclol on acute hepatic failure induced by lipopolysaccharide and galactosamine in mice. Eur J Pharmacol 534:194–201
Soares JB, Pimentel-Nunes P, Afonso L, Rolanda C, Lopes P, Roncon-Albuquerque R Jr et al (2012) Increased hepatic expression of TLR2 and TLR4 in the hepatic inflammation-fibrosis-carcinoma sequence. Innate Immun 18:700–708
Agrawal V, Smart K, Jilling T, Hirsch E (2013) Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2. PLoS ONE 8:e63990
McKimmie CS, Moore M, Fraser AR, Jamieson T, Xu D, Burt C, Pitman NI, Nibbs RJ, McInnes IB, Liew FY, Graham GJ (2009) A TLR2 ligand suppresses inflammation by modulation of chemokine receptors and redirection of leukocyte migration. Blood 113:4224–4231
Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, Vikram A, Bibby K, Morowitz MJ, Firek B, Lu P, Hackam DJ (2014) Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 306:G1021–G1032
Peti W, Page R (2013) Molecular basis of MAP kinase regulation. Protein Sci 22:1698–1710
Wu YL, Lian LH, Wan Y, Nan JX (2010) Baicalein inhibits nuclear factor-κB and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem Biol Interact 188:526–534
Lian LH, Wu YL, Wan Y, Li X, Xie WX, Nan JX (2010) Anti-apoptotic activity of gentiopicroside in D-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. Chem Biol Interact 188:127–133
Jin Q, Jiang S, Wu YL, Bai T, Yang Y, Jin X, Lian LH, Nan JX (2014) Hepatoprotective effect of cryptotanshinone from Salvia miltiorrhiza in D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Phytomedicine 21:141–147
Mencarelli A, Distrutti E, Renga B, D’Amore C, Cipriani S, Palladino G, Donini A, Ricci P, Fiorucci S (2011) Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS ONE 6:e22978
Ewaschuk J, Endersby R, Thiel D, Diaz H, Backer J, Ma M, Churchill T, Madsen K (2007) Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology 46:841–850
Zhang LL, Gao CY, Fang CQ, Wang YJ, Gao D, Yao GE, Xiang J, Wang JZ, Li JC (2011) PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells. Cardiovasc Res 92:484–493
Yao J, Pan D, Zhao Y, Zhao L, Sun J, Wang Y, You QD, Xi T, Guo QL, Lu N (2014) Wogonin prevents lipopolysaccharide-induced acute lung injury and inflammation in mice via peroxisome proliferator-activated receptor gamma-mediated attenuation of the nuclear factor-kappaB pathway. Immunology 143:241–257
Imani Fooladi AA, Mahmoodzadeh Hosseini H, Nourani MR, Khani S, Alavian SM (2013) Probiotic as a novel treatment strategy against liver disease. Hepat Mon 13(2):e7521
Kasravi FB, Adawi D, Molin G, Bengmark S, Jeppsson B (1997) Effect of oral supplementation of lactobacilli on bacterial translocation in acute liver injury induced by d-galactosamine. J Hepatol 26:417–424
Liu L, Yuan S, Long Y, Guo Z, Sun Y, Li Y, Niu Y, Li C, Mei Q (2009) Immunomodulation of Rheum tanguticum polysaccharide (RTP) on the immunosuppressive effects of dexamethasone (DEX) on the treatment of colitis in rats induced by 2,4,6-trinitrobenzene sulfonic acid. Int Immunopharmacol 9:1568–1577
Acknowledgments
We thank Dr. Kerong Zhang and Dr. Charles Hocart from Australian National University for revising the manuscript. This work was supported by grants from the National Natural Science Foundation of China (Grant No. 31270922, 81260662, 81360394), Inner Mongolia Natural Science Foundation (Grant No. 2012MS1156) and the Excellent Young Scientist Foundation of Inner Mongolia Agricultural University (Grant No. 2014XYQ-19).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuzhen Wang, Jiming Xie and Yunxu Li have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Xie, J., Li, Y. et al. Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur J Nutr 55, 821–831 (2016). https://doi.org/10.1007/s00394-015-0904-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0904-3