Abstract
Objective
The primary objective is to assess whether the POC assays to measure infliximab residual trough level in the serum of IBD patients were non-inferior to the ELISA techniques available on the market, and to determine which of them was the most robust. The second is to compare three different ELISA kits for monitoring anti-infliximab antibodies (ATI).
Methods
The assays were carried out on patients’ sera using four ELISA kits from four different suppliers (three with a monoclonal antibody and one polyclonal) and two rapid techniques provided by BÜHLMANN (Quantum Blue®) and R-Biopharm (Ridaquick) for monitoring infliximab levels. ATI were measured by three ELISA sets (Grifols, Theradiag, and R-Biopharm) which have different positivity limits and different units.
Results
We measured infliximab residual level and ATI in the serum of 90 IBD patients (85 treated with infliximab and five with adalimumab). All of the infliximab assays were very well correlated when analyzed with Spearman nonparametric correlation (0.93 ≤ r ≤ 0.99), and the two POC assays were also excellently correlated (r = 0.98). The ATI monitoring kits revealed a correlation ranging from 0.73 to 0.96 when comparing positive and negative patients. When normalizing the quantitative values between the different ELISA tests (expressed arbitrarily by using multiples of the positivity limits defined by each supplier), the Spearman r coefficient ranged from 0.81 to 0.93.
Conclusion
The available evidence allows us to conclude that all of the infliximab monitoring assays correlate well and may be used for IFX monitoring; albeit variations in measured IFX concentration among different assays remain present, these assays could be interchangeable. The ATI monitoring techniques are all capable of detecting ATI-positive patients, but because of the difference in the positivity limits and the measurement units, it is better to follow a patient rate with one definite kit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-TNF therapy has been first developed 20 years ago and is now approved for the treatment of rheumatoid arthritis (RA), inflammatory bowel diseases (IBD), psoriasis, ankylosis spondylitis, juvenile idiopathic arthritis (JIA), and hidradenitis suppurativa [1]. In addition, TNF blockade is also used in Behçet disease, non-infectious ocular inflammation, adult-onset Still disease, and pyoderma gangrenosum [2]. Currently, there are five biological agents targeting the TNF pathway approved for treatment: infliximab, a chimeric antihuman monoclonal antibody; etanercept, a TNFR2 dimeric fusion protein associated with an IgG1 Fc; adalimumab and golimumab, two fully human monoclonal antibodies; and certolizumab, a PEGylated Fab fragment. Close monitoring of patient’s anti-TNF trough levels has proved useful in many ways, as well as cost-effective [3, 4]. Low infliximab trough levels are associated with future loss of response to treatment [5,6,7], while high trough levels correlate with future response to treatment during intensification therapy [7, 8]. Besides, relying not only on the clinical decision but also on drug monitoring and clinical data has been proved more effective in patients with Crohn’s disease (CD) who lose response to anti-TNF treatment, and is associated with better endoscopic outcomes and mucosal healing [9, 10]. Several algorithms based on therapeutic drug monitoring (TDM) have been proposed and assessed, suggesting efficiency in treatment de-escalation [11] and follow-up [12, 13]; however, those studies were mostly retrospective. A prospective study revealed the importance of therapeutic drug monitoring (TDM) with drug-tolerant assays to evaluate the patient clinical remission and guide the therapeutic decisions. The prospective TAXIT study showed that the rate of clinical relapse at 1 year in the TDM arm (treat-to-target) was significantly lower [14]. In addition, the approach enabled clinicians to reduce drug dosage among patients whom blood levels of infliximab were > 7 µg/mL, without hindering clinical response.
As of now, a moderate number of ELISA tests are available on the market, with comparability studies showing mixed results [15,16,17,18,19]. For instance, Lee et al. compared three different ELISAs and concluded that the Theradiag Lisa-Tracker was the most accurate one [20] but presented some false-positive samples in Vande Casteele et al.’s work [19]. All in all, currently available tests seem to be interchangeable, but it is recommended that one test should be used for the entire follow-up of one patient [21]. The need for harmonization grows as there currently is no clear recommendation on which technique to use.
Another point to consider is that the ELISA techniques require an incompressible amount of time (turnaround time of 4–8 h), whereas early dosages of anti-TNF therapies yield valuable information regarding endpoint clinical response [22,23,24]. In this context, Lu et al. developed a point-of-care technique which allowed clinicians to get the drug level within 1 h, and could then tailor drug dosage directly [25]. While the technique seems promising as it allows rapid and accurate drug monitoring, it still requires serum instead of total blood, which hinders the technique from being used by clinicians directly on site. The use of dry blood spot (DBS) via a capillary puncture could be a way of bypassing this issue [26] and might be used in the future either with ELISAs or with the point-of-care technique described above [27]. Two point-of-care techniques now coexist on the market (BÜHLMANN Quantum Blue® Infliximab and Ridaquick IFX from R-Biopharm), and both of them consist of a sandwich immunoassay. Recombinant tumor necrosis factor alpha (TNF-α) is conjugated to gold colloids, and infliximab present in the sample will bind to the conjugate and is revealed with a highly specific monoclonal antibody, and while several studies compared the robustness of those techniques versus the ELISA gold standard [28, 30] with both infliximab and adalimumab [29], none of them (to our knowledge) tested them against one another.
Here, we compared the BÜHLMANN Quantum Blue® Infliximab and the R-Biopharm Ridaquick® against one another and against most of the ELISAs on the market (Theradiag, Sanquin, R-Biopharm, and Grifols). Primary objective was to assess whether these techniques were non-inferior to ELISA technique and which of them was the most robust.
Materials and Methods
Patients
Residual trough level of infliximab and anti-infliximab antibodies (ATI) was measured in the serum of 90 CD patients followed in day hospital of gastroenterology. Eighty-five were undergoing a treatment with Remicade®, and five were treated with adalimumab. We also included to the starting population ten randomly selected patients with high IgM rheumatoid factor blood levels as controls.
Measurement of IFX Trough Levels and Antibodies to Infliximab (ATI)
We have used several commercially available ELISAs from different suppliers to monitor infliximab trough levels (Theradiag, R-Biopharm, Sanquin, and Grifols) and for the anti-infliximab antibodies measurement (drug-sensitive assays; R-Biopharm, Grifols, and Theradiag). Two point-of-care techniques were also tested and compared to ELISAs, supplied by R-Biopharm (RIDA®QUICK IFX Monitoring) and BÜHLMANN (Quantum Blue®) (Table 1).
Statistical Analysis
Quantitative data were summarized by median and interquartile range [IQR]. A D’Agostino and Pearson omnibus normality test was used to assess the normality of continuous variables. Quantitative differences between the results of serum IFX were analyzed using a Wilcoxon test, given the non-normal distribution of the assays. Spearman rank test was used for correlation analysis. Comparison of all pairs of IFX concentration means, distributions by quartile, was analyzed with one-way analysis of variance and Tukey’s multiple comparison test. For the quartile comparison, a one-side Cochrane–Armitage trend test has been used.
Regarding ADA, only a qualitative comparison was used, given the difference of units. The comparison of the number of fold increases in the threshold of positivity was also realized. A p value less than 0.05 was considered statistically significant. GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) was used for all statistical analysis.
Results
Comparison of Different Assays for the Measurement of IFX Trough Levels
We measured the infliximab trough level in the serum of 85 patients presenting IBD and treated with infliximab, using four different ELISA kits from multiple suppliers (Theradiag, R-Biopharm, Sanquin, and Grifols) and two Quick methods (R-Biopharm and BÜHLMANN).
We determined the median of the different assays (Table 2). The median values were very close regardless of the used technique and varied from 1.2 to 2.2 µg/mL. We then divided into four quartiles the infliximab levels that we measured with the available kits (0–3, 3–5, 5–7, and > 7 µg/mL). The results show a homogeneous distribution of the values in all of the three first quartiles, and the values are more scattered in the last one because of the difference in the high measurement threshold depending on the BÜHLMANN assay which has a broader linear range for trough determination up to 20 µg/mL (Supplementary Fig. 1).
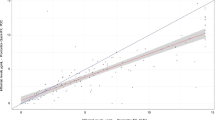
Nonparametric Spearman correlation between the different suppliers’ kits. a Patients trough level distribution within the different assays, the red line representing the median, b correlation between Theradiag and R-Biopharm (ELISA), c correlation between Theradiag and Sanquin (ELISA), d correlation between Theradiag and Grifols (ELISA), e correlation between Sanquin and R-Biopharm (ELISA), f correlation between Grifols and R-Biopharm (ELISA), g correlation between Grifols and Sanquin (ELISA), h correlation between Quantum Blue® (BÜHLMANN quick test) and Ridaquick (R-Biopharm quick test)
We used a nonparametric correlation with a Spearman r coefficient to compare the different results, which revealed an agreement ranging from 0.93 to 0.97 between the different ELISAs [the highest r value was between Theradiag and R-Biopharm ELISA in addition to Grifols and R-Biopharm (0.97); the lowest was between Sanquin and Theradiag (0.93)] and from 0.95 to 0.99 between the ELISAs and the Rapid monitoring kits [the best correlation was between R-Biopharm ELISA and Ridaquick (0.99) and the lowest between both of POCs and Sanquin ELISA (0.95)]. The rapid monitoring assays had a strong correlation with each other, with an r coefficient of 0.98 (Table 3, Fig. 1).
In order to complete this latest nonparametric analysis, we decided to study the infliximab trough levels using Bland–Altman plots and to analyze the techniques two by two. Three different gaps of infliximab trough level were defined according to the recommended therapeutic levels in Crohn’s disease (infratherapeutic: IFX < 3 µg/mL—therapeutic zone: IFX 3–7 µg/mL—supratherapeutic: IFX > 7 µg/mL). We then obtained the average of the two measurements and the difference between both of them (Supplementary Fig. 2). All techniques showed a good agreement up to 3 µg/mL of infliximab trough level, but were less correlated in the therapeutic and supratherapeutic groups. POCs overestimated the infliximab levels superior to 3 µg/mL compared to ELISA techniques.
Comparison of assays to measure antibodies to infliximab (ATI). a Normalized quantitative values comparison of ATI independently of IFX trough levels (conclusive and inconclusive ATI) between the different ELISAs expressed with the fold of the positivity limit defined for each assay. b Normalized quantitative values comparison of ATI (conclusive) between the different ELISAs in patients with IFX < 0.3 µg/mL expressed with the fold of the positivity limit defined for each assay
Comparison of Different Assays for the Measurement of ATI
Anti-infliximab antibodies levels were quantified independently of the IFX trough levels in the 85 sera of the same patients treated by infliximab using three drug-sensitive ELISAs (R-Biopharm, Theradiag, and Grifols). Due to differences in measurement units between three suppliers, we compared the positive and negative rates between them also using a nonparametric correlation; the Spearman r coefficient varied from 0.74 to 0.90, and the highest r value was between Grifols and Theradiag (0.90), while the lowest was between R-Biopharm and Theradiag (0.74) (Table 4).
We also tried to normalize the quantitative values between the different ELISA tests which have different units, and we arbitrarily chose to express them by using multiples of the positivity limits defined by each supplier (Fig. 2). We then compared the data using a Spearman r coefficient that ranged from 0.81 to 0.93 (the highest r value was between Theradiag and Grifols 0.93 and the lowest between Theradiag and R-Biopharm 0.81) (Table 5).
We know that the presence of IFX in the sera interferes with the detection of antibodies to infliximab due to the formation of complexes between infliximab and antibodies to infliximab (ATI inconclusive). We finally compared the presence and the quantities of ATI in samples where IFX trough levels are very low or undetectable (< 0.5 µg/mL). The correlation between Theradiag, Grifols and R-Biopharm ranged from 0.56 to 1 when comparing positive/negative rates (Theradiag/Grifols r = 1, Theradiag–Grifols/R-Biopharm r = 0.56) and from 0.6 to 0.89 when comparing the normalized quantitative values.
Discussion
We report a comparison between two rapid monitoring techniques and four commonly used ELISA techniques for the measurement of infliximab and anti-infliximab antibodies in serum samples of CD patients. The results demonstrate the comparability between all ELISA kits with a great correlation ranging between 0.93 and 0.97. The best coefficient was found between the Grifols and R-Biopharm kits, both of them using a monoclonal antibody to quantify trough concentration of infliximab in patients’ sera and both having a very similar calibration curve, with a detection range from 0.2 to 14.4 µg/mL for Grifols and 0.5–12 µg/mL for R-Biopharm, when using the main dilution recommended by the supplier. This value was also found between Theradiag and R-Biopharm ELISA kits. The lowest r value was between Theradiag and Sanquin (0.93). Nevertheless, all ELISAs are perfectly correlated. However, it seems better to perform the follow-up of a patient with the same assay. The use of the Theradiag assay could be useful in the case of anti-TNF switch between infliximab and adalimumab as it allows measuring the cumulative trough levels between these two drugs.
The comparison between the ELISA methods and the two point-of-care (rapid test) also shows a great correlation (0.95–0.99), with the best Spearman r value being between R-Biopharm and both of the POC assays (0.98–0.99) and the lowest between both of POCs and Sanquin ELISA (0.95). The two POC assays had a good correlation (0.98), demonstrating that we can indifferently use either ELISA techniques or POC assays only for punctual infliximab residual level measurement. On the other hand, when testing inter- and intra-assay coefficients of variability, the ELISA techniques showed CV ranging around 7–8%, while the POC assays showed CVs were around 15–20%. From this point of view, the ELISAs are more precise for application of follow-up of patients, the trade-off being the time delay in result availability as compared to the rapid tests. Although the quantification limit can be modified by the dilution factor, the obtained results when using the supplier’s first recommended dilution vary between the different techniques. POC assays offer a standard range from 0.4 to 20 µg/ml for BÜHLMANN and from 0.5 to 10 µg/ml for R-Biopharm, which is comparable to the ELISA; the therapeutic zone (3–7 µg/ml) is included in the given limits (3–7 µg/ml). The current delay to obtain a result with the standard ELISA techniques is around 90–120 min, whereas the rapid methods are then much shorter (around 30 min including serum generation from venous blood). However, in every assay even the two POCs used to monitor infliximab, measurements are taken on serum and not whole blood samples, which add centrifugation to the process, thus hindering time to results. These are not in fact real point-of-care assays as defined for diabetes testing but rather quick assays. These two tests present two main advantages over ELISA techniques: Firstly, shorter time to obtain results, which would theoretically allow clinicians to rapidly optimize patients’ treatments and improve clinical care, and secondly the possibility to analyze single samples on demand while the ELISA methods lengthen the time-to-result by working in series. Nevertheless, when using those techniques in everyday practice, things get more complicated. ELISA techniques may remain more reliable to use when de-escalating the treatment and when treating to target because of its limited CVR measurement in time, which may allow us to better achieve an almost precise level of infliximab in the patient serum. Nevertheless, changes in the dosing regimen using an ELISA can only be performed at the next infusion of the patient, typically eight weeks later. On the other hand, POC techniques may be advantageous in case of non-responding patients, since they can allow the detection of low infliximab concentrations within the same hospital visit of the patient. Nevertheless, the immediate treatment adaptation would only be possible when POC measuring ATI will be available and combined with infliximab POC. Moreover, the importance of having an exact concentration in non-responders may be less necessary. Nevertheless, there are currently no POC ATI assays available to discriminate between immune- and non-immune-mediated pharmacokinetic treatment failure.
Despite a great correlation when using Spearman test, the Bland–Altman plots showed controversial results. In fact, when the patients have a low infliximab trough level, all the techniques were comparable, despite a small percentage of patients overestimated by POC techniques. Things get more complicated when the infliximab level is higher than 3 µg/mL. Some of the ELISA techniques (such as Sanquin) and especially POC overvalue the trough level compared to the other techniques, which can predict difficulties to optimize patients. A part of them will have a high level of infliximab (> 7 µg/mL) when measurements are taken with POC techniques and will be in the therapeutic gap when dosed with ELISA techniques.
Finally, the POCs are quantitative monitoring methods; therefore, their COFRAC accreditation will also be quantitative so their EQC will probably be based on ELISA values with ELISA CVRs. Since the POC coefficient of variation of reproducibility is around 15–20 versus 7–8% for ELISAs, their accreditation will be difficult.
Regarding the measurement of anti-infliximab antibodies with those drug-sensitive techniques, the comparison between Theradiag, R-Biopharm, and Grifols showed a very good correlation when comparing the positive and negative sera (Spearman r between 0.73 and 0.90). The same comparison using the sera with an undetectable trough infliximab level (< 0.5 µg/mL) showed an excellent correlation between Grifols and Theradiag (r = 1) and a poorer correlation between them and R-Biopharm (r = 0.56). A study in Drug test anal (02/2017) showed that we can transform a drug-sensitive technique into a drug-tolerant technique by pretreating the sample in order to dissociate the drug–antibody complexes to measure the anti-infliximab antibodies levels in non-responding patients with a detectable infliximab level in serum.
We then normalized the quantitative values of anti-infliximab antibodies and arbitrarily chose to express them by using the multiples of the positivity limits defined by each supplier (10 ng/mL for Theradiag, 20 ng/mL for R-Biopharm, and 5AU/mL for Grifols) (Fig. 2). The graphic shows dispersing values with Grifols compared to R-Biopharm and Theradiag which presented a less scattered data. This can be explained by the lower positivity limit of Grifols.
These results highlight the fact that every assay can be used to identify a positive level of antibodies in a patient serum. However, since the kits do not have standardized quantification units and therefore generate very different results in absolute value, they should not be interchanged when monitoring a patient. Besides, the main discrepancy was observed in the Grifols ELISA kit in which patients with a high level of rheumatoid factor showed false-positive responses as if they had anti-infliximab antibodies; this result was absent when using the other kits.
Several ongoing works are trying to standardize the monitoring of ATI by using highly specific antibodies (MA-IFX10F9 for example) that can be utilized as potential universal calibrators and thus improve the measures’ sensitivity and specificity.
In conclusion, despite the good correlation between the different assays, it is more accurate to use the same test when monitoring a patient’s infliximab and anti-infliximab antibodies trough level given the fact that they do not all have the same detection limit and differ within the measuring techniques. A standardization of all those methods could be the key for a complete interchangeable use during TDM. Standards to harmonize these different assays are already described in the literature for both IFX and ATI and can eventually be used to harmonize TDM [31, 32]. Indeed, some groups are working on the monitoring of infliximab through level with LC–MS techniques. The actual results remain controversial. Some are well correlated with ELISA [33], but others show an important variation between LC–MS assays techniques and ELISA commercial kits [34]. The main difficulties when using LC–MS are the determination of a suitable internal standard (IS) when monitoring infliximab and the specificity of the detected signature peptide. Moreover, the LC–MS assay is more difficult to apply for a simple follow-up of the patients.
References
Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27:55–62.
Karampetsou MP, Liossis S-NC, Sfikakis PP. TNF- antagonists beyond approved indications: stories of success and prospects for the future. QJM. 2010;103:917–928.
Steenholdt C, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927.
Krieckaert CLM, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis. 2015;74:361–368.
Steenholdt C, Bendtzen K, Brynskov J, Thomsen OØ, Ainsworth MA. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol. 2011;46:310–318.
Roblin X, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohn’s Colitis. 2015;9:525–531.
Ben-Horin S, Chowers Y. Tailoring anti-TNF therapy in IBD: drug levels and disease activity. Nat Rev Gastroenterol Hepatol. 2014;11:243–255.
Steenholdt C, et al. Changes in serum trough levels of infliximab during treatment intensification but not in anti-infliximab antibody detection are associated with clinical outcomes after therapeutic failure in Crohn’s disease. Journal of Crohn’s and Colitis. 2015;9:238–245.
Kelly OB, Donnell SO, Stempak JM, Steinhart AH, Silverberg MS. Therapeutic drug monitoring to guide infliximab dose adjustment is associated with better endoscopic outcomes than clinical decision making alone in active inflammatory bowel disease. Inflam Bowel Dis. 2017;23:1202–1209.
Paul S, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568–2576.
Amiot A, et al. Therapeutic drug monitoring is predictive of loss of response after de-escalation of infliximab therapy in patients with inflammatory bowel disease in clinical remission. Clin Res Hepatol Gastroenterol. 2016;40:90–98.
Moore C, Corbett G, Moss AC. Systematic review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J Crohn’s Colitis. 2016;10:619–625.
Papamichael K, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.03.031.
Vande Casteele N, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320.e3–1329.e3.
Freeman K, et al. Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Grifols® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling. Health Technol Assess. 2016;20:1–288.
Silva-Ferreira F, Afonso J, Pinto-Lopes P, Magro F. A systematic review on infliximab and adalimumab drug monitoring: levels, clinical outcomes and assays. Inflamm Bowel Dis. 2016;22:2289–2301.
Bader L, et al. Assays for infliximab drug levels and antibodies: a matter of scales and categories. Scand J Immunol. 2017. https://doi.org/10.1111/sji.12572.
Freeman K, et al. Test accuracy of drug and antibody assays for predicting response to antitumour necrosis factor treatment in Crohn’s disease: a systematic review and meta-analysis. BMJ Open. 2017;7:e014581.
Vande Casteele N, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36:765–771.
Lee MW, et al. Comparison of infliximab drug measurement across three commercially available ELISA kits. Pathology. 2016;48:608–612.
Steenholdt C, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohnʼs disease. Ther Drug Monit. 2013;35:530–538.
Davidov Y, et al. Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohn’s and Colitis. 2016. https://doi.org/10.1093/ecco-jcc/jjw182.
Kobayashi T, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis—Results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51:241–251.
Baert F, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol. 2014;12:1474.e2–1481.e2.
Lu J, et al. Fiber optic-SPR platform for fast and sensitive infliximab detection in serum of inflammatory bowel disease patients. Biosens Bioelectron. 2016;79:173–179.
Vande Casteele N, et al. Letter: dry blood spots for anti-TNF treatment monitoring in IBD. Aliment Pharmacol Ther. 2013;37:1024–1025.
Lu J, et al. Immunoassay for detection of infliximab in whole blood using a fiber-optic surface plasmon resonance biosensor. Anal Chem. 2017;89:3664–3671.
Afonso J, et al. Proactive therapeutic drug monitoring of infliximab: a comparative study of a new point-of-care quantitative test with two established ELISA assays. Aliment Pharmacol Ther. 2016;44:684–692.
Bian S, et al. Development and validation of an optical biosensor for rapid monitoring of adalimumab in serum of patients with Crohn’s disease. Drug Test Anal. 2017. https://doi.org/10.1002/dta.2250.
Magro F, et al. Clinical performance of an infliximab rapid quantification assay. Ther Adv Gastroenterol. 2017;10:651–660.
Stappen V, et al. An optimized anti-infliximab bridging enzyme-linked immunosorbent assay for harmonization of anti-infliximab antibody titers in patients with inflammatory bowel diseases. IBD. 2015;9:2172–2177.
Gils A, et al. Harmonization of infliximab and anti-infliximab assays facilitates the comparison between originators and biosimilars in clinical samples. IBD. 2016;22:969–975.
Peng X, et al. Development and validation of LC–MS/MS method for the quantitation of infliximab in human serum. Chromatographia. 2015;78:521–531.
Jourdil J-F, et al. Infliximab quantitation in human plasma by liquid chromatography-tandem mass spectrometry: towards a standardization of the methods? Anal Bioanal Chem. 2017;409:1195–1205.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Distribution of IFX trough levels between assays in the different quartiles. For the quartile comparison, a one-side Cochrane–Armitage trend test has been used. (TIFF 77 kb)
Supplementary Figure 2
Bland–Altman plots of infliximab trough level comparing different techniques two by two. The difference between the two measurements (µg/mL) is plotted on the Y-axis and the average of the two measurements (µg/mL) on the X-axis. Dotted lines represent the 95% limits of agreement. Gap 1: IFX trough level < 3µg/mL, Gap 2: IFX trough level between 3 and 7µg/mL, Gap 3: IFX trough level >7µg/mL. T: Theradiag ELISA. RB: R-Biopharm ELISA. S:Sanquin ELISA. G:Grifols ELISA. RBP: R-Biopharm POC. BP: Buhlmann POC. (TIFF 236 kb)
Rights and permissions
About this article
Cite this article
Nasser, Y., Labetoulle, R., Harzallah, I. et al. Comparison of Point-of-Care and Classical Immunoassays for the Monitoring Infliximab and Antibodies Against Infliximab in IBD. Dig Dis Sci 63, 2714–2721 (2018). https://doi.org/10.1007/s10620-018-5144-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5144-y