Abstract
Background
Eradication therapies for Helicobacter pylori infection are advancing as new acid inhibitory reagents approved. The aim of this study was to assess the efficacy and safety of vonoprazan-based triple treatment.
Materials and Methods
Triple therapy with vonoprazan and two antibiotics (amoxicillin and clarithromycin or metronidazole) received focus in this analysis. We performed a multicenter retrospective study of patients who received vonoprazan-based eradication therapy between February 2015 and February 2016 and conducted a review of the literature.
Results
The eradication rate among the 799 patients in our multicenter study was 94.4% (95% confidence interval [CI] 92.6–96.2%) in the per-protocol analysis for first-line treatment (with vonoprazan 20 mg, amoxicillin 750 mg, and clarithromycin 200 or 400 mg, twice a day for 7 days) and 97.1% (95% CI 93.0–101.1%) for second-line treatment (with vonoprazan 20 mg, amoxicillin 750 mg, and metronidazole 250 mg, twice a day for 7 days). The overall incidence of adverse events was 4.4% in an intention-to-treat analysis with no patients hospitalized. In a literature review, six reports, in which 1380 patients received vonoprazan-based first-line eradication therapy, were included and were all reported by Japanese researchers. The eradication success rates in per-protocol analysis were between 85 and 93%, which was roughly the same among the studies.
Conclusions
Vonoprazan-based triple therapy was effective and safe for Helicobacter pylori eradication in real-world experience, confirmed by a multicenter study and a review of the pertinent literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
East Asian countries such as China, South Korea, and Japan are high-risk countries for gastric cancer due to the high prevalence of Helicobacter pylori (H. pylori) infection [1]. The eradication of H. pylori is one of the strategies for preventing gastric carcinogenesis. Guidelines for the management of H. pylori infection in Japan (2009 revised edition) recommend the elimination of H. pylori in all infected subjects [2]. The Ministry of Health, Labour and Welfare of Japan has announced that the Japanese national health insurance system covers the H. pylori eradication with a proton pump inhibitor (PPI)-based triple regimen. For first-line therapy, a regimen composed of a double dose of a PPI, amoxicillin, and clarithromycin for 7 days was first approved. For second-line therapy, a regimen composed of a double dose of PPI, amoxicillin, and metronidazole was approved. The Japanese national health insurance system did not cover any regimens other than these two. The Japanese Society of Helicobacter Research published a revised guideline in 2013 recommending third-line salvage therapy of high-dose amoxicillin with a PPI, and quinolone with amoxicillin and a PPI [3]. Neither of those regimens, nor any sequential or concomitant therapies, are covered at by the Japanese health insurance system for H. pylori eradication.
A novel potassium-competitive acid blocker (P-CAB), vonoprazan was recently approved in Japan in 2014. Vonoprazan inhibits gastric H+, K+-ATPase, and it is an enzyme that catalyzes the final step in the gastric secretion pathway and inhibits gastric secretion almost equally among various patients [4]. One phase III randomized controlled trial (RCT) of vonoprazan-based eradication therapy was performed as a double-blind, multicenter, parallel-group comparative study by Takeda Pharmaceutical Company, and vonoprazan-based triple therapy was shown to be as effective as PPI-based triple therapy and well tolerated [5]. The first-line eradication rate was 92.6% when vonoprazan plus amoxicillin and clarithromycin was administered for 7 days. The second-line eradication rate was 98.0% using vonoprazan plus amoxicillin and metronidazole. Some researchers have confirmed this high eradication rate of vonoprazan-based therapy retrospectively in clinical use [6,7,8,9,10]. We performed a multicenter study with a larger number of the patients treated with vonoprazan-based triple therapy and conducted a review of the pertinent literature.
Methods
Multicenter Study
Study Design
This was a retrospective multicenter study designed to investigate the usefulness of the vonoprazan-based regimen in clinical practice. The efficacy and safety were evaluated for vonoprazan-based triple therapy. This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of Asahikawa Kousei Hospital approved the study protocol (#2868).
Patients
The four institutes included in this study were Asahikawa Kousei Hospital, Asahikawa Medical University, International University of Health and Welfare Hospital, and Hokkaido Cancer Society Asahikawa. The patients who underwent H. pylori eradication therapy between February 2015 and February 2016 were included in the H. pylori eradication databases. The medical records were reviewed, and we collected the patients’ information, including their gender, age, endoscopic diagnosis, eradication therapy, adverse events, and success in eradication. A total of 799 consecutive patients received vonoprazan-based treatment, including 694 patients for whom it was a first-line therapy, 73 for whom it was a second-line therapy, 30 who received vonoprazan–amoxicillin–metronidazole as a primary therapy, and 2 who received vonoprazan–clarithromycin–metronidazole as a primary therapy. The 694 who received vonoprazan-based triple therapy in combination with amoxicillin and clarithromycin as the first-line therapy were retrospectively analyzed. Seventy-three patients who had a history of previous eradication therapy and received vonoprazan with amoxicillin and metronidazole were defined as the second-line eradication group. These antibiotics could be substituted for generic drugs in our multicenter study.
H. pylori Eradication Success Rate and Adverse Events
The presence of H. pylori was diagnosed, according to the Japanese guideline (3), by at least one of the following methods: rapid urease test, 13C-urease breath test, H. pylori immunoglobulin G serological test, H. pylori stool antigen test. All patients were treated with vonoprazan-based treatment. The eradication treatment consisted of 20 mg of vonoprazan twice a day, 750 mg of amoxicillin twice a day, and 200 mg or 400 mg of clarithromycin twice a day for a week for first line. For the second-line treatment, 20 mg of vonoprazan twice a day, 750 mg of amoxicillin twice a day, and 250 mg of metronidazole twice a day were administered. From four weeks after the end of the treatment, the eradication success was assessed based on the results of the 13C-urea breath test (<0.25%) or the stool antigen test (negative). The first object of this study was to elucidate the eradication rate, as determined by intention-to-treat (ITT) and per-protocol (PP) analyses.
The second goal of this study was to determine the rate of adverse events related to the eradication therapy. Physicians asked about the presence or absence of adverse events during consultation hours and then evaluated the success of the eradication treatment.
Literature Review
A literature review was carried out according to the general principles of systematic reviewing methodology [11]. The eligibility criteria were included as follows; studies of adult patients infected with H. pylori and treated with vonoprazan-based first-line regimens that reported the eradication rates with detailed methods and results.
Search Strategy
MEDLINE and the Cochrane Library were searched in September 2016. The keywords “pylori” and “vonoprazan” were used to develop an appropriate search strategy. First, the titles and abstracts of all retrieved studies were reviewed for the identification of potential reviews. The full texts of studies deemed relevant were then obtained and reviewed in detail. Studies were excluded if they focused on pharmacokinetics, if they were reviews, or if they were not full papers. Conference abstracts or letters were excluded due to a lack of details for data extraction. The reference lists from the reviewed studies were also checked to identify other potentially relevant studies that had not been identified by the electronic search.
Data Collection
From the reviewed studies, the following data were extracted: first author, publication year, journals, number of included patients, study design, patient’s characteristics, eradication protocols, eradication rates, and adverse events. The total eradication rates and adverse event rates were calculated.
Results
Patient Characteristics
The patient characteristics are shown in Table 1. A total of 694 patients were enrolled as the first-line H. pylori eradication treatment group; a total of 641 subjects completed the first-line eradication triple therapy. Fifty-three patients dropped out and did not undergo subsequent urea breath tests or stool tests. Seventy-three patients who had failed H. pylori eradication therapy before were enrolled as the second-line treatment group. The previous eradication therapies were PPI-based treatment in 60 patients and vonoprazan-based in 13. A total of 68 subjects completed second-line eradication therapy, with 5 patients dropping out.
Treatment Outcome
The eradication rates in first-line and second-line therapy are shown in Table 2. The overall outcomes for first-line eradication therapy were 87.2% (95% confidence interval [CI] 84.7–89.2%) in the ITT analysis and 94.4% (95% CI 92.6–96.2%) in the PP analysis. Of those failing first-line therapies, 25 subjects further received and completed second-line vonoprazan-based triple therapy. Some of these cases were included in this second-line eradication group. The total eradication rate for the second-line therapy was 90.4% (95% CI 83.7–97.2%) in the ITT analysis and 97.1% (95% CI 93.0–101.1%) in the PP analysis, with five patients dropping out from the assessment.
Adverse Events
The adverse events of all patients are shown in Table 3. The overall incidence of adverse events was 4.4% in an ITT analysis, and five patients (0.6%) who had adverse events discontinued the eradication therapy. The incidence of adverse events was 4.6% during the first-line eradication phase and 0% during second-line eradication. All adverse events were cured without intervention. No patients were hospitalized because of adverse events.
Literature Review
The PRISMA guidelines for systematic reviews were followed [11]. A flowchart outlining the selection of the papers for our literature review is shown in Fig. 1. A total of 24 reports were initially retrieved. After screening the titles and abstracts, eight records were excluded. Sixteen records were fully reviewed, and abstracts, letters, a review, and a pharmacokinetic study were excluded.
Six full-text articles with a total of 5165 patients who received first-line H. pylori eradication therapy were included. The characteristics of the subjects, study designs, and outcomes are shown in Table 4. One randomized controlled trial (RCT), four retrospective studies, and one prospective study all originated from Japan. Among them, a total of 1380 patients received vonoprazan-based eradication therapy. The eradication regimen approved and covered under the Japanese health insurance is 20 mg of vonoprazan, 750 mg of amoxicillin, and 200 or 400 mg of clarithromycin, twice a day for 7 days. One RCT was a phase III clinical study that Murakami et al. published in 2015 showed an H. pylori eradication rate of 92.6% using this regimen. In addition, retrospective studies showed first-line eradication rates of 85–91.5% according to PP analyses.
Discussions
The primary aim of the present study was to confirm the usefulness of vonoprazan-based eradication therapy in the real-world setting. In comparison with previous literatures (Table 4), this multicenter study involved a larger number of patients resulting in very high success rates and low rates of adverse events. This was a retrospective and observational study of first- and second-line therapies with no comparator group, and there was a shortage of clinical information (e.g., the patient characteristics and bacterial natures).
The findings from this multicenter study and literature review suggested that vonoprazan-based eradication therapy has satisfactory efficacy because the eradication rates are around 90%. Vonoprazan demonstrated a more rapid and sustained acid inhibitory effect than esomeprazole and then increased the intragastric pH [4], resulting in improved eradication rates. It is stable in acid, dissolves in the stomach, moves to the small intestine, and is not influenced by gastric emptying [12]. Regarding the pharmacokinetics, triple therapy with vonoprazan, amoxicillin, and clarithromycin increases the vonoprazan and clarithromycin exposure. Cytochrome P450 (CYP) 3A4, which metabolizes both clarithromycin and vonoprazan, is most likely involved in the mechanism of pharmacological interaction [13]. The rate of adverse events in a Phase III study in Japan was 30.4% [5]. We were afraid that a strong acid inhibitor might increase severe adverse events. However, very few problematic adverse events were seen in our clinical study and other retrospective studies.
Some eradication-failure patients remained following the first-line vonoprazan-based treatment. A number of clinical variables have been suggested to play a role in the failure of eradication therapy [14]. With regard to host-related factors, adherence, polymorphism of CYP2C19, cigarette smoking, and diabetes mellitus were associated with treatment failure [14]. With regard to the H. pylori-related factors, antibiotic sensitivity has emerged as the single most important predictor of treatment success [15]. According to a multivariate analysis, clarithromycin resistance was the only significant factor for treatment failure [16]. Our high eradication rate in the second-line therapy (97.1% in PP analysis) may support their conclusion.
At present, standard triple therapy using amoxicillin, clarithromycin, and a PPI is recommended for first-line treatment in China, South Korea, and Japan [1]. Triple therapy proposed at the first Maastricht conference [17] has become popular around the world. However, the eradication rate of this traditional therapy is decreasing, as the prevalence of antibiotic-resistant strains of H. pylori is increasing. The most recent data show only a maximum success rate of 70% with clarithromycin resistance observed in more than 20% of patients in Europe and North America [14]. The latest Maastricht IV/Florence Consensus Report recommends prior susceptibility testing before PPI-clarithromycin-containing triple therapy [18]. In regions of high clarithromycin resistance, bismuth-containing quadruple therapies are the first choice. However, bismuth drugs may not be available in some areas. Sequential or concomitant treatment may be an option. The Maastricht IV Consensus Report stated that “While no new drug has been developed …”. However, a new drug has just appeared. A very recent statement developed by the Canadian Association of Gastroenterology has mentioned the agent [15]. One potential method of improving acid inhibition would be to use newer anti-secretory agents, such as P-CABs.
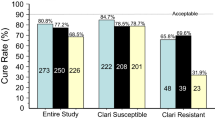
Vonoprazan was developed because the effects of PPIs are insufficient for patients with gastroduodenal ulcer and gastroesophageal reflux disease. For first-line eradication, vonoprazan is prescribed with amoxicillin and clarithromycin twice daily for 7 days. Because the clinical efficacy has not been fully demonstrated, we conducted a multicenter study and a review of the literature. The reported eradication rates are 83–89% in ITT analyses and 95–92% in PP analyses (Table 4). Some authors have considered the issue of clarithromycin resistance. For clarithromycin-sensitive and clarithromycin-resistant H. pylori, the respective success rates of the vonoprazan-based triple therapy are 97.6 and 82.0% according to Murakami [5], 100 and 87.5% according to Noda [9], 100 and 76.1% according to Matsumoto [10]; the respective rates of resistance of clarithromycin are 30.4, 42.1, and 44.7%. Our clinical observation showed 87.2% in an ITT analysis and 94.4% in a PP analysis. The rate of clarithromycin resistance is 27% in our region of Japan (unpublished data), which is not as high as the resistance rates mentioned above.
For second-line eradication, vonoprazan, amoxicillin, and metronidazole were used in our study, because this combination is covered by the national health insurance system in Japan. Murakami and Matsumoto reported eradication rate as 98.0% [5] and 100% [10], respectively. These rates are very high; therefore, details of the reports in the former eradication regimen and data analysis should be carefully considered. The four other studies selected in our review do not mention the success rate for second-line treatment. However, three of the letters, excluded through the selection process for our review, reported the efficacy of vonoprazan-based second-line therapy. One report investigated the eradication rate of a vonoprazan-based regimen with amoxicillin and clarithromycin as a second-line treatment in patients who failed the first-line treatment with a PPI, amoxicillin, and clarithromycin [19]. Two other brief reports described the eradication rate when vonoprazan with amoxicillin and metronidazole was used as a second-line regimen [20, 21]. One of those two reports cited an eradication rate for H. pylori of 87.0% (20/23) in patients who failed vonoprazan-based first-line treatment [20]. In another letter with 151 patients who received second-line therapy, 95 patients retested for H. pylori infection after the therapy was successfully eradicated (100% in a PP analysis) [21]. This letter does not describe the details of the former first-line treatment. Our 73 patients in second-line therapy were a mixture of the PPI-failure and vonoprazan-failure patients and were successfully eradicated at a rate of 90.4% in an ITT analysis and 97.1% in a PP analysis. Murakami also examined a mixture of first-line-failed patients at 98% (49/50) in a PP analysis [5]. Since these reports were based on a single-arm study, more detailed studies comparing PPI and P-CAB are needed in order to clarify the optimum second-line treatment for PPI-failure or vonoprazan-failure patients.
The eradication rate with vonoprazan-based therapy is also expected to decrease with increasing resistance to antibiotics among H. pylori strains. The cure rate with vonoprazan among populations with clarithromycin resistance is estimated at 82% [22]. Therefore, regional antibiotic resistance patterns should be considered when choosing a first-line therapy [18]. A vonoprazan-based triple regimen with metronidazole instead of clarithromycin for first-line treatment may result in a better success rate and bring economical benefits as well. For populations with high clarithromycin resistance, such as Europe or North America [14, 15, 18], other strategies should be considered, such as vonoprazan-containing bismuth quadruple therapy, concomitant therapy, or sequential therapy [23].
One principal limitation of our study and review is that vonoprazan is currently approved by no countries other than Japan. Since only one RCT has been reported thus far, we conducted a literature review instead of a meta-analysis. More clinical trials are needed to determine the efficacy and safety of vonoprazan in many areas. The eventual inclusion of vonoprazan in other regimens around the world is expected.
In conclusion, this multicenter study and review confirmed the high efficacy and safety of first- and second-line vonoprazan-based triple therapy. The future direction of vonoprazan-based therapy is the modification of this regimen worldwide.
References
Lee SY. Current progress toward eradicating Helicobacter pylori in East Asian countries: differences in the 2013 revised guidelines between China, Japan, and South Korea. World J Gastroenterol. 2014;20:1493–1502.
Asaka M, Kato M, Takahashi S, et al. Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20.
Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. (Japanese article) Tokyo Japan: Sentan-igakusha; 2016: 46-58.
Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium-competitive acid blockers: advanced therapeutic option for acid-related diseases. Pharmacol Ther. 2016;168:12–22.
Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446.
Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol. 2016;111:949–956.
Shichijo S, Hirata Y, Niikura R, et al. Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: a multicenter retrospective study in clinical practice. J Dig Dis. 2016;17:670–675.
Shinozaki S, Nomoto H, Kondo Y, et al. Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J Med Sci. 2016;32:255–260.
Noda H, Noguchi S, Yoshimine T, et al. A Novel potassium-competitive acid blocker improves the efficacy of clarithromycin-containing 7-day triple therapy against Helicobacter pylori. J Gastrointestin Liver Dis. 2016;25:283–288.
Matsumoto H, Shiotani A, Katsumata R, et al. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig Dis Sci. 2016;61:3215–3220. doi:10.1007/s10620-016-4305-0.
Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15.
Otake K, Sakurai Y, Nishida H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther. 2016;33:1140–1157.
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239.
Sakurai Y, Shiino M, Okamoto H, Nishimura A, Nakamura K, Hasegawa S. Pharmacokinetics and safety of triple therapy with vonoprazan, amoxicillin, and clarithromycin or metronidazole: a phase 1, open-label, randomized, crossover study. Adv Ther. 2016;33:1519–1535.
Fallone CA, Chiba N, van Zanten SV, et al. The toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.
Lee JY, Kin N, Kim MS, et al. Factors affecting first-line therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235–1243. doi:10.1007/s10620-014-3093-7.
Malfertheiner P, Mégraud F, O’Morain C, et al. The European Helicobacter pylori Study Group (EHPSG). Current European concepts in the management of Helicobacter pylori infection–the Maastricht Consensus Report. Eur J Gastroenterol Hepatol.. 1997;9:1–2.
Malfertheiner P, Megraud F, O’Morain CA, et al.; European Helicobacter Study Group. Management of Helicobacter pylori infection–the maastricht IV/florence consensus report. Gut 2012; 61: 646–664.
Inaba T, Iwamuro M, Toyokawa T, Okada H. Promising results of Helicobacter pylori eradication with vonoprazan-based triple therapy after failure of proton pump inhibitor-based triple therapy. Aliment Pharmacol Ther. 2016;43:179–180.
Katayama Y, Toyoda K, Kusano Y, et al. Efficacy of vonoprazan-based second-line Helicobacter pylori eradication therapy in patients for whom vonoprazan-based first-line treatment failed. Gut 2016; doi:10.1136/gutjnl-2016-312028.
Fukuda D, Akazawa Y, Takeshima F, Nakao K, Fukuda Y. Safety and efficacy of Vonoprazan-based triple therapy against Helicobacter pylori infection: a single-center experience with 1118 patients. Therap Adv Gastroenterol. 2016;9:747–748.
Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut. 2017;66:384–386.
Yeo YH, Shiu SI, Ho HJ, et al.; Taiwan Gastrointestinal Disease and Helicobacter Consortium. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut. 2016; doi:10.1136/gutjnl-2016-311868.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Rights and permissions
About this article
Cite this article
Tanabe, H., Ando, K., Sato, K. et al. Efficacy of Vonoprazan-Based Triple Therapy for Helicobacter pylori Eradication: A Multicenter Study and a Review of the Literature. Dig Dis Sci 62, 3069–3076 (2017). https://doi.org/10.1007/s10620-017-4664-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4664-1