Abstract
Management of pancreatic cystic lesions relies on patients’ clinical presentation, imaging, and endoscopic ultrasound. Current research in basic science, radiology, and endoscopy is evolving and making progress in this condition which is relatively common in the general population. This review focuses on the recent endoscopic ultrasound approaches to the diagnosis of these pancreatic disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overview
The endoscopic management of pancreatic cyst encompasses more aspects than only the endoscopic procedure itself. When excluding pancreatic pseudocysts which arise usually in the realm of pancreatitis, the majority of the remaining pancreatic cysts fall within the category of pancreatic cystic neoplasms, or are at least managed as presumed pancreatic cystic neoplasms [1]. This requires expertise when assessing pancreatic cysts endosonographically, particularly when defining their relation to the pancreatic ductal system and when assessing findings concerning advanced dysplasia both endosonographically and on fine needle aspiration or biopsy specimens. Further, the endosonographer assumes frequently the role of a triaging physician making most important decisions with substantial impact on the patient’s outcome. This includes triaging patients who undergo surgery, surveillance imaging, or discontinue surveillance. A particularly important aspect of endoscopic management is addressing patient’s wishes and their anxiety of developing pancreatic cancer, particularly as most patients will undergo surveillance imaging and not surgery. Over the last decade, this is becoming a more recognized problem given the rising prevalence of pancreatic cysts in light of aging population and improved imaging modalities [2, 3].
Endoscopic Assessment of a Pancreatic Cyst
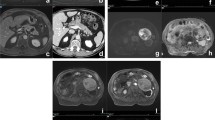
Endoscopic ultrasound (EUS) allows high-resolution imaging of the entire pancreatic gland. The focus is laid on the detection and description of cyst morphology (Fig. 1), which allows to draw conclusions on the cyst type and its malignant potential. Generally, cyst size, morphology of the cyst wall, communication between cyst and main pancreatic duct, associated solid components or masses, and findings within the cyst like mural nodules are the main features assessed by EUS. All of them are associated with malignant transformation of pancreatic neoplastic cysts, although the exact rate of such a transformation remains unclear for the general gastroenterology patient population and most knowledge on this topic is extrapolated from surgical series [4–6].
As most pancreatic cysts will not undergo resection, data on the accuracy of EUS assessing the pancreatic cyst type are mainly based on surgical resected cysts, allowing a gold-standard histopathological evaluation. In such cohorts, Ahmad et al. [7, 8] demonstrated that EUS alone has limited ability differentiating benign from malignant and mucinous from non-mucinous lesions, with an accuracy of approximately 50–75%. The strength of EUS lays in the high sensitivity to detect pancreatic cysts. EUS and magnetic resonance imaging (MRI) are more sensitive, particularly for small cysts compared to a computer tomography (CT) imaging, although the majority of these small cysts, particularly cysts under 1 cm, have limited or no clinical significance and usually lead to surveillance with cross-sectional imaging [1, 2, 5, 9].
Another important aspect of EUS is its ability to visualize communications between the pancreatic duct and the cyst, which if present is suggestive for branch duct (BD-IPMN) or mixed-type (MT-IPMN) IPMN. BD-IPMN is thought to be the most common pancreatic cystic neoplasm. However, the connection to the pancreatic duct cannot always be discerned.
Cystic mural nodules, particularly if present in IPMN and mucinous cystic neoplasms (MCN), are associated with increased risk of high-grade dysplasia and invasive cancer (Fig. 2). Surgical series showed a risk of malignancy in the presence of mural nodule in 25–60% of cases [10]. In the absence of mural nodules at the index imaging, up to 17% of patients develop mural nodules during cyst surveillance, although that number appears for the authors of this article to be too high for the usual gastroenterology population which undergoes imaging surveillance for presumed mainly low-risk pancreatic cystic neoplasms [11–13]. An important limitation of EUS is that mural nodules can be misinterpreted as mucin globules, which had been shown to account for 65% of intracystic lesions on EUS [14]. This reflects the most significant limitation of EUS, which is its low interobserver agreement, particularly for establishing differential diagnoses of pancreatic cysts and when assessing cyst characteristics like mural nodules [7, 8, 14]. Educating endosonographers about such pitfalls is important, as it has shown to increase the diagnostic accuracy of EUS [14, 15].
A recent meta-analysis summarized endosonographic features predicting the presence of malignancy in pancreatic cystic neoplasms. Cyst size larger than 3 cm had the highest risk predicting the presence of malignancy (odds ratio 62.4), followed by the presence of mural nodule (odds ratio 9.3) and a dilated main pancreatic duct (odds ratio 7.3) [16].
EUS-Guided Fine Needle Aspiration and Biopsy
The main advantage of EUS, which is usually considered in pancreatic cyst larger than 15 mm [17], specifically in the presence of concerning features, is its ability to sample the cyst fluid containing epithelial cells shed within the cyst fluid and more recently also the cyst wall itself by targeted needle puncture or miniature forceps [18–20]. This permits evaluation of cytology, chemistry, e.g., amylase and tumor markers levels, and most recently molecular markers, e.g., DNA, which tests for genetic mutations associated with high-grade dysplasia and pancreatic cancer. Khashab et al. [21] demonstrated that EUS-guided fine needle aspiration (EUS-FNA) increases the correct cyst diagnosis compared with CT and MRI by 36 and 54%, respectively. The two principal questions in EUS-FNA are to distinguish serous from mucinous cystic lesions and to identify mucinous lesions with highest likelihood of developing invasive cancer, which intuitively are cystic lesions with high-grade dysplasia. Along these lines, the pace and rate of developing invasive pancreatic cancer from high-grade dysplasia are currently not known.
Historically, the viscosity of cyst fluid was tested to distinguish between serous and mucinous cysts and amylase levels to distinguish cysts with connection to the pancreatic duct from cysts without connection to the pancreatic duct [22]. These objective markers are, however, poor predictors distinguishing serous from mucinous cysts and not useful when assessing malignancy [22].
Cytology on EUS-FNA was reported to have specificity as high as 100% when distinguishing premalignant from malignant pancreatic mucinous cysts, but the sensitivity is low [23, 24]. This is a consequence of a generally low cellularity in the fluid of pancreatic cystic neoplasms. Lowering the cytological threshold for malignant cells to high-grade atypical cells increases the sensitivity to 72%, but at the price of a lower specificity (85%) [25]. Whether the generally limited yield of cytology can be increased by targeted cell wall puncture or by the most recent introduction of EUS-guided miniature forceps biopsy remains to be defined. Recent pilot studies showed a diagnostic yield of cytology from FNA cyst wall puncture of 81% and using EUS-guided miniature forceps of 91% [18, 19]. In terms of distinguishing mucinous from non-mucinous cysts based on cytology, Thosani et al. [26] reported in a meta-analysis a pooled sensitivity and specificity of only 63 and 88%, respectively.
The addition of tumor markers to the cyst fluid analysis showed further improvement in test characteristics. Brugge et al. [15] established carcinoembryonic antigen (CEA) as a marker discriminating mucinous and non-mucinous lesions. The optimal cutoff in their report was set at 192 ng/mL, yielding a sensitivity and specificity of 73 and 84%, respectively. The combination of CEA with cytological examination and EUS findings increased the sensitivity to 91%, but it lowered the specificity to 31%. Other tumor markers like CA 19-9, CA 125, CA 15-3, and CA 72-4 had worse test characteristics [15]. More recent studies showed even higher sensitivities of CEA to differentiate mucinous from serous cystic lesions [27, 28]. IPMNs, however, which are the most common pancreatic mucinous lesions, cannot be reliably distinguished from other cystic neoplasms as shown recently by Moris et al. [29], who reported a sensitivity of only 21.4% and specificity of 83.3% when utilizing amylase, CEA, and cytology.
Although commonly recognized, CEA cannot distinguish malignant from non-malignant cysts, as shown in two meta-analyses. Ngamruengphong and colleagues [30] calculated a pool sensitivity and specificity of only 63 and 63%, respectively. Van der Waaij et al. [31] reported a sensitivity of 48% and specificity of 98% when utilizing as CEA level with a cutoff greater than 800 ng/mL for mucinous lesions.
Given the limited test characteristics of cytology with CEA and amylase levels, new molecular biologic markers were introduced in the last decade. Particularly altered DNA was evaluated as surrogate marker for advanced dysplasia and invasive carcinoma, which are known to be associated with genetic alterations. The PANDA study [32] (pancreatic cyst DNA analysis) was one of the first studies addressing K-ras mutations in pancreatic cystic lesions. Identification of K-ras mutation yielded a sensitivity of only 45% but a specificity of 96%, respectively, distinguishing mucinous from non-mucinous cysts. More importantly, the majority of included patients had pancreatic carcinoma or IPMN, which was the main criticism of the study. As such, a patient cohort does not reflect the usual gastroenterology patient population which undergoes pancreatic cystic neoplasm evaluation, even when EUS high-risk features are present. Rockacy et al. [33] assessed EUS-FNA findings in 113 patients with pancreatic cysts of whom 51 patients eventually underwent surgical resection. The presence of solid component on EUS achieved highest odds ratio of 17.7 to be associated with a non-benign course, whereas K-ras mutations had an odds ratio of only 2.3 and in combination with loss of heterozygosity of 9.9. Along these lines, Al-Haddad et al. [34] assessed a comprehensive DNA analysis including K-ras mutations, loss of heterozygosity, with cytology, and CEA on 48 patients with suspected mucinous cystic lesions who underwent surgical resection. Considering cytology, CEA, and comprehensive DNA analysis, sensitivity, specificity, and accuracy of 67, 60, and 73%, respectively, were achieved. In a patient cohort who underwent mainly surveillance imaging, comprehensive DNA analysis yielded a sensitivity of 83.3% and specificity of 90.6% [35]. GNAS is another recently described genetic mutation which is present in 66% of IPMNs, particularly in IPMN with intestinal histology followed by pancreatobiliary and gastric histology [36, 37]. Presence of either GNAS or K-ras mutation was reported as high as 96% in IPMNs [36]. Given the cumulative character of genetic mutations in advancing dysplasia, a combination of genetic mutations was proposed to be of higher accuracy than only one genetic mutation. When applying next-generation genetic sequencing, the combination of K-ras, GNAS, and RNF43 mutations was proposed to define IPMN and distinguish MCN which have been positive only for K-ras and RNF43 mutations and serous cystic neoplasms which have mutations in the VHL tumor suppressor gene [38].
When assessing for risk factors for malignancy in IPMNs, 4 different histology subtypes were described which have different malignant potential, with the gastric IPMN subtype having a much lower likelihood harboring advanced dysplasia and cancer than the pancreatobiliary IPMN subtype [39–41]. Evaluating the expression of mucin (MUC1, MUC2, and MUC5AC) was shown to differentiate among histologic subtypes [38]. However, this discrimination has not been implemented in the EUS-FNA workup of pancreatic cystic neoplasms.
Despite the latest technological advancement, the test performance to define and differentiate pancreatic cystic neoplasms and their risk and degree of harboring advanced dysplasia remains suboptimal. Jones et al. [42] reported the impact on decision making for the workup of pancreatic cysts with next-generation sequencing utilizing 39 cancer genes including K-ras, GNAS, VHL, SMAD4, AKT1, and BRAF. The authors analyzed 92 cysts and reported that the majority of cysts diagnosed based on cytology and CEA level were confirmed by next-generation sequencing, whereas only 12% of cyst diagnoses were changed based on next-generation sequencing. Comparable findings were reported by Shen et al. [43], who found significant agreement between standard EUS-FNA including cytology with CEA level and molecular diagnosis provided by PathFinderTG. Therefore, the impact on clinic management from molecular markers results has probably only limited value for the workup of most pancreatic cystic neoplasms, particularly as the natural history of mucinous cysts including IPMNs is under current investigation [11–13, 44–46]. The strength of molecular analysis lays in the differentiation between cysts with malignant potential from cysts with barely any malignant potential, which are serous cystic neoplasm (SCN) [42]. Following successful pilot studies, additional diagnostic markers including microRNA, proteomics, and metabolomics are under current investigation and might find their way into clinical practice [47–50].
As a consequence, the majority of pancreatic cysts which are evaluated by gastroenterologists and considered for EUS evaluation are managed as BD-IPMN, unless EUS, MRI, or CT imaging is convincing for the diagnosis of MD-IMPN or MT-IMPN, MCN, or SCN (Fig. 3). Several societies proposed guidelines addressing the management of suspected IPMN and more recently for all asymptomatic pancreatic cysts [1, 5]. These guidelines will be addressed in the later chapter. Nonetheless, it is important to state that endosonographic and cyst fluid analysis are yet not sufficiently accurate to predict the histology and the biology for all pancreatic cysts, which have substantial impact on cyst prognosis. Consequently, diagnostic uncertainties will remain, which are reflected by the fact that patients develop invasive pancreatic cancer despite close surveillance according to guidelines. On the other hand, patients still undergo surgical resection for cysts which are at minimal risk of malignant transformation [45, 51]. In addition, most guidelines are based on surgical literature with very selective patient cohorts and therefore limiting their generalization. This is particularly acknowledged by the latest American Gastroenterology Association (AGA) guidelines, stating that most of their recommendations are conditional recommendations based on very low-quality evidence [1]. Therefore, one can argue that the guidelines are not more than management suggestions and that the entire responsibility detecting, assessing the risk, and triaging patients accordingly remained in the hand of the endosonographer, with all the uncertainly of diagnostic markers as shown above.
New Endoscopic Imaging Modalities
In the last decade, new endoscopic imaging modalities were described to improve the endoscopic diagnostic accuracy of suspected neoplastic pancreatic cysts and their degree of dysplasia. With several exceptions, most techniques utilize the EUS-FNA as the platform to introduce new imaging devices into the pancreatic cyst.
Pancreatoscopy
Peroral pancreatoscopy has been described to directly visualize the epithelial lining of the main pancreatic duct. In retrospective surgical series, villous elevations along with vascular red color markings were found in severely dysplastic IPMNs and invasive pancreatic adenocarcinoma [52, 53]. Addition of intraductal ultrasonography showed that lesions which protruded more than 4 mm had a high risk of invasive cancer [53]. The introduction of the digital spyglass system improved the technical feasibility of single-operator cholangiopancreatoscopies. However, this technique is still limited to few high-volume tertiary care referral centers [54]. It is also self-explanatory that this technique will be limited to a handful of indications, e.g., when the length and distribution of a MD-IPMN or MT-IPMN remain unclear on conventional imaging during the planning of a surgical resection.
Another way to utilize the spyglass system was delineated by Aparicio et al. [55]. They reported intraluminal cyst inspection with the initial fiber-optic spyglass system utilizing EUS-FNA platform. This allowed inspection and guided biopsies of the cyst. However, no large case series have reported the utility of this technique.
Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) is a real-time high-resolution imaging modality allowing in vivo histopathology-like evaluation [56]. Three multicenter studies addressed the utility of a needle-based confocal laser endomicroscopy (nCLE) of pancreatic cystic neoplasms, using EUS platform.
The INSPECT study evaluated the yield of nCLE for pancreatic cystic neoplasms. The authors reported that a detection of epithelial villous structures yielded a sensitivity and specificity of 59 and 100%, respectively, which included the detection of IPMN, MCN, and adenocarcinoma [57]. In the DETECT study, the combined use of fiber-optic spyglass cystoscopy and nCLE in pancreatic cystic neoplasms addressed test characteristics to diagnose mucinous cysts. The reported sensitivities were 90% of cystoscopy, 80% for nCLE, and 100% for both with a specificity of 100% [58]. In the CONTACT study, any pancreatic cystic neoplasm was evaluated. The authors defined the presence of a superficial vascular network pattern as diagnostic for pancreatic serous cystadenoma yielding a sensitivity and specificity of 69 and 100%, respectively, with a high interobserver agreement [59]. Of note, acute pancreatitis was reported as complication in 3–7% of patients [57–59].
Similar to pancreatoscopy, nCLE is a technically challenging procedure which is limited to few tertiary care centers. Beside the costs, its relatively high rate of pancreatitis prohibits its use for most patients. Further, no data are available in how many cases the impact on nCLE resulted in changing the clinical management of a patient with pancreatic cystic neoplasm in comparison with the standard methods.
Pancreatic Cyst Ablation
Beside its utility in the diagnosis of pancreatic cysts, EUS was reported as a platform for focal ablative methods of pancreatic cysts, which include either targeted injection or lavage of a solution causing epithelial destruction or the application of a high-energy probe resulting in focal thermocoagulation and eventually focal necrosis.
EUS-Guided Ethanol Injection
Most studies addressing ablation of pancreatic cysts reported the use of ethanol of various concentrations, ranging from 5 to 99%. Under EUS-guidance, the cyst was lavaged with ethanol for 3–5 min [60–63]. Following the index procedure, patients were followed clinically with cross-sectional imaging. Treatment response was defined as cyst size reduction or resolution of cyst. The only randomized control trial comparing ethanol with normal saline lavage reported a pancreatic cyst reduction in 24% after ethanol lavage compared to 15% after lavage with normal saline [62]. Kandula et al. [64] reported in a meta-analysis complete cyst resolution in 56% and partial cyst resolution in 24% following ethanol lavage. Complications included abdominal pain in 6.5% and acute pancreatitis in 3.9%.
EUS-Guided Ethanol and Paclitaxel Injection
In addition to ethanol lavage, two studies reported an additional treatment with paclitaxel injection following ethanol lavage as treatment for pancreatic cystic neoplasms [65, 66]. Cyst resolution was achieved in 62 and 79% based on subsequent imaging [65, 66]. However, when addressing histologic findings following cyst ablation with ethanol lavage and paclitaxel injection in four patients with persisting pancreatic cysts who underwent surgical resection, ablated epithelial lining was found in 0–100% [65]. Given this broad range of ablative success rate on histologic specimens, this technique requires additional assessment on its histologic and real impact. It is intuitive to assume that cysts which resolved following EUS-guided ethanol lavage with or without adjuvant paclitaxel injection have complete ablated epithelium. However, this has not been proved yet, particularly in human histologic specimens.
EUS-Guided Radiofrequency Ablation
Radiofrequency ablation (RFA) delivers ablative energy by high-frequency alternating electromagnetic energy which results in thermocoagulation. Several animal studies reported EUS-guided RFA treatment of the porcine pancreas, which resulted in coagulative necrosis of the ablated tissue [67]. Despite the wide utility of RFA for Barrett’s esophagus and, for example, liver tumor ablation, RFA ablation was not tested for pancreatic cysts in humans yet [68, 69].
Miscellaneous Ablation Techniques
Additional new ablative techniques include the utility of laser energy (neodymium-doped yttrium aluminum garnet [Nd:YAG] laser), high-intensity focused ultrasound, and photodynamic therapy. These techniques have in common that high temperature is delivered focally and results in denaturation of the tissue by thermocoagulation [70, 71]. Most tests were performed in the realm of animal studies and solid pancreatic lesions or normal pancreatic tissue. However, one can assume that these modalities might find their application also for pancreatic cystic lesions [70, 72–74].
Current Limitations of Focal Pancreatic Cyst Ablation
Focal ablative techniques for pancreatic cystic neoplasms have several major limitations.
First, considering the enigmatic natural history of pancreatic cystic lesions, it is unknown which cyst eventually develops pancreatic cancer. Current guidelines recommend surgery for the highest risk group, which still includes up to 80% of patients who would have not required surgical intervention. That is, however, only known after reviewing the surgical specimen, which would not be the case following a focal ablation. At the same time, surgical studies have reported up to 20–50% of patients having already invasive cancer on the surgical specimens, which is the other extreme. In such cases, ablation would have not offered an adequate treatment.
Second, due to the unclear efficacy of focal ablation, the risk of persisting dysplastic epithelium following ablation remains to be a major concern, as shown by Oh et al. [65].
Lastly, not all pancreatic cancers arise from mucinous cystic lesions, particularly in IPMN. In fact, up to 17% of pancreatic cancer arises independently of the IPMN lesion, which is suspected to be due to a “field defect” in the entire pancreas. These cases would have most likely not benefit from focal ablation of pancreatic neoplastic cysts [75].
Summary
The endosonographer has the “quarterback” position in the detection of pancreatic cysts and their exact diagnosis, assessing their risk of malignancy and their further stratification to recommend surgical resection, surveillance, or no surveillance. Although EUS-FNA offers the most important tool in that field, current imaging and cyst fluid analysis modalities are not optimal, and patients will undergo surgery for cystic lesions which would have not required resection and at the same time, patients will develop pancreatic cancer, which can be unresectable at the diagnosis, under the eyes of the gastroenterologist during close surveillance. Major effort is currently undertaken to understand the natural history of pancreatic cystic neoplasms and to find new biomarkers which allow recognition of patients with pancreatic cysts at risk to progress from high-grade dysplasia to invasive cancer, as that is the optimal target group to offer surgical resection. Whether focal ablative methods will gain acceptance remains to be elucidated as well.
-
Prevalence of pancreatic cysts is rising, and most cysts are managed as presumed pancreatic cystic neoplasms (PCN), particularly as BD-IPMN.
-
EUS is a very sensitive tool for detecting and defining cyst morphology, but it is not sensitive or specific to predict malignancy.
-
EUS-FNA permits evaluation of cytology, chemistry, tumor, and molecular markers which have to be interpreted individually to triage patients adequately for surgical resection, surveillance, or no surveillance.
-
Pilot studies defined new diagnostic markers for PCNs including microRNA, proteomics, and metabolomics which need to be evaluated for their general diagnostic utility.
-
Peroral pancreatoscopy and confocal laser endomicroscopy among other advanced endoscopic techniques are unlikely to gain wide acceptance in the general PCN workup given their costs, limited availability, and procedure risk profile.
-
While waiting for an ideal marker defining which patients will progress from high-grade dysplasia to invasive cancer, the endosonographer will continue to stratify patients to undergo surgical resection, surveillance, or no surveillance, with all the significant uncertainties of current diagnostic tools.
References
Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:e822.
Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol. 2016;14:e583.
Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807.
Roch AM, Schmidt CM. Management of mixed-type intraductal papillary mucinous neoplasm. Adv Surg. 2016;50:1–15.
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International Association of P: International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197.
Marchegiani G, Fernandez-del Castillo C. Is it safe to follow side branch IPMNs? Adv Surg. 2014;48:13–25.
Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64.
Ahmad NA, Kochman ML, Lewis JD, Ginsberg GG. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol. 2001;96:3295–3300.
Adimoolam V, Sanchez MJ, Siddiqui UD, et al. Endoscopic ultrasound identifies synchronous pancreas cystic lesions not seen on initial cross-sectional imaging. Pancreas. 2011;40:1070–1072.
Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 2007;133:72–79; quiz 309-310.
Kobayashi G, Fujita N, Maguchi H, et al. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: a Japan Pancreas Society multicenter study. Pancreas. 2014;43:532–538.
Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364–370.
Tanno S, Nakano Y, Nishikawa T, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57:339–343.
Zhong N, Zhang L, Takahashi N, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol 2012;10:192–198, 198 e191–192.
Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336.
Anand N, Sampath K, Wu BU: Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol 2013;11:913–921; quiz e959–960.
Walsh RM, Zuccaro G, Dumot JA, et al. Predicting success of endoscopic aspiration for suspected pancreatic cystic neoplasms. JOP. 2008;9:612–617.
Hong SK, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775–782.
Basar O, Yuksel O, Yang D, et al. The micro-forceps for pancreatic cysts a game changer. Pancreas. 2016;45:1494–1551.
Rogart JN, Loren DE, Singu BS, Kowalski TE. Cyst wall puncture and aspiration during EUS-guided fine needle aspiration may increase the diagnostic yield of mucinous cysts of the pancreas. J Clin Gastroenterol. 2011;45:164–169.
Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42:717–721.
Sakorafas GH, Sarr MG. Cystic neoplasms of the pancreas; what a clinician should know. Cancer Treat Rev. 2005;31:507–535.
Zhan XB, Wang B, Liu F, Ye XF, Jin ZD, Li ZS. Cyst fluid carcinoembryonic antigen concentration and cytology by endosonography-guided fine needle aspiration in predicting malignant pancreatic mucinous cystic neoplasms. J Dig Dis. 2013;14:191–195.
Maire F, Couvelard A, Hammel P, et al. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701–706.
Pitman MB, Genevay M, Yaeger K, et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than “positive” cytology. Cancer Cytopathol. 2010;118:434–440.
Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756–2766.
Attasaranya S, Pais S, LeBlanc J, McHenry L, Sherman S, DeWitt JM. Endoscopic ultrasound-guided fine needle aspiration and cyst fluid analysis for pancreatic cysts. JOP. 2007;8:553–563.
Hammel P, Levy P, Voitot H, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230–1235.
Moris M, Raimondo M, Woodward TA, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration cytology, carcinoembryonic antigen, and amylase in intraductal papillary mucinous neoplasm. Pancreas. 2016;45:870–875.
Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis. 2013;45:920–926.
van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389.
Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102.
Rockacy MJ, Zahid M, McGrath KM, Fasanella KE, Khalid A. Association between KRAS mutation, detected in pancreatic cyst fluid, and long-term outcomes of patients. Clin Gastroenterol Hepatol. 2013;11:425–429.
Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87.
Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136–142.
Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:9.
Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802–3808.
Maker AV, Carrara S, Jamieson NB, et al. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg. 2015;220:243–253.
Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–516.
Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799.
Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–1720.
Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83:140–148.
Shen J, Brugge WR, Dimaio CJ, Pitman MB. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer. 2009;117:217–227.
Roch AM, Ceppa EP, Al-Haddad MA, et al. The natural history of main duct-involved, mixed-type intraductal papillary mucinous neoplasm: parameters predictive of progression. Ann Surg 2014;260:680–688; discussion 688–690.
Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017;66:495–506. doi:10.1136/gutjnl-2015-310162.
Crippa S, Fernandez-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–219.
Farrell JJ, Toste P, Wu N, et al. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol. 2013;108:1352–1359.
Park WG, Wu M, Bowen R, et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest Endosc. 2013;78:e292.
Jabbar KS, Verbeke C, Hyltander AG, Sjovall H, Hansson GC, Sadik R. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J Natl Cancer Inst. 2014;106:djt439.
Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–4724.
Kwong WT, Hunt GC, Fehmi SM, et al. Low rates of malignancy and mortality in asymptomatic patients with suspected neoplastic pancreatic cysts beyond 5 years of surveillance. Clin Gastroenterol Hepatol. 2016;14:865–871.
Yamaguchi T, Hara T, Tsuyuguchi T, et al. Peroral pancreatoscopy in the diagnosis of mucin-producing tumors of the pancreas. Gastrointest Endosc. 2000;52:67–73.
Hara T, Yamaguchi T, Ishihara T, et al. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34–43.
Parsi MA, Stevens T, Bhatt A, Jang S, Vargo JJ. Digital, catheter-based single-operator cholangiopancreatoscopes: can pancreatoscopy and cholangioscopy become routine procedures? Gastroenterology. 2015;149:1689–1690.
Aparicio JR, Martinez J, Niveiro M, et al. Direct intracystic biopsy and pancreatic cystoscopy through a 19-gauge needle EUS (with videos). Gastrointest Endosc. 2010;72:1285–1288.
Nakai Y, Isayama H, Shinoura S, et al. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases. Dig Endosc. 2014;26(Suppl 1):86–94.
Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–1013.
Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–1214.
Napoleon B, Lemaistre AI, Pujol B, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26–32.
Gan SI, Thompson CC, Lauwers GY, Bounds BC, Brugge WR. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746–752.
DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664–668.
DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–723.
DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–866.
Kandula M, Moole H, Cashman M, Volmar FH, Bechtold ML, Puli SR. Success of endoscopic ultrasound-guided ethanol ablation of pancreatic cysts: a meta-analysis and systematic review. Indian J Gastroenterol. 2015;34:193–199.
Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–179.
Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–642.
Gaidhane M, Smith I, Ellen K, et al. Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of the Pancreas in a Porcine Model. Gastroenterol Res Pract. 2012;2012:431451.
Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392–401.
Kim HJ, Seo DW, Hassanuddin A, et al. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039–1043.
Di Matteo F, Martino M, Rea R, et al. EUS-guided Nd:YAG laser ablation of normal pancreatic tissue: a pilot study in a pig model. Gastrointest Endosc. 2010;72:358–363.
Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35:967–975.
Chan HH, Nishioka NS, Mino M, et al. EUS-guided photodynamic therapy of the pancreas: a pilot study. Gastrointest Endosc. 2004;59:95–99.
Yusuf TE, Matthes K, Brugge WR. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: a pilot study in a porcine model (with video). Gastrointest Endosc. 2008;67:957–961.
Di Matteo F, Martino M, Rea R, et al. US-guided application of Nd:YAG laser in porcine pancreatic tissue: an ex vivo study and numerical simulation. Gastrointest Endosc. 2013;78:750–755.
Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–580.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Rights and permissions
About this article
Cite this article
Bartel, M.J., Raimondo, M. Endoscopic Management of Pancreatic Cysts. Dig Dis Sci 62, 1808–1815 (2017). https://doi.org/10.1007/s10620-017-4544-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4544-8