Abstract
Background/Aim
How to prevent the small intestinal damage induced by NSAIDs is an urgent issue to be resolved. In the present study, we examined the effects of soluble dietary fibers on both anti-inflammatory and ulcerogenic effects of indomethacin in arthritic rats.
Methods
Male Wistar rats weighing 180–220 g were used. Arthritis was induced by injecting Freund’s complete adjuvant (killed M. tuberculosis) into the plantar region of the right hindpaw. The animals were fed a regular powder diet for rats or a diet supplemented with soluble dietary fibers such as pectin or guar gum. Indomethacin was administered once a day for 3 days starting 14 days after the adjuvant injection, when marked arthritis was observed. The volumes of the hindpaw were measured before and after indomethacin treatment to evaluate the effect of indomethacin on edema. The lesions in the small intestine were examined 24 h after the final dosing of indomethacin.
Results
Hindpaw volume was increased about 3 times 14 days after injection of the adjuvant. Indomethacin (3–10 mg/kg, p.o.) decreased hindpaw volume dose-dependently, but caused severe lesions in the small intestine at doses of 6 and 10 mg/kg. The addition of pectin (1–10 %) or guar gum (10 %) to the diet markedly decreased the lesion formation without affecting the anti-edema action of indomethacin. The same effects of pectin were observed when indomethacin was administered subcutaneously.
Conclusions
It is suggested that soluble dietary fibers can prevent intestinal damage induced by NSAIDs without affecting the anti-inflammatory effect of these agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent progress in endoscopies such as capsule endoscopy and double-balloon endoscopy has revealed that nonsteroidal anti-inflammatory drugs (NSAIDs) [1–4] and aspirin [5–7] often cause mucosal damage, not only in the stomach and duodenum, but also in the small intestine, and the intestinal lesions are more common than previously thought. It has been reported that some probiotics and anti-ulcer agents protected the small intestinal mucosa against NSAIDs in pilot clinical studies in healthy volunteers [8–10] and experimental animals [11–14]. However, at present, effective therapies for the prevention of intestinal damage are not available in patients. Therefore, how to manage NSAID-/aspirin-induced small intestinal damage remains an urgent issue [15]. In previous studies, we found that soluble dietary fibers (DFs) such as pectin and guar gum could prevent the adverse effects of NSAIDs on the small intestine in cats [16, 17]. However, there are few reports about whether soluble DFs affect the main pharmacological action of NSAIDs, such as anti-inflammatory action, or not.
On the other hand, it has been reported that patients with rheumatoid arthritis are more susceptible to NSAID-induced gastric damage than other NSAID users [18]. This is a very important problem for patients because they cannot avoid using NSAIDs. Kato et al. [19, 20] have reported that NSAIDs caused more severe lesions in the gastrointestinal mucosa in rats associated with adjuvant-induced arthritis (a rat model of rheumatoid arthritis) than those in normal rats. Therefore, in the present study, we firstly examined the effect of arthritis on indomethacin-induced small intestinal lesions and secondly examined the effect of soluble DFs on the anti-inflammatory action and ulcerogenic effect of indomethacin in the arthritic rats. The results showed that arthritis aggravated the intestinal damage and that soluble DFs were able to prevent the intestinal damage aggravated by arthritis without affecting the anti-inflammatory action of indomethacin.
Materials and Methods
Ethics Approval
Experimental protocols were approved by the Animal Research Committee at the Faculty of Agriculture, Tottori University, Tottori, Japan.
Animals
Seven-week-old male Wistar rats (Shimizu Laboratories, Shizuoka, Japan) weighing 180–220 g were used.
Diets
The animals were usually given a regular powder diet (RPD) for rats (CE-2; Clea Japan, Osaka, Japan). In experiments examining the effect of DFs on the formation of intestinal lesions and the anti-inflammatory activity of indomethacin, a dietary-fiber-free diet (FFD) (F2LCP; Oriental Bioservice, Kyoto, Japan), the FFD supplemented with insoluble DF (α-cellulose; Sigma, St. Louis, Missouri, USA), or the RPD supplemented with soluble DFs (pectin and guar gum; Wako, Osaka, Japan) was used (Table 1).
Composition of diets: RPD contained 24.9 % crude protein, 4.4 % crude fat, 4.1 % crude fiber, 6.9 % crude ash, 2.5 % minerals, and 51 % soluble non-N materials such as starch and sucrose. FFD (F2LCP; Oriental Bioservice) contained 19.6 % protein, 17.1 % fat, 4.6 % minerals, 2.3 % vitamins, and 53.8 % sucrose.
Drugs
The following drugs and chemicals were used: carboxymethylcellulose (CMC), Freund’s complete adjuvant (FCA; killed M. tuberculosis, H37Ra), indomethacin, and ether (Wako, Osaka, Japan). Drugs for oral or subcutaneous administration were suspended in a 1 % CMC solution. The drugs were prepared just prior to the experiments and administered at a volume of 0.2 mL/100 g body wt.
Induction and Measurement of Small Intestinal Damage
Indomethacin (3, 6, or 10 mg/kg) was administered orally or subcutaneously once or once daily for 3 days in unfasted rats. The animals were killed by ether overdose 24 h after the final dose of indomethacin. The small intestines were spread out on paper and opened along the anti-mesenteric side, and the contents were removed. The length (mm) of the individual lesions was measured under a dissecting microscope with a 1-mm square grid eyepiece (×10), and the sum of the lengths of all of the lesions in each intestine was used as the lesion index. The individual measuring the lesions did not know the treatments given to the animals.
Induction of Arthritis by FCA and Evaluation of Inflammation (Edema)
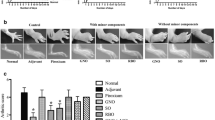
Arthritis in rats was induced by the method described by Winter and Nuss [21]. Briefly, 50 μl of Freund’s complete adjuvant (FCA; 10 mg/ml killed M. tuberculosis, suspended in paraffin oil) was injected into the plantar region of the right hindpaw under ether anesthesia. Inflammation was estimated by measuring the change of volume of the paw in both hindlegs according to the method of Winter and Nuss [21] (Fig. 1a).
Adjuvant-induced arthritis in rats. Fifty microliters of FCA was injected into the plantar region of the right hindpaw under ether anesthesia. A Measurement of inflammation (edema) of a hindpaw. A hindleg was dipped into the tube filled with water containing 1 % detergent until the edge of the bristle. The weight of fluid overflowing from the tube was measured and used to determine the volume of the paw. B Photograph of the hindlegs 14 days after injection of FCA. Color of the legs becomes reddish, and edema is observed in both left (mild) and right (severe) legs. C Photograph of small intestine 24 h after the administration of indomethacin (10 mg/kg, p.o.). Many lesions are observed in the mucosa
Statistics
All data are expressed as mean ± SEM. Differences between groups were analyzed by Student’s t test for paired group comparisons, or analysis of variance (Dunnett’s multiple range test) if more than 2 variables were considered, with the significance level set at P < 0.05.
Results
Time Course of Adjuvant Arthritis
The injection of FCA into the right hindpaw caused acute inflammation (severe edema, reddish appearance, and hyperthermia) in the paw topically. The edema peaked within 3 days (primary phase), and at 10 days or later it gradually increased again, and another peak value was observed 21 days later (secondary phase) (Fig. 2). The volume of the left hindpaw did not change during the first 7 days, but then gradually increased and peaked at 21 days (Fig. 2). Figure 1b shows the reddish edema of both hindpaws 14 days after the injection of FCA. At day 21, edema, a reddish appearance, and skin ulcers were observed systematically in the forepaws, tail, ears, and nose.
Time-dependent changes in the volume of hindpaws after the injection of FCA. FCA was injected into the plantar region of the right hindpaw at day 0, and the volumes of both hindpaws were measured at various days after the injection of FCA until day 28. Volumes of the right (open circle) and left (filled circle) paws at various days are expressed as the % of volume at day 0 regarded as 100 %. Data show the mean values and the SEs of six rats
Effect of FCA Injection on Small Intestinal Damage Induced by Indomethacin
Indomethacin (10 mg/kg) was administered orally once before or 3, 7, 14, or 28 days after the injection of FCA or vehicle (50 μl of liquid paraffin). The gastrointestinal lesions were examined 24 h after the administration of indomethacin. Under the applied conditions, indomethacin caused few lesions in the stomach, but many lesions in the middle and lower parts of the small intestine (Fig. 1c). The lesion index of the small intestine in the normal rats administered indomethacin was 84.2 ± 19.7 mm (n = 6). As shown in Fig. 3A, the lesion indices in FCA-treated rats were larger than those in the vehicle group. The lesion indices of the FCA group were significantly (P < 0.05) larger than those of the vehicle group at days 14 and 28 (Fig. 3A).
Effect of FCA on the formation of small intestinal ulcers induced by indomethacin. A Time-dependent changes in the ulcerogenic effect of indomethacin. FCA (filled square) or vehicle (open square) was injected into the plantar region of the right hindpaw at day 0. Indomethacin (10 mg/kg, p.o.) was administered at various days after the injection of FCA until day 28. B Dose-related response of ulcerogenic effect of indomethacin. Indomethacin (3, 6, and 10 mg/kg, p.o.) was administered at 14 days after FCA injection. The intestinal lesions were measured 24 h after the administration of indomethacin. Data show the mean values and the SEs of 6–8 rats. a: P < 0.05 versus vehicle
The effect of FCA on the lesion formation at doses of 3, 6, and 10 mg/kg indomethacin was examined at day 14. In the group given vehicle, indomethacin at 3 mg/kg did not cause any lesions, but caused lesions at 6 and 10 mg/kg dose-dependently; that is, the lesion indices were 8.2 ± 6.0 mm (n = 6) and 100.0 ± 26.4 mm (n = 8), respectively. The administration of FCA alone did not cause any lesions in the small intestine, but it markedly increased the lesions induced by indomethacin; that is, the lesion indices at doses of 6 and 10 mg/kg were 111.3 ± 39.0 mm (n = 6, P < 0.05 vs. vehicle) and 242.2 ± 42.9 mm (n = 8, P < 0.05 vs. vehicle), respectively (Fig. 3B).
Effects of FFD on Ulcerogenic and Anti-inflammatory Actions of Indomethacin in Arthritic Rats
To determine the role of dietary fiber in the formation of intestinal lesions by indomethacin, rats were given RPD or FFD starting 2 days before the administration of indomethacin until the end of the experiment. Indomethacin (3–10 mg/kg) was given orally once daily for 3 days starting at 14 days after the injection of FCA.
Indomethacin at a dose of 3 mg/kg did not cause any lesions in the small intestine in both groups given RPD or FFD. However, indomethacin at doses of 6 and 10 mg/kg caused many severe lesions in the group given RPD; that is, the lesion indices were 176.2 ± 32.0 mm (n = 6) and 194.3 ± 26.9 mm (n = 8), respectively. However, in the group given FFD, the ulcerogenic action of indomethacin was markedly decreased; that is, the lesion indices at doses of 6 and 10 mg/kg were 2.0 ± 2.0 mm (n = 6, P < 0.01 vs. RPD) and 12.1 ± 3.9 mm (n = 7, P < 0.01 vs. RPD), respectively (Fig. 4A).
Effect of FFD on ulcerogenic and anti-inflammatory effects of indomethacin in arthritic rats. The rats fed RPD or FFD from 12 days after the injection of FCA. Indomethacin (3, 6, and 10 mg/kg, p.o.) was administered once daily for 3 days starting at day 14. The intestinal lesions (A) and the volume of the paw (B) were measured 24 h after the final doses of indomethacin (day 17). Data show the mean values and the SEs of 6–8 rats. a: P < 0.05, b: P < 0.01 versus RPD with indomethacin (A) and with vehicle (B)
The volume of the hindpaw increased slightly during 3 days of treatment with vehicle (1 % CMC instead of indomethacin) in the group given RPD; that is, the volume of the right hindpaw was 113.6 ± 7.9 % (n = 6). Indomethacin (3–10 mg/kg) decreased the paw volume dose-dependently, and the peak effects were observed at a dose of 6 mg/kg in both RPD and FFD groups (Fig. 4B). The effects at 3 mg/kg were almost the same and significant (P < 0.05 vs. vehicle) in both groups, but those at 6 and 10 mg/kg were weak in the group given FFD (Fig. 4B). The paw volumes at a dose of 6 mg/kg of indomethacin in both groups were 70.0 ± 7.0 % (n = 6, P < 0.01 vs. vehicle) and 84.5 ± 2.6 % (n = 8, P < 0.01 vs. vehicle), respectively.
Effects of Cellulose on Ulcerogenic and Anti-inflammatory Actions of Indomethacin in Arthritic Rats
To determine the role of insoluble DF in the formation of intestinal lesions by indomethacin, rats were given RPD, FFD, or FFD supplemented with cellulose. Indomethacin (10 mg/kg) was given orally once daily for 3 days starting at 14 days after the injection of FCA.
As shown in the previous section, FFD markedly decreased the lesions caused by indomethacin. However, the addition of cellulose (3 or 10 %) to the FFD increased the lesion index dose-dependently (Fig. 5A). The lesion index in the 10 % cellulose group was 98.6 ± 11.9 mm (n = 7), and it was significantly (P < 0.05) greater than that of FFD alone.
Effect of cellulose on ulcerogenic and anti-inflammatory effects and changes in hematocrit levels caused by indomethacin in arthritic rats. The rats fed RPD alone or FFD with or without cellulose (3 and 10 %) from 12 days after the injection of FCA. Indomethacin (10 mg/kg, p.o.) was administered once daily for 3 days starting at day 14. The intestinal lesions (A), the paw volume (B), and the hematocrit levels (C) were measured 24 h after the final dose of indomethacin (day 17). Data show the mean values and the SEs of 6–8 rats. a: P < 0.05, b: P < 0.01 versus RPD with indomethacin (A) and with vehicle (B, C)
The addition of cellulose (3 and 10 %) to the FFD concentration-dependently increased the anti-inflammatory action of indomethacin (Fig. 5B).
The level of hematocrit in the vehicle group was 40.9 ± 0.6 % (n = 7). Indomethacin at 10 mg/kg markedly decreased the level in the group given RPD to 32.0 ± 3.1 % (n = 8, P < 0.01 vs. vehicle). In the group given FFD, the decrease in the hematocrit level by indomethacin was mild; that is, the level was 38.3 ± 0.9 % (n = 7, P < 0.05 vs. RPD). The addition of cellulose (3 and 10 %) to the FFD decreased the level again concentration-dependently, and the level at 10 % was 31.3 ± 1.3 % (n = 7, P < 0.01 vs. vehicle) (Fig. 5C).
Effects of Pectin and Guar Gum on Ulcerogenic and Anti-inflammatory Actions of Indomethacin in Arthritic Rats
To determine the effect of soluble DFs in the formation of intestinal damage by indomethacin, pectin (1–10 %) or guar gum (10 %) was added to the RPD. Indomethacin (10 mg/kg) was given orally once a day for 3 days starting at 14 days after the injection of the FCA. Pectin decreased the lesion index concentration-dependently, and the effects of 3 and 10 % were significant (P < 0.05 and 0.01 vs. RPD alone) (Fig. 6A). Guar gum (10 %) also significantly (P < 0.01 vs. RPD alone) decreased the intestinal lesions. The addition of pectin or guar gum to the RPD did not affect the anti-inflammatory action of indomethacin (Fig. 6B).
Effect of pectin and guar gum on the ulcerogenic and anti-inflammatory effects of indomethacin in arthritic rats. The rats fed RPD alone or RPD supplemented with pectin (PEC, 1–10 %) or guar gum (GG, 10 %) from 12 days after the administration of FCA. Indomethacin (10 mg/kg) was administered orally once daily for 3 days starting at day 14. The intestinal lesions and the paw volume were measured 24 h after the final dose of indomethacin (day 17). Data show the mean values and the SEs of 6–8 rats. a: P < 0.05, b: P < 0.01 versus RPD with indomethacin (A) and with vehicle (B)
The effects of pectin on the ulcerogenic and anti-inflammatory actions of indomethacin administered subcutaneously were examined in arthritic rats. Indomethacin (10 mg/kg) was given subcutaneously once a day for 3 days starting at 14 days after the injection of the FCA. As shown in Fig. 7A, B, similar results to those of oral indomethacin were observed; namely, pectin (10 %) added to the RPD significantly prevented the intestinal lesion formation (P < 0.05 vs. RPD), but did not affect the decrease in paw volume by indomethacin (P < 0.01 vs. RPD).
Effect of pectin on ulcerogenic and anti-inflammatory effects and decrease in body weight caused by indomethacin given subcutaneously in arthritic rats. The rats fed RPD alone or RPD supplemented with pectin (PEC, 10 %) from 12 days after the administration of FCA. Indomethacin (IND, 10 mg/kg) was administered subcutaneously once daily for 3 days starting at day 14. The intestinal lesions (A) and the paw volume (B) were measured 24 h after the final dose of indomethacin (day 17), and body weight (C) was measured every morning at 10 a.m. Data show the mean values and the SEs of 6–8 rats. a: P < 0.05, b: P < 0.01 versus RPD with indomethacin (A) and with vehicle (B), and versus control (C)
The body weight in the vehicle group slightly increased during 3 days of treatments. Indomethacin (10 mg/kg, s.c.) gradually decreased the body weight in the group given RPD alone, and the effects were significant (P < 0.01) after 2 and 3 doses (Fig. 7C). However, in the group given RPD supplemented with pectin (10 %), indomethacin did not cause a decrease in body weight during 3 days of treatment (Fig. 7C).
Discussion
Kato et al. [19, 20] have reported that NSAIDs produced more GI lesions in arthritis rats than those in normal rats, supporting the findings of Fries et al. [18] showing that rheumatoid arthritis patients are more sensitive to gastric damage from NSAIDs than other patients. In the present study, we firstly examined the effect of arthritis on indomethacin-induced intestinal damage. The results showed that the lesion indices of indomethacin-induced intestinal lesions in the arthritic rats were larger than those in the rats treated with vehicle, and the effect was significant when indomethacin was administered 14 or 28 days after the injection of adjuvant, when arthritis became marked, confirming the findings of Kato et al. [20] in the small intestine.
Small intestinal damage caused by NSAIDs/aspirin is more common in patients taking the drugs for more than 3 months than previously thought [1–7]. However, effective therapy for this intestinal damage is not available at present, although several possibilities have been proposed [8–14]. In previous studies, we found that soluble DFs such as pectin and guar gum could prevent the intestinal damage induced by NSAIDs/aspirin in cats [16, 17]. In the present study, we examined the effects of soluble DFs on both anti-inflammatory (anti-edema) action and ulcerogenic effect of indomethacin using an arthritic rat model. Indomethacin (6 and 10 mg/kg, p.o.) given once daily for 3 days starting at 14 days after adjuvant injection significantly decreased the edema (anti-inflammatory action), but produced severe lesions in the small intestine in the rats given a regular powder diet (RPD). Pectin (1–10 %) added to the diet markedly prevented the formation of small intestinal lesions concentration-dependently, but it did not affect the anti-inflammatory action of indomethacin (10 mg/kg, p.o.), even at the highest concentration of 10 %. Similar results were obtained when guar gum (10 %) was added to the diet. These results suggest that soluble DFs can prevent the intestinal side effects of NSAIDs without affecting their anti-inflammatory action, indicating the usefulness of soluble DFs for the treatment of small intestinal damage in NSAID/aspirin users including rheumatoid arthritis patients.
We previously reported that insoluble DFs, in contrast to soluble DFs, play an important role in the formation of NSAID-induced intestinal lesions in normal rats and cats [22, 23]. In the present study, we examined this possibility in arthritic rats. As mentioned above, indomethacin produced severe lesions in the small intestine in the rats given a regular powder diet (RPD) containing crude DF at a concentration of 4.1 %. In the rats given a fiber-free diet (FFD), the intestinal lesions due to indomethacin were markedly decreased, but in the rats given FFD supplemented with cellulose (1–10 %), indomethacin again produced intestinal lesions in a concentration-dependent manner. These results correspond with the previous findings that insoluble DFs play an important role in the formation of intestinal ulcers in rats and cats and also support the idea that the intestinal ulcers are initiated by rubbing of the mucosa with insoluble DFs under the conditions of decreased mucus and increased intestinal motility caused by NSAIDs [16, 23, 24].
On the other hand, the anti-edema action of indomethacin was mildly decreased in the rats given the FFD compared with that in the rats given the regular powder diet (RPD). These results are explained as follows: In the group given the regular diet, indomethacin produced severe lesions in the small intestine, resulting in a decrease in body fluid due to intestinal bleeding. This might have caused a decrease in edema in the paw and enhanced the apparent action of indomethacin on the edema. This is supported by the facts that the hematocrit level in the group given FFD and indomethacin was significantly higher than that in the animals given the regular diet and indomethacin, and the addition of cellulose to the FFD again caused intestinal lesions by indomethacin and a decrease in the hematocrit level with an increase in anti-inflammatory action.
We have proposed that multiple factors, such as intestinal hypermotility, decreased mucus secretion, enterobacteria, bile acid, and indigestive solid components of food are involved in the pathogenesis of NSAID-induced small intestinal damage [15, 24, 25]. Several possibilities are considered as mechanisms by which soluble DFs prevented the intestinal damage induced by indomethacin, namely the effect of soluble DFs on intestinal motility, mucus, enterobacteria, bile acid, and the intestinal absorption of indomethacin, among others. Previously, we reported that pectin prevented indomethacin-induced intestinal damage in cats, but it neither affected the absorption of indomethacin from the gastrointestinal tract nor inhibited the intestinal hypermotility caused by indomethacin [16]. In the present study, pectin prevented the formation of intestinal lesions induced by indomethacin administered subcutaneously. These results rule out the possibility that pectin prevented the formation of intestinal damage by affecting the absorption of indomethacin or by inhibiting the intestinal motility. We suggested in a study in cats that both soluble DFs and mucin from pig stomach protected the small intestine against NSAID-induced damage by compensating for a decrease in barrier function caused by NSAIDs due to their viscous nature because a good correlation was found between the viscosities of mucin and the soluble DFs (guar gum, pectin, and polydextrose) and the protective activities of these substances on the intestinal mucosa [16]. This may partly explain the protective activity of pectin on the intestinal mucosa in the present study in arthritic rats. In addition, in the present study, pectin prevented the decrease in body weight induced by repeated administration of indomethacin for 3 days. These results strongly support the usefulness of soluble DFs as a new therapy for NSAID-induced intestinal damage.
We have reported that pectin prevented the small intestinal damage induced not only by NSAIDs but also by enteric-coated aspirin in cats [16, 17]. These findings and the results of the present study show that soluble DFs such as pectin and guar gum can prevent the intestinal lesions caused by NSAIDs/aspirin without affecting the anti-inflammatory action of these agents. In this context, the question arises of what level of soluble DFs is necessary to protect the small intestine against NSAIDs/aspirin. It is very difficult to answer this question because it is well known that food materials contain different amounts of soluble and insoluble DFs, and each individual takes different kinds of meals every day. However, from the results in cats [16], it is suggested that a small amount (less than 1 %) of soluble DFs would be sufficient to decrease the intestinal lesions induced by NSAIDs. We expect that the clinical usefulness of soluble DFs in decreasing adverse events in the small intestine in patients will be revealed in the near future.
References
Graham DY, Opekum AR, Willingham FF, et al. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59.
Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178.
Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141.
Matsumoto T, Kudo T, Esaki M, et al. Prevalence of nonsteroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496.
Leung WK, Bjarnason I, Wong VW, et al. Small bowel enteropathy associated with chronic low-dose aspirin therapy. Lancet. 2007;369:614.
Shiotani A. Low-dose aspirin-induced gastrointestinal diseases: past, present, and future. J Gastroenterol. 2008;43:581–588.
Endo H, Hosono K, Inamori M, et al. Characteristics of small bowel injury in symptomatic chronic low-dose aspirin users: the experience of two medical centers in capsule endoscopy. J Gastroenterol. 2009;44:544–549.
Bjarnason I, Smethurst P, Fenn CG, et al. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1981;34:407–411.
Watanabe T, Sugimori S, Kameda N, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–1282.
Fujimori S, Seo T, Gudis K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc.. 2009;69:1339–1346.
Watanabe T, Nishio H, Tanigawa T, et al. Probiotic Lactbacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol.. 2009;297:G506–G513.
Mizoguchi H, Ogawa Y, Kanatsu K, et al. Protective effect of rebamipide on indomethacin-induced intestinal damage in rats. J Gastroenterol Hepatol. 2001;16:1112–1119.
Kamei K, Kubo Y, Kato N, et al. Prophylactic effect of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci. 2008;53:2657–2666.
Satoh H, Amagase K, Takeuchi K. Mucosal protective agents prevent exacerbation of NSAID-induced small intestinal lesions caused by antisecretory drugs in rats. J Pharmacol Exp Ther. 2012;348:227–235.
Satoh H, Takeuchi K. Management of NSAID/aspirin-induced small intestinal damage by GI-sparing NSAIDs, anti-ulcer drugs and food constituents. Curr Med Chem. 2012;19:82–89.
Satoh H, Hara T, Murakawa D, et al. Soluble dietary fiber protects against nonsteroidal anti-inflammatory drug-induced damage to the small intestine in cats. Dig Dis Sci. 2010;55:1264–1271.
Satoh H, Amagase K, Takeuchi K. The role of food for the formation and prevention of gastrointestinal lesions induced by aspirin in cats. Dig Dis Sci. 2013;58:2840–2849.
Fries JF, Miller SR, Spitz PW, et al. (1989) Toward an epidemiology of gastropathy associated with nonsteroidal antiinflammatory drug use. Gastroenterology. 6:647-55.
Kato S, Tanaka A, Kunikata T, et al. Changes in gastric mucosal ulcerogenic responses in rats with adjuvant arthritis: role of nitric oxide. Aliment Pharmacol Ther. 1999;13:833–840.
Kato S, Ito Y, Nishio H, et al. Increased susceptibility of small intestine to NSAID-provoked ulceration in rats with adjuvant-induced arthritis: involvement of enhanced expression of TLR4. Life Sci. 2007;81:1309–1316.
Winter CA, Nuss GW. Treatment of adjuvant arthritis in rats with anti-inflammatory drugs. Arthritis Rheum. 1966;9:394–404.
Satoh H, Guth PH, Grossman MI. Role of food in gastrointestinal ulceration produced by indomethacin in the rat. Gastroenterology. 1982;83:210–215.
Satoh H, Shiotani S, Otsuka N, et al. Role of dietary fibres, intestinal hypermotility and leukotrienes in the pathogenesis of NSAID-induced small intestinal ulcers in cats. Gut. 2009;58:1590–1596.
Satoh H. Role of dietary fiber in formation and prevention of small intestinal ulcers induced by nonsteroidal anti-inflammatory drug. Curr Pharm Des. 2010;16:1209–1213.
Takeuchi K, Satoh H. NSAID-induced small intestinal damage—roles of various pathogenic factors. Digestion. 2015;91:218–232.
Acknowledgments
The authors are greatly indebted to Dr. Yasutada Akiba, Center for Ulcer Research and Education/University of California, Los Angeles Medical School, Los Angeles, CA, USA, for his valuable discussions and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Satoh, H., Matsumoto, H., Hirakawa, T. et al. Soluble Dietary Fibers Can Protect the Small Intestinal Mucosa Without Affecting the Anti-inflammatory Effect of Indomethacin in Adjuvant-Induced Arthritis Rats. Dig Dis Sci 61, 91–98 (2016). https://doi.org/10.1007/s10620-015-3889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3889-0