Abstract
Background
Pyruvate kinase isoenzyme M2 (PKM2) is an essential enzyme involved in the regulation of aerobic glycolysis in cancer cells and promotes the translation between glycolytic flux and biosynthesis of cellular building blocks.
Aim
Our present study aims to explore the expression pattern and underlying cellular functions of PKM2 in pancreatic ductal adenocarcinoma (PDAC) under metabolic stress.
Methods
Oncomine database and a tissue microarray (n = 90) were used to investigate the expression pattern of PKM2 and its clinicopathological findings. In vitro proliferation, apoptosis and invasion assays were used to determine the role and related mechanism of PKM2 in PDAC.
Results
Data from Oncomine database and our tissue microarray show that PKM2 is significantly elevated in PDAC specimens compared with the corresponding normal tissues. Kaplan–Meier survival analysis shows that higher expression of PKM2 is closely correlated with a poor prognosis of patients with PDAC. Under metabolic stress, suppression of PKM2 expression in PANC-1 and AsPC-1 cells results in decreased cell survival, increased caspase-3/7 activity, and reduced invasive potential, and these effects can be reversed by reintroduction of PKM2. Furthermore, sh-PKM2 cells show a significant decreased Warburg effect compared with sh-Ctrl cells as demonstrated by reduced glucose consumption and lactate production. Treatment with 2-deoxy-d-glucose, a glycolysis inhibitor, completely blocks the influences of PKM2 on cell survival and invasion.
Conclusions
Our study reveals that silencing of PKM2 exhibits a tumor-suppressive role through altered Warburg effect and suggests that targeting PKM2 might serve as a potential therapeutic target for PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human malignancies worldwide with an overall 5-year survival rate of <6 % [1]. Due to the aggressive behaviors, such as abnormal growth, early local invasion, and tumor metastasis, the average survival time in PDAC patients is usually <6 months after the time of diagnosis [2]. Currently, surgical resection is the most effective treatment of PDAC. Because the majority of PDAC patients are diagnosed at advanced stage, only 10–15 % of PDAC patients are eligible for surgical resection [3, 4]. Therefore, it is of great importance to find new prognostic factors and develop novel therapeutic approaches to improve the poor outcome of patients with PDAC.
Reprogrammed glucose metabolism is a one of the hallmarks of cancer, known as Warburg effect [5]. Pyruvate kinase (PK) is a rate-limiting enzyme in executing aerobic glycolysis in tumor cells [6]. PK is composed of four isoforms: L, R, M1, and M2 [6–8]. Up-regulated expression of the M2 type of pyruvate kinase (PKM2) has been reported in multiple cancers including hepatocellular carcinoma (HCC) [9, 10], tongue cancer [11], glioma [12], colorectal cancer [13], and gallbladder cancer [14]. And high PKM2 expression is closely associated with shorter overall survival in patients with esophageal squamous cell cancer [15], tongue cancer [11], and HCC [9, 10]. PKM2 has been demonstrated to be involved in cell proliferation, survival, migration, invasion, and angiogenesis. In HCC, PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and prevents cell apoptosis via modulating Bim stability [10, 16]. In colon cancer cells, secreted PKM2 protein facilitates cell migration via PI3K/Akt and Wnt/β-catenin signaling pathway [13]. PKM2 not only controls glycolytic intermediates to divert to the biosynthesis of cellular building blocks, but also regulates gene expression to promote tumor progression [17]. For example, PKM2 promotes HCC cell proliferation through up-regulating the expression of HIF1α and Bcl-xL in culture [18]. Besides its function as a glycolytic enzyme in the cytoplasm, PKM2 also acts as a protein kinase in the nucleus [19]. It has been reported that PKM2 functions as a protein kinase in controlling the fidelity of chromosome segregation, cell-cycle progression, and tumorigenesis of tumor cells [20]. However, the possible clinical significance and underlying cellular functions of PKM2 in human PDAC remain largely unknown.
In current study, we demonstrate that PKM2 expression is up-regulated in PDAC and is closely linked to a poorer prognosis. Knockdown of PKM2 in PDAC cell lines impairs cell survival, and invasive potential under metabolic stress and reintroduction of PKM2 can completely reverse this effect. Mechanistically, the oncogenic activity of PKM2 is markedly blocked by 2-DG, a glycolysis inhibitor, indicating that PKM2-mediated cellular functions is dependent on enhanced Warburg effect. These findings provide evidence of the roles of PKM2 in PDAC and indicate that PKM2 might be a suitable therapeutic target for PDAC.
Materials and Methods
Clinical Tissue Samples
The commercial tissue microarray (HPan-Ade180Sur-01) used in this study was purchased from Shanghai Outdo Biotech Inc. The achievable clinical data of PDAC patients in this cohort are reported in Table 1. All specimens were collected between September 2004 and December 2008. The clinical stages were classified according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system. The follow-up time was calculated from the date of surgery to the date of death, or the last known follow-up. All specimens were collected in full accordance with the ethical and legal standards.
Cell Culture and Transfection
Human PDAC cells including PANC-1, MiaPaca-2, AsPC-1, SW1990, Capan-1, HPAC, and the nonmalignant hTERT-HPNE cells were all obtained from American Type Culture Collection (ATCC). Cells were cultured in indicated medium supplemented with 10 % (v/v) fetal bovine serum (FBS) and 1 % antibiotics (penicillin and streptomycin) at 37 °C in a humidified incubator under 5 % CO2 condition according to ATCC protocols. The metabolic stress was induced by Cocl2 (50 μM) and serum deprivation. 2-DG was obtained from Sigma (St. Louis, USA). All reagents were diluted to preferable concentrations in the culture medium before use. Specific shRNA targeting PKM2 and a negative control were obtained from GenePharma (Shanghai, China). A PKM2 expression plasmid was obtained from GeneCopoeia™. The transfection was performed according to the manufacturer’s protocol.
Quantitative Real-Time PCR
Total RNA was extracted from PDAC cells with Trizol reagent (Takara, Shiga, Japan) and reverse transcribed into cDNA with PrimeScript RTMasterMix (Takara, Japan). The mRNA expression of indicated genes was measured using ABI Prism 7500 Sequence Detection System with SYBR Green (Applied Biosystems, Life Technology). The RT-PCR primer sequences are as follows: PKM2, forward 5′-AAGGGTGTGAACCTTCCTGG-3′, reverse, 5′-GCTCGACCCCAAACTTCAGA-3′; β-actin, forward 5′-GCACAGAGCCTCGCCTT-3′, reverse, 5′-GTTGTCGACGACGAGCG-3′. The ΔΔC t comparative method was used to calculate the relative mRNA expression level and β-actin was used as internal control.
Immunohistochemistry
Firstly, the commercial slide was deparaffinized and rehydrated. Then neutralization of endogenous peroxidase and blocking were performed by treatment with 3 % hydrogen peroxide in methanol and 10 % (v/v) bovine serum albumin (Sangon, Shanghai), respectively. After washed with 1× phosphate-buffered saline (PBS) for three times, the slide was incubated with primary antibody (PKM2, #38237, Abcam) at 4 °C overnight. Then the sections were labeled by HRP (rabbit) second antibody for 1 h at 37 °C. Visualization was performed by 3,3′-diaminobenzidine tetrahydrochloride (DAB) and counterstained by hematoxylin. Immunoreactivity was evaluated on the basis of staining intensity. Intensity score was defined as: −, negative; +, weak; ++, moderate; and +++, strong. The cohort was divided into two groups based on the intensity score: − to +, low expression; and ++ to +++, high expression.
Western Blot
The whole cell extracts were collected in cell lysis buffer (Beyotime, China). Equal amounts of protein (20 μg) were separated by SDS-PAGE and then electrophoretically transferred onto PVDF membranes. After blocked with 5 % bovine serum albumin for 1 h at room temperature, the membranes were incubated with primary antibodies at 4 °C overnight. After washed with 1× TBST for three times, membranes were incubated with HRP-conjugated secondary antibodies (Abmart, China) and visualized using ECL Plus kit (Millipore).
Cell Survival and Apoptosis Assay
A total of 1 × 104 cells with indicated gene types were seeded into a 96-well plate per well in the presence of 50 μM CoCl2. After incubation for 48 h under serum deprivation, cell viability and caspase-3/7 activity were measured. Cell viability was evaluated by Cell Counting Kit-8 (CCK-8, Dojindo, Japan) following the manufacturer’s protocols, and the absorbance was measured at 450 nm using a Multifunctional Microplate Reader (Tecan). The caspase-3/7 activity in each group was detected according to manufacturer’s instructions (Promega).
Cell Invasion Assay
The invasive potential of PANC-1 or AsPC-1 cells was measured by transwell model (Corning, NY, USA) according to the manufacturer’s instructions. Briefly, 3 × 104 cells in 100 μl medium were seeded into the upper chamber of matrigel-coated transwell inserts (BD Bioscience, USA). The lower chambers were supplemented with 600 μl of RPMI-1640 or DMEM medium containing 2 % FBS. After incubation under metabolic stress for 48 h, the non-invading cells that remained on the upper surface were removed. The invaded cells were fixed with 4 % paraformaldehyde and stained with 0.1 % crystal violet. The number of invaded cells was counted under a light microscope in six random fields. Each experiment was performed at least in triplicate.
Measurement of Glucose Utilization and Lactate Production
PANC-1 or AsPC-1 cells were seeded in fresh phenol red-free medium and the culture medium was collected in the first 24 h after indicated treatment. All the treatment in PANC-1 or AsPC-1 cells was based on the metabolic stress condition. The glucose and lactate levels were detected by glucose assay kits (Life technologies) or lactate assay kits (Biovision) according to manufacturer’s instructions, respectively. The value of each well was normalized to the amounts of protein.
Statistical Analysis
Data were presented as the means ± standard deviations (SDs). Statistical analyses and graphical representations were performed with SPSS 16.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 5 (San Diego, CA, USA) software. Overall survival rate was calculated according to the Kaplan–Meier method and the difference in survival curves was evaluated by the log-rank test. The Student’s t test was used to determine the statistically significant differences among indicated experimental results. p < 0.05 was considered as significant.
Results
Up-Regulated PKM2 Predicts Poor Prognosis in PDAC
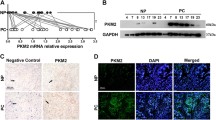
To observe the expression pattern of PKM2 in PDAC, we first analyzed the differential expression of PKM2 mRNA between PDAC tissues and normal pancreas tissues by Oncomine microarray gene expression datasets. We found that PKM2 was significantly up-regulated in PDAC tissues compared with normal pancreas using Logsdon pancreas dataset (Fig. 1a) and Pei pancreas dataset (Fig. 1b). Similar result was also observed in 39 paired PDAC tumor and non-tumor tissues using Badea pancreas dataset (Fig. 1c). Consistent with this finding from Oncomine database, PKM2 was also over-expressed in all the PDAC cell lines detected compared with the non-malignant hTERT-HPNE cells (Fig. 1d). To further illustrate the underlying roles in PDAC, we analyzed the clinical significance of PKM2 using a tissue microarray with corresponding patients’ parameters (Fig. 1e). As shown in Table 1, elevated PKM2 expression in PDAC tissues was significantly associated with tumor size (p = 0.010), tumor location (p = 0.016), and TNM stage (p = 0.004), whereas no significant correlations were observed between PKM2 expression and age, gender, neuronal invasion, and histological grade. Meanwhile, PDAC patients with higher PKM2 expression had remarkably shorter survival time than those with lower PKM2 expression (p = 0.0043, Fig. 1f). Collectively, these data above indicate that up-regulated PKM2 predicts poor prognosis in PDAC.
Up-regulated PKM2 predicts poor prognosis in PDAC. a PKM2 was upregulated in PDAC tissues comparing with the normal pancreatic tissues revealed using the Logsdon pancreas dataset from Oncomine database. b Elevated PKM2 expression in PDAC tissues revealed by Pei pancreas dataset. c PKM2 is upregulated in PDAC tissues comparing with their normal counterparts revealed using the Badea pancreas. d PKM2 mRNA expression in 6 PDAC cell lines and the non-malignant hTERT-HPNE cells. e Representative images of PKM2 immunoreactivity in PDAC tissues. f Kaplan–Meier curves for PDAC patients grouped based on PKM2 expression
PKM2 Contributes to Cell Survival and Invasion Under Metabolic Stress
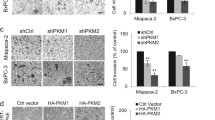
PDAC is histologically characterized by intense desmoplasia and poor vascular, which constitutes a hypoxic microenvironment. To simulate this type of metabolic stress in PDAC, we cultured PDAC cells with 50 μM CoCl2 in the presence of serum deprivation. Because up-regulated PKM2 expression is closely associated with tumor size and TNM stage in patients with PDAC (Table 1), we hypothesized whether elevated PKM2 contributes to tumor progression under metabolic stress in PDAC. To confirm this hypothesis, we first silenced PKM2 expression in PANC-1 and AsPC-1 cells. As shown in Fig. 2a, sh-PKM2 markedly reduced PKM2 expression in PANC-1 and AsPC-1 cells. By comparison, in sh-Ctrl and sh-PKM2 PANC-1 cells, the OD450 value was 0.99 ± 0.08 and 0.67 ± 0.07, respectively; similar result was also observed in AsPC-1 cells (Fig. 2b). Meanwhile, suppression of PKM2 also remarkably promoted cell apoptosis as demonstrated by enhanced caspase-3/7 activity, indicating that PKM2 exhibits a critical role in cell apoptosis under metabolic stress (Fig. 2c). To further analyze the potential roles of PKM2 on cell invasion, we performed Transwell assay. Indeed, cell invasion ability of sh-PKM2 cells was significantly decreased compared with sh-Ctrl cells (Fig. 2d). And surprisingly, all these effects induced by suppression of PKM2 can be completely blocked by reintroduction of PKM2, indicating that endogenous PKM2 favors cell survival and invasion under metabolic stress in PDAC (Fig. 2b–d).
PKM2 contributes to cell survival and invasion under metabolic stress. a Knockdown and reintroduction of PKM2 were confirmed by Western blotting. The cell viability (b) caspase-3/7 activity (c) and invasion ability (d) of PANC-1 or AsPC-1 cells under metabolic stress were measured at 48 h after knockdown and reintroduction of PKM2. sh-Ctrl vs sh-PKM2, *p < 0.05; **p < 0.01
PKM2-Mediated Cellular Functions Are Dependent on Enhanced Warburg Effect
Because PKM2 is an essential enzyme in aerobic glycolysis (Warburg effect), which characterizes by glucose utilization and lactate production, we analyzed the glucose and lactate level in the culture medium of PANC-1 and AsPC-1 cells. Indeed, both glucose utilization (Fig. 3a) and lactate production (Fig. 3b) were significantly reduced in sh-PKM2 of PANC-1 and AsPC-1 cells compared with the sh-Ctrl cells. Reintroduction of PKM2 expression can completely restore the Warburg effect to normal level in PANC-1 and AsPC-1 cells (Fig. 3a, b). To demonstrate whether the cellular functions of PKM2 are associated with Warburg effect, we determined cell survival and invasion assay in the presence of 2-DG, a glycolysis inhibitor. The result showed that no significant differences in cell viability (Fig. 3c) and invasive potential (Fig. 3d) among sh-Ctrl cells, sh-PKM2 cells, and sh-PKM2 + pcDNA3.1-PKM2 cells were observed upon 2-DG treatment. Taken together, these data above indicate that the oncogenic activities of PKM2 in PANC-1 and AsPC-1 cells are mediated by enhanced Warburg effect.
PKM2-mediated cellular functions are dependent on enhanced Warburg effect. Glucose utilization (a) and lactate production (b) of PANC-1 or AsPC-1 were measured at 24 h after knockdown and reintroduction of PKM2. In the presence of 2-DG, cell viability (c) and invasion ability (d) of PANC-1 or AsPC-1 cells under metabolic stress were measured after knockdown and reintroduction of PKM2. sh-Ctrl versus sh-PKM2, *p < 0.05
Discussion
In the present study, we determined the clinical significance and cellular functions of PKM2 in PDAC. The results showed that up-regulated PKM2 promotes tumor progression under metabolic stress by regulation of aerobic glycolysis. First, we found that PKM2 was up-regulated in PDAC specimens and closely associated with the prognosis of PDAC patients. Second, suppression of PKM2 exhibited a tumor-suppressive role, whereas reintroduction of PKM2 did the opposite. At last, we demonstrated that PKM2-mediated oncogenic activities were dependent on enhanced Warburg effect. Thus, dysregulated PKM2 signaling contributed to the enhanced Warburg effect and ultimately to the development and progression of PDAC cells.
Previous studies have demonstrated that PKM2 is mainly expressed in all proliferating cells (embryonic cells and adult stem cell), especially cancer cells [21–23]. Consistent with the observations from other groups [11, 15, 16, 24, 25], PKM2 is significantly up-regulated in PDAC. Apart from its functions as a glycolytic enzyme, PKM2 has also been reported to translocate to the nucleus under specific conditions [17]. We found that PKM2 immunoreactivity was mainly distributed in the cytoplasm but not the nucleus, indicating that the metabolic function of PKM2 is critical for its roles in PDAC. And in line with previous reports focused on the prognostic value of PKM2 in tumors [10, 11, 16, 25], we confirmed that elevated PKM2 expression was associated with tumor size and TNM stage as well as a poor prognosis, indicating the importance of PKM2 in evaluating the prognosis of PDAC patients.
PKM2 has been demonstrated to act as an oncogene in many types of cancers [26–29]. In PDAC, it has been demonstrated that PKM2 has an important role in the proliferation, invasion, migration, and apoptosis of PANC-1 and SW1990 pancreatic cancer cells, which may be associated with the expression of ERK1/2 and p38 of the MAPK signaling cascade [30]. Different form the experimental conditions in this study, we mimicked the undernourished microenvironment of PDAC by treatment with CoCl2 and serum deprivation in current study. And consistent with previous reports in HCC and colorectal cancer [10, 13, 16], suppression of PKM2 in PDAC cells under metabolic stress also resulted in an antitumor effect. In brain tumor cells, depletion of PKM2 expression largely inhibited Warburg effect and tumor growth [31]. Similar findings were observed in our study. By treatment with 2-DG in PANC-1 and AsPC-1 cells under metabolic stress, we confirmed that PKM2-mediated oncogenic functions in PDAC cells were closely correlated with enhanced Warburg effect. However, the clear mechanisms involved in PKM2-mediated cell survival and invasion under metabolic stress warrant further investigation.
Taken together, we demonstrated the expression pattern and clinical significance of PKM2 in PDAC and revealed that elevated PKM2 favors PDAC cell survival and invasion under metabolic stress condition. Our study propose that PKM2 is a valuable prognostic factor in PDAC and the metabolic PKM2-related signaling might be a potential cascade in PDAC progression.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620.
Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview Nature reviews. Gastroenterol Hepatol. 2009;6:699–708.
Hartwig W, Werner J, Jager D, Debus J, Buchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476–e485.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
Gruning NM, Rinnerthaler M, Bluemlein K, et al. Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab. 2011;14:415–427.
Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261:13807–13812.
Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987;262:14366–14371.
Chen Z, Lu X, Wang Z, et al. Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget. 2015;6:2538–2548.
Liu WR, Tian MX, Yang LX, et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget. 2015;6:846–861.
Yuan C, Li Z, Wang Y, et al. Overexpression of metabolic markers PKM2 and LDH5 correlates with aggressive clinicopathological features and adverse patient prognosis in tongue cancer. Histopathology. 2014;65:595–605.
Yang W, Xia Y, Ji H, et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122.
Yang P, Li Z, Wang Y, Zhang L, Wu H, Li Z. Secreted pyruvate kinase M2 facilitates cell migration via PI3K/Akt and Wnt/beta-catenin pathway in colon cancer cells. Biochem Biophys Res Commun. 2015;459:327–332.
Li J, Yang Z, Zou Q, et al. PKM2 and ACVR 1C are prognostic markers for poor prognosis of gallbladder cancer. Clin Transl Oncol. 2014;16:200–207.
Zhan C, Shi Y, Lu C, Wang Q. Pyruvate kinase M2 is highly correlated with the differentiation and the prognosis of esophageal squamous cell cancer. Dis Esophagus. 2013;26:746–753.
Hu W, Lu SX, Li M, et al. Pyruvate kinase M2 prevents apoptosis via modulating Bim stability and associates with poor outcome in hepatocellular carcinoma. Oncotarget. 2015;6:6570–6583.
Yang W, Lu Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett. 2013;339:153–158.
Dong T, Yan Y, Chai H, et al. Pyruvate kinase M2 affects liver cancer cell behavior through up-regulation of HIF-1alpha and Bcl-xL in culture. Biomed Pharmacother. 2015;69:277–284.
Wong N, Ojo D, Yan J, Tang D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015;356:184–191.
Jiang Y, Li X, Yang W, et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. 2014;53:75–87.
Gali P, Bourdin M. Ontogenesis of pyruvate kinase in the brain and liver tissues of the rat. Biochimie. 1978;60:1253–1260.
Reinacher M, Eigenbrodt E. Immunohistological demonstration of the same type of pyruvate kinase isoenzyme (M2-Pk) in tumors of chicken and rat. Virchows Archiv B Cell Pathol Incl Mol Pathol. 1981;37:79–88.
Max-Audit I, Testa U, Kechemir D, Titeux M, Vainchenker W, Rosa R. Pattern of pyruvate kinase isozymes in erythroleukemia cell lines and in normal human erythroblasts. Blood. 1984;64:930–936.
Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23:560–566.
Zhao Y, Shen L, Chen X, et al. High expression of PKM2 as a poor prognosis indicator is associated with radiation resistance in cervical cancer. Histol Histopathol. 2015;30:1313–1320.
Chen L, Shi Y, Liu S, Cao Y, Wang X, Tao Y. PKM2: the thread linking energy metabolism reprogramming with epigenetics in cancer. Int J Mol Sci. 2014;15:11435–11445.
Chiavarina B, Whitaker-Menezes D, Martinez-Outschoorn UE, et al. Pyruvate kinase expression (PKM1 and PKM2) in cancer-associated fibroblasts drives stromal nutrient production and tumor growth. Cancer Biol Ther. 2011;12:1101–1113.
Macintyre AN, Rathmell JC. PKM2 and the tricky balance of growth and energy in cancer. Mol Cell. 2011;42:713–714.
Papadaki C, Sfakianaki M, Lagoudaki E, et al. PKM2 as a biomarker for chemosensitivity to front-line platinum-based chemotherapy in patients with metastatic non-small-cell lung cancer. Br J Cancer. 2014;111:1757–1764.
Feng J, Ma T, Ge Z, et al. PKM2 gene regulates the behavior of pancreatic cancer cells via mitogen-activated protein kinase pathways. Mol Med Rep. 2015;11:2111–2117.
VanderHeiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Rights and permissions
About this article
Cite this article
Li, C., Zhao, Z., Zhou, Z. et al. PKM2 Promotes Cell Survival and Invasion Under Metabolic Stress by Enhancing Warburg Effect in Pancreatic Ductal Adenocarcinoma. Dig Dis Sci 61, 767–773 (2016). https://doi.org/10.1007/s10620-015-3931-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3931-2