Abstract
Backgroud
Wogonoside (WO), a flavonoid extracted from Huangqin, plays multiple physiological roles. However, it has remained elusive how WO regulates hepatic fibrogenesis until now.
Aim
The purpose of the study was to investigate the potential protective effects of WO against liver fibrosis induced by carbon tetrachloride (CCl4).
Methods
In this study, male rats were randomly allocated into four groups: a control group, the CCl4 group, the CCl4 and WO (4 mg/kg) group, and CCl4 and WO (8 mg/kg) group. Hepatic fibrosis was induced by subcutaneous injection of CCl4 twice a week for a continuous 6-week period. Then the rats were intragastrically administrated with WO daily for 4 weeks before being killed.
Results
As expected, histopathological assessment, Masson trichrome staining, and Sirius red staining demonstrated that WO drastically ameliorated the hepatic fibrosis caused by CCl4. WO significantly attenuated the CCl4-induced upregulations of liver indices including alanine aminotransferase, aspartate aminotransferase, tumor necrosis factor-α, interleukin-1β, IL-6, hexadecenoic acid and laminin in serum, as well as hydroxyproline, malondialdehyde and phosphatidylinositol 3-kinase (PI3K)/protein Kinase B(Akt)/mechanistic target of rapamycin (mTOR)/nuclear factor-kappa B signalings in liver. Meanwhile, WO also effectively recovered the depletions of superoxide dismutase, glutathione and IL-10. Furthermore, we evaluated the effects of WO on the alpha smooth muscle actin, type I collagen expressions, and PI3K/Akt/ mTOR/ribosomal protein S6 kinase 70 kDa (p70S6K) signaling in transforming growth factor (TGF-β) stimulated hepatic stellate cell-T6 cells.
Conclusions
These results suggested that WO had significant protective effects against liver fibrosis induced by CCl4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the high frequency and severity of fibrotic diseases, there are few effective therapies because of the limited knowledge of its underlying pathological process. A wide array of pathogenic factors including metabolic disorders, hepatotoxins, hepatitis B virus (HBV), hepatitis C virus (HCV), and alcoholism are highly related to hepatic fibrogenesis [1]. In the range of 10–20 % hepatic fibrosis cases eventually progresses to irreversible cirrhosis or even liver cancer [2]. Hence, the alleviation of fibrogenesis at an early stage is considered a feasible strategy for curing chronic liver dysfunction. Recovering fibrotic tissues to their previous normal functional state is an active area for research and new studies focused on its cellular and molecular mechanisms burst out. Besides the etiology of fibrosis, developing anti-fibrotic treatments against the liver diseases are of great importance for therapy. Consequently, preventing, delaying, and improving liver fibrosis are main trends in the treatment of chronic liver diseases. Therefore, there is an urgent need finding safer drugs for the treatment of hepatic fibrosis.
As a crucial transcriptional factor, NF-κB is associated with the mediation of inflammatory cytokines in various disorders [3]. Additionally, PI3K/Akt signaling is the upstream molecule driving mTOR. The PI3K/Akt/mTOR pathway has been proven to play an essential role in a variety of diseases including hepatic fibrosis [4].
Natural products have established a strong position in drug research and development. Nonetheless, their underlying mechanisms are still unknown, which hampers drug discovery. The root of Scutellaria baicalensisg Georgi, namely Huangqin, is traditionally used as a functional food component for the intervention of various diseases and health promotion [5]. Wogonoside (WO), a flavonoid extracted from Huangqin, plays multiple physiological roles [6]. However, it has remained elusive how WO regulates hepatic fibrogenesis until now.
Herein, we hypothesize that WO exerts its pharmacological regulative effects towards hepatic fibrogenesis via NF-κB and PI3K/Akt/mTOR pathway in CCl4-induced rats. It is the first study on the hepatoprotective effects of WO and the first time to elucidate its potential underlying mechanism.
Materials and Methods
Reagents
WO powder (purity 99 %), purchased from Sigma (St. Louis, MO, USA), was dissolved in dimethyl sulfoxide (DMSO) as a stock solution and diluted with normal saline before each experiment. Anti-transforming growth factor (TGF-β), anti-Matrix metalloproteinase-2(MMP)-2, anti-MMP-9, anti-phospho-Smad-3, anti-Smad-3, Anti-phospho-p70S6K, anti-p70S6k, anti-α-SMA, anti-phospho-PI3K, anti-PI3K, anti-phospho-Akt, anti-Akt, anti-phospho-mTOR, anti-mTOR, anti-phospho-p65 and anti-p65 primary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The horseradish peroxidase-conjugated anti-rabbit antibody was supplied by Bioworld Technology Inc. (MN, USA). TGF-β was acquired from Sigma (Sigma-Aldrich, USA).
Animals
Forty male Wistar rats (8–12 weeks old) were obtained from Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China). Water and food, supplied in special steel containers, were provided ad libitum. During the study, rats were kept in an air-conditioned room at 23 ± 2 °C with a 12-h light/dark cycle. All the experimental procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Design
The rats were randomly divided into four groups (n = 10): the control group, the CCl4 group, the CCl4 and WO (4 mg/kg) group, the CCl4 and WO (8 mg/kg) group. Hepatic fibrosis was induced by subcutaneous injection of carbon tetrachloride (1:1 in olive oil) in rats at a dose of 3 ml/kg twice-weekly for consecutive 6 weeks. The rats in the control group were injected with an equal volume of olive oil without CCl4.
When the hepatic fibrosis model was established and identified, the rats in the WO groups intragastrically received 4 and 8 mg/kg WO dissolved in normal saline daily for consecutive 4 weeks. Accordingly, rats in the control and model groups received an equal volume of normal saline. The body weights of all rats were recorded once a week.
At the end of the experimental period, the rats were weighed (W body), anesthetized with 20 % urethane, and then killed. The livers were immediately harvested and weighed (W liver) after blood was collected from the abdominal aorta. The blood was centrifuged at 3000 rpm for 8 min and then the serum samples were stored at −80 °C for pending tests. Three of the liver samples were removed and fixed in 10 % neutral formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), while the other liver samples were homogenized for the assays of MDA, SOD, GSH, Hyp, and for Western-blot analysis.
Calculation of Liver Index
Liver index was calculated by the following formula:
Determination of Liver Functional Enzymes in Serum
Serum concentrations of ALT and AST were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Evaluation of Oxidative Stress
Liver samples were homogenized with cold normal saline. After being centrifuged at 12,000 rpm for 10 min at 4 °C, the supernatant of the homogenate was collected into tubes and stored at −80 °C. The protein contents were determined with a BCA protein assay kit. The levels of SOD, GSH, and MDA were evaluated with commercially available kits from Jiancheng Biological Engineering Institute (Nanjing, China) according to the manufacturer’s protocols.
Assay of Collagenic Parameters
Serum concentrations of hexadecenoic acid (HA) and laminin (LN) were measured by radioimmunoassay (RIA) using commercial kits (Beifang Inst. Biotechnology, Beijing, China). Radioimmunoassays were carried out as described [7].
Hyp contents were measured using the kits obtained from Jiancheng Institute of Biotechnology (Nanjing, China) according to the manufacturer’s protocols.
Measurement of Inflammatory Mediators Content in Serum
Serum TNF-α, IL-6, IL-1β, and IL-10 (R&D, Minneapolis, MN, USA) levels were measured using the enzyme-linked immunosorbent assay method with the appropriate commercial kits.
Histopathological Examination
Liver tissues were excised from the left lobe of livers. Then the liver specimens were fixed with 10 % neutral formalin and embedded in paraffin blocks. The paraffin-embedded liver tissues were cut into 4-µm sections with a microtome (Leica, Nussloch, Germany) and stained with hematoxylin and eosin (H&E) (Sigma, Korea) for photomicroscopic assessment (200× magnification). Masson stain was performed to confirm the CCl4-induced hepatic fibrosis evidenced by fiber extension and collagen accumulation.
For Sirius Red staining, the samples were stained with 0.1 % picro-Sirius Red Sigma (Sigma-Aldrich, USA) at room temperature for 1 h, rinsed in acidified water, dehydrated with ethanol and xylene. After being mounted in a resinous medium, the liver sections were observed under a light microscope. Sirius Red-positive areas were quantified by Image-Pro Plus (Media Cybernetics, Bethesda, MD, USA). The percentage of hepatic fibrotic area was calculated according to: 100× (area occupied with red color/area of whole tissue).
Cell Culture and Treatment
HSC-T6 cells were provided by Fuxiang Biological Co., Ltd (Shanghai, China). The cells were maintained in Dulbecco’s modified Eagle’s culture medium containing 10 % fetal bovine serum (Hyclone, South America), 100 IU/ml penicillin and 100 IU/ml streptomycin (Amresco, USA) in an environment of 5 % CO2 at 37 °C. In general, HSC cells were seeded at a density of 6 × 104/ml. Twenty-four hours later, the cells were pre-treated with TGF-β (5 ng/ml) for 2 h and then treated with various concentrations of WO (5, 10, and 20 μM) for 24 h. At the end of cell culture, cells were collected for Western-blot analysis.
Western Blotting
The liver tissues and the HSC-T6 cells were homogenized, washed with PBS, and incubated in lysis buffer (Sigma, St. Louis, MO, USA) to obtain extracts of lung proteins. The samples were loaded to 10 % SDS-PAGE gels and then electrotransferred to nitrocellulose. The blots were incubated with the appropriate concentrations of specific antibodies. After washing, the blots were incubated with horseradish peroxidase-conjugated secondary anti-rabbit antibody. The membranes were stripped and reblotted with anti-GAPDH antibody (Sigma) to verify the equal loading of protein in each lane. Quantification of protein expression was normalized to GAPDH using a densitometer (Imaging System). Three samples were tested and the representative results are shown.
Statistical Analysis
All data are expressed as mean ± SD and analyzed with one-way analysis of variance (ANOVA) with Tukey multiple comparison test. A p value <0.05 was considered statistically significant.
Results
Effects of WO on Body Weight and Liver Index in Rats
To verify the effects of WO on the food consumption and excretion in CCl4-stimulated rats, we recorded their body weight once a week. Liver weights were also immediately measured after the separation of liver tissues for the determination of liver indices. The results indicated that the body weight of rats in the CCl4 group were significantly reduced compared with those in the control group. After the treatment with WO, the rats in the WO group gained body weight faster than that those in the model group. It was obvious that the liver indices of CCl4-induced rats predominantly increased compared to those of the control rats. Treatment with WO effectively lessened the severity of liver injury (Fig. 1).
Effects of WO on Liver Functional Enzymes in Serum
Serum ALT and AST activities were measured to assess the effects of WO on liver functions. The activities of ALT and AST were significantly higher in the CCl4-treated group compared with those in other groups (p < 0.001). However, rats treated with WO showed a significant reduction of ALT and AST activities (p < 0.001), as revealed in Fig. 2. These data demonstrated that treatment with WO effectively suppressed the activities of liver functional enzymes in CCl4-induced rats.
Effects of WO on serum ALT (a) and AST (b). Rats were subcutaneously injected with carbon tetrachloride (1:1 in olive oil) in rats at a dose of 3 ml/kg twice weekly for a consecutive 6 weeks and then intragastrically received 4 and 8 mg/kg WO daily for another consecutive 4 weeks. Values are expressed as mean ± SD. Compared with control: ### p < 0.001; Compared with model: ***p < 0.001
Effects of WO on Collagenic Parameters
The levels of HA, LN, and Hyp were investigated to evaluate the collagen content in serum. The productions of HA and LN remarkably increased in CCl4-stimulated rats in comparison to the control group rats (p < 0.001). Administration of WO (8 mg/kg) obviously downregulated HA and LN productions, which was more potent than the administration of WO (4 mg/kg).
The levels of Hyp in CCl4-treated rats were dramatically higher than those in control group rats (p < 0.001). By contrast, the Hyp productions in rats treated with WO (8 mg/kg) were significantly inhibited (p < 0.001), which were more pronounced than those in rats administrated with WO (4 mg/kg) (p < 0.01) (Fig. 3).
Effects of WO on the content of serum HA (a), LN (b), and liver Hyp (c). Rats were subcutaneously injected with carbon tetrachloride (1:1 in olive oil) in rats at a dose of 3 ml/kg twice weekly for a consecutive 6 weeks and then intragastrically received 4 and 8 mg/kg WO daily for another consecutive 4 weeks. Values are expressed as mean ± SD. Compared with control: ### p < 0.001; Compared with model: **p < 0.01; ***p < 0.001
Effects of WO on Oxidative Stress
Lipid peroxidation in liver was determined by measuring the generations of MDA, SOD, and GSH. As illustrated in Fig. 4, CCl4 stimulation significantly declined the SOD activities and GSH contents, respectively (p < 0.001), while WO treatment effectively restored the levels of GSH and SOD. On the contrary, exposure to CCl4 displayed a strikingly high MDA level (p < 0.001), whereas this situation was ameliorated after the administration of WO.
Effects of WO on the production of SOD (a), MDA (b), GSH (c) in liver tissues. Rats were subcutaneously injected with carbon tetrachloride (1:1 in olive oil) at a dose of 3 ml/kg twice weekly for a consecutive 6 weeks and then intragastrically received 4 and 8 mg/kg WO daily for another consecutive 4 weeks. Values are expressed as mean ± SD. Compared with control: ### p < 0.001; Compared with model: *p < 0.05, **p < 0.01, ***p < 0.001
Effects of WO on the Levels of Inflammatory Mediators in Serum
Next, we evaluated the generation of inflammatory cytokines in serum. As shown in Fig. 5, the levels of TNF-α, IL-1β and IL-6 were elevated in the CCl4-stimulated group than those in the control group (p < 0.001). Rats treated with WO displayed significant suppression of these cytokines. In response to CCl4, IL-10 content was pronouncedly decreased (p < 0.001), whereas treatment with WO (8 mg/kg) remarkably increased IL-10 level (p < 0.05).
Effects of WO on the levels of serum TNF-α (a), IL-1β (b), IL-6 (c), and IL-10 (d). Values are expressed as mean ± SD. Rats were subcutaneously injected with carbon tetrachloride (1:1 in olive oil) at a dose of 3 ml/kg twice weekly for a consecutive 6 weeks and then intragastrically received 4 and 8 mg/kg WO daily for another consecutive 4 weeks. Values are expressed as mean ± SD. Compared with control: ### p < 0.001; Compared with model: *p < 0.05; **p < 0.01; ***p < 0.001
Effects of WO on TGF-β, Smad-3, MMP-2, and MMP-9
To further confirm the therapeutic effects of WO on hepatic fibrosis, we detected the expressions of several key molecules in hepatofibrosis. As illustrated in Fig. 6, the expressions of TGF-β, MMP-2, MMP-9, and the phosphorylation of Smad-3 were significantly upregulated in response to CCl4. The treatment with WO (8 mg/kg) effectively ameliorated these situations, which was slightly more potent than the treatment with WO (4 mg/kg).
Effects of WO on the expressions of TGF-β, p-smad-3, smad-3, MMP-2, and MMP-9 in liver tissues. Rats were subcutaneously injected with carbon tetrachloride (1:1 in olive oil) at a dose of 3 ml/kg twice weekly for a consecutive 6 weeks and then intragastrically received 4 and 8 mg/kg WO daily for another consecutive 4 weeks. Values are expressed as mean ± SD. Compared with control: ## p < 0.01; ### p < 0.001; Compared with model: *p < 0.05; **p < 0.01
Effects of WO on Histopathological Changes and Collagen Deposition
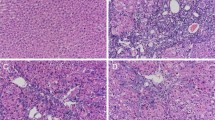
Histopathological observations revealed no appreciable alterations in the control group; the hepatic lobular architecture was normal, and little connective tissue proliferation was found (Fig. 7a). By contrast, liver tissues in CCl4-injured rats had hepatocellular necrosis, massive fatty changes, infiltration of lymphocytes and collagen accumulation (Fig. 7b), while the administration of WO obviously lessened the severity of steatosis, necrosis, inflammation, fibrosis, and significantly lowered the collagen deposition (Fig. 7c, d). Sirius Red staining was performed for detecting the degree of liver fibrosis. It is obvious that Sirius Red-stained areas in the CCl4 group were significantly increased compared with those of the control group. Treatment with WO decreased the proportion of fibrotic areas (Fig. 8). Masson stain was carried out to confirm CCl4-induced liver fibrosis evidenced by apparent collagen deposition, portal inflammation or necrosis, fatty degeneration, and fiber extension. The area percent of trichrome staining in the WO-treated group was obviously decreased in the CCl4-treated-only group (Fig. 9). These data indicated that WO attenuated the CCl4-induced liver fibrosis.
Histological examination of liver by H&E staining (light microscopy, ×400 magnification): a (control): black arrow indicates the portal area; b (CCl4 group) black arrow indicates liver tissue hyperplasia, green arrow indicates lipid droplet vacuoles; c (CCl4 + WO, 4 mg/kg): black arrow indicates liver tissue hyperplasia, green arrow indicates lipid droplet vacuoles, black star indicates portal area; d (CCl4 + WO, 8 mg/kg): green arrow indicates limited lipid droplet vacuoles
Histological changes of Masson-staining in hepatic tissue. a (control): green arrow indicates the normal amount of fibrous tissue in portal area; b (CCl4 group): green arrow indicates the hyperplastic fibrous tissue in necrosis of liver tissue; c (CCl4 + WO, 4 mg/kg): green arrow indicates the hyperplastic fibrous tissue in portal area; d (CCl4 + WO, 8 mg/kg): green arrow indicates the limited hyperplastic fibrous tissue in portal area
Sirius Red staining on the sections of a (control): black arrow indicates the normal amount of fibrous tissue around great vessels, green arrow indicates the limited normal amount of fibrous tissue around central veios; b (CCl4 group): black star indicates the obviously expansile portal area, black arrow indicates the migration of hyperplastic fibrous tissue from hepatic lobule to portal area; c (CCl4 + WO, 4 mg/kg): green arrow indicates the hyperplastic fibrous tissue in liver; d (CCl4 + WO, 8 mg/kg): green arrow indicates the limited fibrous tissue
Effects of WO on PI3K/Akt/mTOR In Vivo and In Vitro
To explore the hepatoprotective-related signaling of WO-treated rats, the phosphorylated and non-phosphorylated forms of the pathway components were examined respectively in liver tissues. As shown in Fig. 10, CCl4-injured rats had obvious up-regulated p-PI3K, p-Akt, p-mTOR, and p-p65 expressions in liver tissues (p < 0.001). Notably, WO effectively suppressed the phosphorylation of the key regulators in PI3K/Akt/mTOR and NF-κBp65 pathways with a concentration-dependent manner in CCl4-induced rats.
Effects of WO on PI3K/Akt/mTOR pathway and the expression of NF-κBp65 in liver tissues. Rats were subcutaneously injected with carbon tetrachloride (1:1 in olive oil) in rats at a dose of 3 ml/kg twice weekly for consecutive 6 weeks and then intragastrically received 4 and 8 mg/kg WO daily for another consecutive 4 weeks. Values are expressed as mean ± SD. Compared with control: ### p < 0.001; compared with model: *p < 0.05; **p < 0.01
To further detect the underlying mechanism of WO, we measured the expressions of PI3K/Akt/mTOR/p70S6K in TGF-β induced HSC-T6 cells. As illustrated in Fig. 11, TGF-β significantly upregulated the phosphorylation of PI3K, Akt, mTOR, and p70S6K. On the other hand, treatment with WO remarkably blocked the phosphorylation of PI3K/Akt/mTOR/p70S6K molecules in a dose-dependent manner.
Effects of WO on the α-SMA, type I collagen expressions, and PI3K/Akt/mTOR/p70S6K signaling in TGF-β stimulated HSC-T6 cells. HSC cells were seeded at a density of 6 × 104/ml. Twenty-four hours later, the cells were pre-treated with TGF-β (5 ng/ml) for 2 h and then treated with various concentrations of WO(5 μM, 10 μM, 20 μM) for 24 h. Values are expressed as mean ± SD. Compared with control: ### p < 0.001; compared with model: *p < 0.05; **p < 0.01; ***p < 0.001
Effects of WO on the Expressions of Type I Collagen and α-SMA in TGF-β-Induced HSC-T6 Cells
To further confirm the therapeutic effects of WO on hepatic fibrosis, we detected the expressions of several key molecules in TGF-β-induced HSC-T6 cells. As illustrated in Fig. 11, the expressions of α-SMA and type I collagen significantly upregulated in response to TGF-β. The treatment with WO effectively ameliorated these situations.
Discussion
Accumulating evidence indicates that WO exhibited anticancer, anti-inflammatory, and antithrombotic activities [8]. However, whether WO exerts a potent therapeutic activity against hepatic fibrosis remains poorly defined.
Exposure to CCl4 leads to hepatic injury, which causes the permeability alteration of the plasma and mitochondrial membranes [9]. As a result, cytoplasmic enzymes, including ALT and AST, discharge into circulation [10]. Both ALT and AST reflect the degree of liver cell injury and medically serve as a reliable diagnostic indicator of liver damage [11]. In our study, the CCl4-injury rats gained less weight and had significantly higher ALT and AST levels than the control rats, while the rats in the WO groups showed significant reductions of these levels. These results indicated that WO negatively mediated ALT and AST activities.
Some investigators pointed out the critical role of inflammatory modulation in the intervention of hepatic fibrosis [12]. The promotion of IL-10 as well as the inhibition of TNF-α, IL-1β, and IL-6 could actively ameliorate the hepatic aggravation during fibrogenesis [13]. In the present work, we found that WO increased the production of IL-10 and decreased the levels of other inflammatory cytokines in CCl4-induced rats.
Several pieces of evidence proposed that the common link between chronic liver damage and hepatic fibrosis could be closely associated with oxidative stress [14]. The excessive oxygen-free radicals that occur during the hepatic lesion pathogenesis also conduce to biological membranes lipid peroxidation and severe cell damage. Interestingly, it is widely acknowledged that WO exhibits antioxidant activities in various diseases [15]. Our present data showed that treatment with WO significantly reduced the content of MDA, and restored SOD and GSH activities. These analytical results suggested that the anti-fibrotic effect of WO might be attributed to its antioxidative activity. The lipid peroxidation is not only the standard but also the direct inducer of liver fibrosis [16]. Stimulation with CCl4 leads to the lipid peroxidation and the overexpression of inflammatory cytokines. Liver damage, oxidative stress, inflammation deteriorate each other in hepatic fibrosis [17]. It is hypothesized that WO exerted a potent protective effect through the anti-oxidative, anti-inflammatory, anti-fibrotic, and lesion-released pathways in coordinative manners. More detailed signalings are warranted in the future studies.
As an essential factor for promoting collagen formation, TGF-β is considered as a key activator of hepatic stellate cells (HSCs) and a primary target in the development of anti-fibrotic agents [18]. TGF-β binds to the receptor on cell membrane and activates the Smad complex, which then translocates into the nucleus. The phosphorylation of Smad-3 is the key event for the treatment of hepatic fibrosis. Moreover, MMP-2 establishes the living environment of HSCs and degrades the normal subendothelial matrix. MMP-9 decomposes the type IV collagen and promotes the activation of HSCs. Both MMP-2 and MMP-9 are the major factors in fibrogenesis [19]. The present study indicated that the treatment with WO significantly inhibited the expressions of TGF-β, p-smad-3, MMP-2, and MMP-9, and thus further confirmed the pharmacological effects of WO on CCl4-induced rats.
Continuous accumulation of extracellular matrix (ECM) is responsible for hepatic fibrosis. As the major component of ECM, type I collagen is composed of Hyp [20]. Additionally, HA and LN are important indices to evaluate the extent of hepatic fibrosis [21]. The data showed that treatment with WO predominantly suppressed the levels of Hyp, HA, and LN in CCl4-induced rats and type I collagen in TGF-β-induced HSC-T6 cells. Our experimental results indicated that WO inhibited the hepatofibrosis progression.
As an essential factor for promoting collagen formation, TGF-β is considered a key activator of hepatic stellate cells(HSCs) and the most potent fibrogenic factor for HSC-T6. α-SMA is a typical index for the activated HSCs during fibrotic process [22]. The expressions of α-SMA and type I collagen increased in TGF-β-induced HSC-T6 cells [23]. To our knowledge, NF-κB p65, a central signaling factor for hepatic fibrosis, governs the generation of inflammatory cytokines [24]. In addition, the PI3K/Akt/mTOR pathway is an essential cellular signaling pathway regulating cell growth, adhesion, migration, and survival in response to extracellular stimuli [25]. Research has suggested that PI3K/Akt/mTOR and NF-κB pathways were related to various disorders [26]. It is also known that the PI3K/Akt/mTOR pathway is involved in the process of anti-inflammation and anti-oxidation [27]. As the critical molecule of mTOR, p70S6K mediates protein expression and proliferation through the inhibition of DNA synthesis in HSCs [28]. Previous work proved the critical role of PI3K/Akt signaling in hepatic fibrosis [29]. Zhao also demonstrated the relationship between the phosphorylation of mTOR and fibrogenesis [30]. The suppression of mTOR/p70S6K signaling results in the inhibition of type I collagen accumulation and HSC proliferation [31]. Of notice, WO was considered to exert an inhibitive effect on p-mTOR protein expression. It has been proposed that mTOR could regulate NF-κB in feedback or directly [32]. Our Western-bolt data indicated that WO blunted the phosphorylation of PI3K, Akt, mTOR, and p70S6K. Thus, these analytical results suggested that WO exhibited anti-hepatic fibrosis activity via the PI3K/Akt/mTOR pathway.
Sirius Red is a strong anionic dye that is widely used as an indicator for the quantification of collagen in tissue. Meanwhile, the histopathological analysis of liver sections showed higher steatosis, necrosis, inflammation, and fibrosis in the CCl4 groups compared with those in control group. Masson staining also confirmed the hyperplastic fibrous tissue caused by CCl4. Rats treated with WO displayed significant alleviation of these conditions. These results further confirmed the anti-fibrotic effects of WO.
In conclusion, the present study supported that WO exhibited protective activity on liver fibrosis in vivo and in vitro. WO may be a potential candidate for the prevention and treatment of liver fibrosis. Future studies on liver function will be crucial to assess its effects on clinical interventions.
References
Nagao Y, Kawahigashi Y, Sata M. Association of periodontal diseases and liver fibrosis in patients with HCV and/or HBV infection. Hepat Monthly. 2014;14:e23264.
Wang L, Cheng D, Wang H, et al. The hepatoprotective and antifibrotic effects of Saururus chinensis against carbon tetrachloride induced hepatic fibrosis in rats. J Ethnopharmacol. 2009;126:487–491.
Abhilash PA, Harikrishnan R, Indira M. Ascorbic acid suppresses endotoxemia and NF-kappaB signaling cascade in alcoholic liver fibrosis in guinea pigs: a mechanistic approach. Toxicol Appl Pharmacol. 2014;274:215–224.
Wang Y, Liu Y, Zhang XY, et al. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-kappaB and PI3K/Akt/mTOR pathways. Int Immunopharmacol. 2014;23:77–84.
Chan E, Wong CY, Wan CW, et al. Evaluation of anti-oxidant capacity of root of Scutellaria baicalensis Georgi, in comparison with roots of Polygonum multiflorum Thunb and Panax ginseng CA Meyer. Am J Chin Med. 2010;38:815–827.
He MY, Deng YX, Shi QZ, Zhang XJ, Lv Y. Comparative pharmacokinetic investigation on baicalin and wogonoside in type 2 diabetic and normal rats after oral administration of traditional Chinese medicine Huanglian Jiedu decoction. J Ethnopharmacol. 2014;155:334–342.
Wang R, Yu XY, Guo ZY, Wang YJ, Wu Y, Yuan YF. Inhibitory effects of salvianolic acid B on CCl(4)-induced hepatic fibrosis through regulating NF-kappaB/IkappaBalpha signaling. J Ethnopharmacol. 2012;144:592–598.
Zhang L, Ren Y, Yang C, et al. Wogonoside ameliorates lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2014;37:2006–2012.
Krahlenbuhl S, Reichen J, Zimmermann A, Gehr P, Stucki J. Mitochondrial structure and function in CCl4-induced cirrhosis in the rat. Hepatology (Baltimore, MD). 1990;12:526–532.
Domitrovic R, Rashed K, Cvijanovic O, Vladimir-Knezevic S, Skoda M, Visnic A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem Biol Interact. 2015;230c:21–29.
Lu J, Chen B, Li S, Sun Q. Tryptase inhibitor APC 366 prevents hepatic fibrosis by inhibiting collagen synthesis induced by tryptase/protease-activated receptor 2 interactions in hepatic stellate cells. Int Immunopharmacol. 2014;20:352–357.
Chang YY, Lin YL, Yang DJ, et al. Hepatoprotection of noni juice against chronic alcohol consumption: lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation. J Agric Food Chem. 2013;61:11016–11024.
Goral V, Atayan Y, Kaplan A. The relation between pathogenesis of liver cirrhosis, hepatic encephalopathy and serum cytokine levels: what is the role of tumor necrosis factor alpha? Hepato-gastroenterology. 2011;58:943–948.
Vendemiale G, Grattagliano I, Caruso ML, et al. Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol. 2001;175:130–139.
Sun Y, Zou M, Hu C, et al. Wogonoside induces autophagy in MDA-MB-231 cells by regulating MAPK-mTOR pathway. Food Chem Toxicol. 2013;51:53–60.
MadanKumar P, NaveenKumar P, Manikandan S, Devaraj H, NiranjaliDevaraj S. Morin ameliorates chemically induced liver fibrosis in vivo and inhibits stellate cell proliferation in vitro by suppressing Wnt/beta-catenin signaling. Toxicol Appl Pharmacol. 2014;277:210–220.
Shen H, Sheng L, Chen Z, et al. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology (Baltimore, MD). 2014;60:2065–2076.
Lee JH, Jang EJ, Seo HL, et al. Sauchinone attenuates liver fibrosis and hepatic stellate cell activation through TGF-beta/Smad signaling pathway. Chem Biol Interact. 2014;224c:58–67.
Liang B, Guo XL, Jin J, Ma YC, Feng ZQ. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon–tetrachloride-induced rat liver injury. World J Gastroenterol. 2015;21:5271–5280.
Zhou YN, Sun MY, Mu YP, et al. Xuefuzhuyu decoction inhibition of angiogenesis attenuates liver fibrosis induced by CCl(4) in mice. J Ethnopharmacol. 2014;153:659–666.
Li GY, Gao HY, Huang J, Lu J, Gu JK, Wang JH. Hepatoprotective effect of Cichorium intybus L., a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fibrosis in rats. World J Gastroenterol. 2014;20:4753–4760.
Su KY, Hsieh CY, Chen YW, Chuang CT, Chen CT, Chen YL. Taiwanese green propolis and propolin G protect the liver from the pathogenesis of fibrosis via eliminating TGF-beta-induced Smad2/3 phosphorylation. J Agric Food Chem. 2014;62:3192–3201.
Tang Y, Hu C, Liu Y. Effect of bioactive peptide of Carapax Trionycis on TGF-beta1-induced intracellular events in hepatic stellate cells. J Ethnopharmacol. 2013;148:69–73.
Kocabayoglu P, Lade A, Lee YA et al. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63:141–147.
Li T, Wang G. Computer-aided targeting of the PI3K/Akt/mTOR pathway: toxicity reduction and therapeutic opportunities. Int J Mol Sci. 2014;15:18856–18891.
Mu GG, Zhang LL, Li HY, Liao Y, Yu HG. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig Dis Sci. 2015;60:1067–1080.
Luo L, Wall AA, Yeo JC, et al. Rab8a interacts directly with PI3Kgamma to modulate TLR4-driven PI3K and mTOR signalling. Nat Commun. 2014;5:4407.
Wang Y, Gao J, Zhang D, Zhang J, Ma J, Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53:132–144.
Bai T, Lian LH, Wu YL, Wan Y, Nan JX. Thymoquinone attenuates liver fibrosis via PI3K and TLR4 signaling pathways in activated hepatic stellate cells. Int Immunopharmacol. 2013;15:275–281.
Zhao Y, Ma X, Wang J, et al. Paeoniflorin alleviates liver fibrosis by inhibiting HIF-1alpha through mTOR-dependent pathway. Fitoterapia. 2014;99:318–327.
Gabele E, Reif S, Tsukada S, et al. The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem. 2005;280:13374–13382.
Chen J, Crawford R, Xiao Y. Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J Cell Biochem. 2013;114:245–249.
Conflict of interest
There are no conflicts of interest regarding this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Wen, R., Lin, Q. et al. Wogonoside Shows Antifibrotic Effects in an Experimental Regression Model of Hepatic Fibrosis. Dig Dis Sci 60, 3329–3339 (2015). https://doi.org/10.1007/s10620-015-3751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3751-4