Abstract
Background
Damage-specific DNA binding protein 2 (DDB2) is implicated in the recognition of DNA damage and the initiation of nucleotide excision repair process. The aim of this study was to explore the role of DDB2 in the initiation, progression, and prognosis of colorectal cancer (CRC).
Methods
Totally tissues of 300 CRC and 300 adjacent, 267 colorectal adenoma (CRA) and 214 normal (NOR) were collected. The expression of DDB2 protein was detected by immunohistochemical staining.
Results
DDB2 protein was highly expressed in CRC and CRA compared with NOR (P < 0.001, respectively) in the dynamic sequence of NOR → CRA → CRC; CRC tissue demonstrated increased DDB2 expression compared with non-tumor adjacent tissues (P < 0.001). DDB2 expression was higher in T1–T2 than that in T3–T4 in CRC (P = 0.023); cloddy/nested CRC demonstrated increased DDB2 expression than infiltrative CRC (P = 0.007). Survival analysis showed that high DDB2 expression was associated with favorable survival in colon cancer (adjusted HR 0.20, 95% CI 0.06–0.72, P = 0.014) and female CRC patients (adjusted HR 0.27, 95% CI 0.08–0.92, P = 0.036).
Conclusion
DDB2 protein expression was associated with the initiation, progression, and prognosis of CRC, and might function as a tumor biomarker for the diagnosis and prognosis of CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DDB2 (damage-specific DNA binding protein 2) gene, mapped to chromosome 11p11.2, encodes a protein implicated in the recognition of DNA damage and the initiation of NER process [1]. DDB2 gene was first found to reflect radiation, whose mutation would lead to defect of DNA damage repair, appearance of Xeroderma pigmentosum E type and finally progress to skin cancer [2, 3]. Subsequently, over-expression of DDB2 gene was found to not merely decrease the motility and invasion, but also inhibit the implantation metastasis of tumor at the lung of nude mice [4]. Using Kaplan–Meier plotter tool, Gyorffy et al. [5] suggested a favorable relapse-free survival and overall survival in breast cancer patients with high DDB2 mRNA expression. As for colon cancer, Roy et al. indicated that DDB2 expression could significantly suppress the implantation metastasis of tumor cells in liver and lung of mice. In addition, they observed that DDB2 decreased invasion of cancer mainly through inhibiting epithelial-mesenchymal transition (EMT) of colon cells [6]. Similarly, the tumor suppressor function of DDB2 was then confirmed in ovarian cancer [7]. As a result, DDB2 might be involved in carcinogenesis and progression of different types of cancer.
Colorectal cancer (CRC) is one of the most common malignant tumors in digestive tract. According to the global cancer statistics, CRC new cases ranked the third in males and the second in females; CRC related death was the fourth in males and the third in females [8]. Until now, the role of DDB2 in the occurrence, development of CRC is still elusive, neither is clear concerning the relation of DDB2 expression with clinical biological behavior and prognosis of CRC. In the present study, we therefore investigated DDB2 protein expression in normal (NOR) intestinal mucosa, colorectal adenoma (CRA), and colorectal cancer (CRC) tissues to elucidate the variation tendency of intestinal mucosa cells in the dynamic sequence of NOR → CRA → CRC. The expression difference of DDB2 in CRC tissue and its corresponding non-tumor adjacent tissue was also detected. In addition, we investigated the association of DDB2 expression with clinicopathological parameters and survival of CRC patients in order to shed light on the effect of DDB2 in colorectal carcinogenesis, progression, and prognosis.
Materials
Patients and Tissue Specimens
A total of 300 CRC patients were included from the Department of Anorectal Surgery of the First Affiliated Hospital of China Medical University who underwent surgical resection between October 2012 and July 2015. The corresponding non-tumor adjacent tissues of CRC patients were also collected. We also enrolled 267 colorectal adenoma (CRA) from Endoscopic submucosal dissection (ESD) or surgical operation in our hospital. As for the normal group (NOR), 214 individuals with non-tumor benign anal disease (internal hemorrhoid, mixed hemorrhoid, prolapse of rectum, rectocele) were enrolled. The samples of CRC and CRA were diagnosed based on histological results according to World Health Organization criteria. TNM staging of CRC was performed according to International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) (7th edition, 2010) after postoperative pathological diagnosis. Exclusion criteria for CRC patients: (1) with XP disease, (2) with Hereditary nonpolyposis colorectal cancer (HNPCC), (3) receiving preoperative chemotherapy or radiation. Our follow-up ended in August 2016. Among the 300 CRC cases, intact survival information was acquired from 274 cases patients (mean survival time was 39.9 months; the time of follow-up ranged from 13 to 46 months; 52 of them had died). Overall survival (OS) was calculated for the time from the date of operation to death. History of smoking is defined as at least one cigarette daily for at least 1 year. History of drinking was considered as the average alcohol intake per day for at least 50 g, and duration for at least 1 year. The study was approved by the Institute Research Medical Ethics Committee of the First Affiliated Hospital of China Medical University. We began our study in June, 2016. All the participants provided their written informed consent and consented to participate in our study and have their tissue used for research.
Immunohistochemistry
A 4-μm-thick tissue sections from paraffin-embedded tissues were cut into the poly-l-lysine-coated glass slides and then baked in oven at 70 °C overnight. After deparaffinizing in xylene and rehydrating in graded ethanol, tissue sections were immersed in citrate buffer for antigen retrieval. Endogenous peroxidase was quenched using 3% hydrogen peroxide for 30 min. In order to decrease the nonspecific staining, 10% normal goat serum was subsequently used to block tissue collagen for 30 min. Tissue sections were then incubated with rabbit polyclonal antibody anti-DDB2 (ab77765, 1:1000 dilution; Abcam, Cambridge, UK) for 60 min at room temperature (24–27 °C). After that, biotinylated secondary antibody and streptavidin–biotin–peroxidase were used to incubate tissue sections for 10 min each in turn. Slides were stained with DAB (DAB-0031, Maixin Inc., Fujian, China) chromogenic reagent for 60 s, afterward counterstained with hematoxylin. As a negative control, PBS buffer replaced the antibody against DDB2. UltraSensitive™ SP (Mouse/Rabbit) IHC Kit (KIT-9720, Maixin Inc., Fujian, China) were used in our experiment.
Evaluation of Immunohistochemical Staining
Because DDB2 is a nuclear protein [9], we calculated the nuclear staining in our study. The immunohistochemical results were evaluated and scored independently by two experienced pathologists who were blinded to the patients’ clinicopathological characteristics. And if the differences between the results of the pathologists were more than one grade, more scopes would be selected and the final scores would be discussed and concluded by the two pathologists. We used a semiquantitative scoring criterion to evaluate the expression of DDB2 in positive nucleus. Scoring criterion: (1) staining intensity was classified into 0 (no staining), 1 (light brown), 2 (brown staining), and 3 (heavy brown staining); (2) percentage of stained cells (0, 0–5; 1, 6–25; 2, 26–50; 3, 51–75; 4, 76–100%). Finally, we multiplied staining intensity by percentage of stained cells to get immunoreactivity score (IS), which was classified as: score = 0, negative (-); score = 1–4, weak positivity (+); score = 5–8, moderate positivity (++); score = 9–12, strong positivity (+++). We used IS = 6 (half of the strongest score 12) as cutoff value to distinguish high or low expression of DDB2 protein.
TCGA Data of DDB2
TCGA (The Cancer Genome Atalas) generated comprehensive and multi-dimensional maps of the critical genomic changes in 33 types of cancer. In this study, we analyzed TCGA data to investigate the association of DDB2 mRNA expression with clinicopathological parameters and prognosis of CRC.
Statistical Analysis
The differences of age between groups were assessed using the analysis of variance test. The χ2 test was applied to assess differences between categorical variables such as gender. The difference of DDB2 expression in NOR-CRA-CRC sequence and the difference between CRC and non-tumor adjacent tissues were assessed by nonparametric test. Survival analysis was assessed by Kaplan–Meier method, and log-rank test was used to compare the differences between groups. To evaluate whether the expression of DDB2 is an independent prognostic factor, multivariate Cox proportional hazards model was used. Two-sided P values < 0.05 were considered to be statistically significant. Statistical analysis was performed by SPSS 18.0 software (SPSS, Chicago, IL, USA).
Results
DDB2 Protein Expression in Different Colorectal Diseases
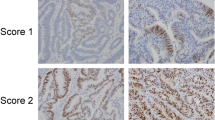
The baseline characteristics and clinicopathological parameters of NOR, CRA, CRC are listed in Table 1. The expression profile of DDB2 protein in NOR, CRA, and CRC is summarized in Table 2. High positive rate of DDB2 protein expression was detected in NOR (94.9%), CRA (99.3%), and CRC (99.7%). According to the results of Mann–Whitney U test between two different groups, DDB2 protein expression was significantly higher in CRC than that in NOR (P < 0.001); DDB2 protein expression was also significantly increased in CRA than that in NOR (P < 0.001); but no significant difference was found between CRC and CRA, (P = 0.563) (Fig. 1). In addition, DDB2 was significantly highly expressed in CRC tissue than that in non-tumor adjacent tissue (P < 0.001) (Table 2; Fig. 2). Four different grades of immunoreactivity score (IS) including negative (-), weak positivity (+), moderate positivity (++), and strong positivity (+++) are displayed in Fig. 3.
We then performed subgroup analysis based on age and gender and got similar results: in both males and females, age > 60 and age < 60, DDB2 protein expression was all increased in CRC or CRA than that in NOR; DDB2 was significantly highly expressed in CRC tissue than that in non-tumor adjacent tissue.
Association Between DDB2 Protein Expression and Individual Clinicopathological Parameters of CRC
We classified 300 CRC individuals based on age, gender, smoking, drinking, TNM stage, tumor invasion, lymph node metastasis and so on. The difference between each group was detected by Mann–Whitney U test (Table 3). The results indicated that DDB2 expression was higher in T1–T2 CRC patients than that in T3–T4 (P = 0.023); cloddy/nested CRC demonstrated increased DDB2 expression than infiltrative CRC (P = 0.007). As for other clinicopathological factors, no significant result was found.
Correlation Between DDB2 Expression and Survival of CRC
Immunoreactivity score (IS) = 6 was used as cutoff value to distinguish high or low expression of DDB2 protein. Log-rank test was adopted to describe the survival status of CRC patients in different groups (Table 1). Cox regression was used to assess the relation of DDB2 protein expression with CRC overall survival. According to the univariate survival analysis, DDB2 expression was not significantly associated with prognosis of CRC (HR = 0.60, 95% CI 0.34–1.09, P = 0.093) (Table 4). After multivariate survival analysis adjusted by age, gender, TNM stage, and differentiation degree, there was still no significant relation between DDB2 protein expression and CRC survival (adjusted HR = 0.64, 95% CI 0.35–1.17, P = 0.147). In the subgroup of chemotherapy, DDB2 expression showed no significant relation with CRC prognosis; in the subgroup of no chemotherapy, high DDB2 expression demonstrated significant relation with better CRC prognosis in both univariate (HR = 0.43, 95% CI 0.19–1.00, P = 0.049) and multivariate models (adjusted HR = 0.40, 95% CI 0.16–0.97, P = 0.043). Subgroup analysis based on gender indicated that CRC patients with high DDB2 expression had favorable overall survival (OS) than individuals with low DDB2 expression in females (adjusted HR = 0.27, 95% CI 0.08–0.92, P = 0.036). As for the subgroup analysis of colon cancer and rectal cancer, high DDB2 expression was related with longer OS than low expression in colon cancer (adjusted HR = 0.20, 95% CI 0.06–0.72, P = 0.014) (Fig. 4).
No significant result was observed in other subgroup analysis on the basis of TNM stage, tumor invasion, lymph node metastasis, growth pattern, differentiation degree, and the maximum diameter of tumor.
Results Based on TCGA Data
According to the data from TCGA, DDB2 mRNA expression was significantly associated with TNM stage (N, M) of colon cancer. No significant relation was found between DDB2 mRNA expression and clinicopathological parameters of rectal cancer (Supplementary Table 1). In addition, DDB2 mRNA expression showed no significant association with prognosis of CRC (Supplementary Table 2).
Discussion
As a key protein implicated in the recognition step of NER pathway, the alternation of DDB2 might change the DNA repair capacity of organism, thus influencing the development and outcome of a variety of cancers including CRC. In order to elucidate the role of DDB2 in colorectal carcinogenesis, we detected the protein expression of DDB2 in the dynamic sequence of NOR → CRA → CRC as well as the expression difference between CRC tissue and non-tumor adjacent tissues by immunohistochemical staining. In addition, the relation of DDB2 protein expression with clinicopathological parameters and survival of CRC patients was also explored. To the best of our knowledge, this is the first investigation about DDB2 expression covering the dynamic developing sequence of CRC. Besides, the association of DDB2 expression with CRC clinical biological behaviors and prognosis has not been studied previously. The results indicated that DDB2 protein was highly expressed in CRC and CRA tissues compared with normal colorectal tissues; high DDB2 expression was associated with longer survival time in colon cancer and female CRC patients.
Mice deficient in DDB2 have been proved to develop spontaneous malignant tumors at a high rate [10], which indicated probable effect of DDB2 in cancer development. Previously, DDB2 protein expression has been studied in skin cancer, ovarian cancer, and colon cancer, which was mainly expressed in cell nucleus. Using tissue microarrays, Stoyanova et al. [11] observed a significant loss of the DDB2 protein expression in basal cell carcinoma (BCC). In ovarian cancer, normal ovary epithelial cells (n = 16) displayed high DDB2 staining, whereas ovarian carcinoma cells (n = 43) in most of tumor tissue exhibited low DDB2 expression [7]. Roy et al. [6] found that DDB2 was down-regulated in colon cancer. In our study, DDB2 protein was highly expressed in CRC and CRA tissues compared with normal colorectal tissues. In addition, CRC tissues demonstrated increased DDB2 protein expression than non-tumor adjacent tissues. The controversies between results of our study and that in skin cancer and ovarian cancer might due to the different types of cancers. Roy et al.’s study included 41 normal colon and 206 colon cancer and found different results with ours. The reasons might be as follows: our study included more available sample size (300 CRC, 267 CRA, and 214 NOR) of dynamic disease sequence. Besides, the ethnicities and detecting methods between Roy et al.’s study and ours were different. In addition, the inclusion criteria and clinicopathological status of the patients might also contribute to the difference. The phenomenon that DDB2 was highly expressed in CRC and CRA might due to significant increased DNA damages in CRC and CRA. Previous investigations have shown that expression levels of NER factors including ERCC1 and XPD correlated with DNA repair ability in various tissues [12,13,14]. Because high DDB2 expression was closely related to enhanced NER capacity [15, 16], colorectal carcinogenesis may arise more DNA damage and thereby induce the high expression of DDB2. DDB2 might therefore be a promising biomarker to predict the development of CRC. Although DDB2 has been reported to suppress epithelial-to-mesenchymal (EMT) in colon cancer [6], the changing mechanism of DDB2 and NER capacity in colorectal carcinogenesis has not been investigated. Further studies concerning the role of DDB2 expression profile in CRC development and its mechanism are still required.
It has been reported that high DDB2 expression could not only reduce the invasion and migration of breast cancer, but also inhibit the implantation metastasis of breast cancer cells at lung in nude mice [4]; in ovarian cancer cell line and nude mice, over-expression of DDB2 could suppress the growth of ovarian cancer [7]. Considering the influence of DDB2 on multiple biological activities of tumor, we analyzed the relation between DDB2 expression and clinicopathological parameters of CRC patients. We did not found certain relation of DDB2 protein expression with lymph node metastasis, distant metastasis, and differentiation degree. Significant decreased DDB2 expression was detected in deeper invasion and worse growth pattern: DDB2 was highly expressed in T1–T2 stage than that in T3–T4 stage; the expression of DDB2 was increased in cloddy/nested growth pattern than that in infiltrative growth. Roy et al.’s research in colon cancer suggested a tendency of decreased DDB2 expression in high-grade colon cancer [6], which was similar to our finding of low DDB2 expression in T3–T4 stage and infiltrative growth type CRC. As the invasive depth and growth pattern are key factors to reflect the invasion and migration of tumor cells, DDB2 protein expression might indicate the different stages of CRC progression.
Previous investigations have reported the predictive role of NER pathway genes in prognosis of various types of cancers [17,18,19,20,21]. In this study, we revealed for the first time the relation between DDB2 expression and CRC prognosis. Borderline relation was detected in univariate survival analysis (P = 0.093) while no significant association was found in multivariate survival analysis (P = 0.147). After subgroup analysis based on gender and tumor location, high DDB2 protein expression was found to predict longer survival time in colon cancer and in female CRC patients. In the subgroup of no chemotherapy, high DDB2 expression demonstrated significant relation with better CRC prognosis while no significant result was found in the subgroup of chemotherapy. These results suggested that chemotherapy might influence the association between DDB2 expression and prognosis of CRC patients. Two studies in ovarian cancer and breast cancer also suggested similar results: Han et al. [7] demonstrated association of low DDB2 mRNA expression and worse OS and progression-free survival (PFS) in ovarian cancer on the basis of publicly available Kaplan–Meier plotter tool. Using the same online survival analysis tool, Gyorffy et al. [5] revealed a favorable relapse-free survival (RFS) and OS in high DDB2 mRNA expression group of breast cancer. These findings all indicate a predictive role of high DDB2 protein for better prognosis of cancer, which is anticipated to be applied in the evaluation and treatment of postoperative CRC patients. According the data from TCGA, DDB2 mRNA expression was significantly associated with TNM stage (N, M) of colon cancer but no significant relation was found between DDB2 mRNA expression and clinicopathological parameters of rectal cancer. In addition, DDB2 mRNA expression showed no significant associated with prognosis of CRC. The differences between TCGA data and our findings might arise from the different detecting levels that TCGA data only had mRNA expression profiles.
Several limitations should be acknowledged in the present study. Although the immunohistochemical results were evaluated and discussed by two experienced pathologists, the semiquantification scoring system might still involve subjective factors. In addition, research based on only one ethnicity could not come up with persuasive results. Therefore, further large-scale studies base on different ethnicities as well as molecular investigations are required to confirm our findings.
Conclusion
In summary, DDB2 protein was highly expressed in CRC and CRA than benign colorectal diseases in the dynamic sequence of NOR → CRA → CRC; CRC tissue demonstrated increased DDB2 expression compared with non-tumor adjacent tissues, which suggested that DDB2 is implicated in the pathogenic process of CRC. DDB2 expression was associated with invasion depth and growth pattern of CRC. High DDB2 expression was associated with favorable survival in colon cancer and female CRC patients. Therefore, DDB2 might function as a tumor biomarker for the diagnosis and prognostic evaluation of CRC in the future.
References
Sugasawa K. Regulation of damage recognition in mammalian global genomic nucleotide excision repair. Mutat Res. 2010;685:29–37.
Alekseev S, Kool H, Rebel H, et al. Enhanced DDB2 expression protects mice from carcinogenic effects of chronic UV-B irradiation. Can Res. 2005;65:10298–10306.
Feldberg RS, Grossman L. A DNA binding protein from human placenta specific for ultraviolet damaged DNA. Biochemistry. 1976;15:2402–2408.
Ennen M, Klotz R, Touche N, et al. DDB2: a novel regulator of NF-kappaB and breast tumor invasion. Can Res. 2013;73:5040–5052.
Gyorffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat. 2010;123:725–731.
Roy N, Bommi PV, Bhat UG, et al. DDB2 suppresses epithelial-to-mesenchymal transition in colon cancer. Can Res. 2013;73:3771–3782.
Han C, Zhao R, Liu X, et al. DDB2 suppresses tumorigenicity by limiting the cancer stem cell population in ovarian cancer. Mol Cancer Res. 2014;12:784–794.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Shiyanov P, Nag A, Raychaudhuri P. Cullin 4A associates with the UV-damaged DNA-binding protein DDB. J Biol Chem. 1999;274:35309–35312.
Yoon T, Chakrabortty A, Franks R, Valli T, Kiyokawa H, Raychaudhuri P. Tumor-prone phenotype of the DDB2-deficient mice. Oncogene. 2005;24:469–478.
Stoyanova T, Roy N, Bhattacharjee S, et al. p21 cooperates with DDB2 protein in suppression of ultraviolet ray-induced skin malignancies. J Biol Chem. 2012;287:3019–3028.
Li Q, Yu JJ, Mu C, et al. Association between the level of ERCC-1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res. 2000;20:645–652.
Vogel U, Dybdahl M, Frentz G, Nexo BA. DNA repair capacity: inconsistency between effect of over-expression of five NER genes and the correlation to mRNA levels in primary lymphocytes. Mutat Res. 2000;461:197–210.
Wei Q, Xu X, Cheng L, Legerski RJ, Ali-Osman F. Simultaneous amplification of four DNA repair genes and beta-actin in human lymphocytes by multiplex reverse transcriptase-PCR. Can Res. 1995;55:5025–5029.
Christmann M, Boisseau C, Kitzinger R, et al. Adaptive upregulation of DNA repair genes following benzo(a)pyrene diol epoxide protects against cell death at the expense of mutations. Nucl Acids Res. 2016;44:10727–10743.
Stoyanova T, Roy N, Kopanja D, Raychaudhuri P, Bagchi S. DDB2 (damaged-DNA binding protein 2) in nucleotide excision repair and DNA damage response. Cell Cycle. 2009;8:4067–4071.
Deng N, Liu JW, Sun LP, et al. Expression of XPG protein in the development, progression and prognosis of gastric cancer. PLoS ONE. 2014;9:e108704.
Fu X, Hu J, Han HY, et al. High expression of XPA confers poor prognosis for nasopharyngeal carcinoma patients treated with platinum-based chemoradiotherapy. Oncotarget. 2015;6:28478–28490.
Gomez GV, de Oliveira C, Rinck-Junior JA, de Moraes AM, Lourenco GJ, Lima CS. XPC (A2920C), XPF (T30028C), TP53 (Arg72Pro), and GSTP1 (Ile105Val) polymorphisms in prognosis of cutaneous melanoma. Tumour Biol. 2016;37:3163–3171.
Han JJ, Baek SK, Lee JJ, Kim GY, Kim SY, Lee SH. Combination of TRAP1 and ERCC1 expression predicts clinical outcomes in metastatic colorectal cancer treated with oxaliplatin/5-fluorouracil. Cancer Res Treat. 2014;46:55–64.
Wang S, Wang J, Bai Y, et al. The genetic variations in DNA repair genes ERCC2 and XRCC1 were associated with the overall survival of advanced non-small-cell lung cancer patients. Cancer Med. 2016;5:2332–2342.
Funding
This study is supported by grants from Public Welfare Foundation of Liaoning Province (No. 2015005002) and Fund for Scientific Research of The First Hospital of China Medical University (FHCMU-FSR).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, H., Liu, J., Jing, J. et al. Expression of DDB2 Protein in the Initiation, Progression, and Prognosis of Colorectal Cancer. Dig Dis Sci 63, 2959–2968 (2018). https://doi.org/10.1007/s10620-018-5224-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5224-z