Abstract
Substances added to food are considerable for survival and are the oldest technologies used in preservation, sweetening and coloring. This work was conducted to evaluate the toxicity of the food additives sunset yellow (SY) and brilliant blue (BB) on Allium cepa root meristematic cells. Control and treatment groups were created from germinated roots. Group 1 (control group) did not receive chemicals. Group 2 (SY or BB-treatment group), received increasing doses of SY (25, 50, 100 and 500 ppm) and BB (100, 200, 400 and 500 ppm) with time periods of 24, 48 and 72 h. After different treatment periods, the roots were obtained from all groups and EC50 concentrations, cell death, chromosome aberrations, mitotic index were observed by a light microscopy. Changing antioxidant capacity of roots was determined by FRAP and TEAC assay. Also, DNA damage was measured by comet assay and RAPD–PCR technique. Approximately 50 and 200 ppm were accepted as EC50 value for SY and BB, respectively. Chromosome aberration values were obtained with increasing concentrations and longer treatment times such as chromosome bridge, C-mitosis, micronucleus, chromosome mis-segregation in both groups. Increasing exposure doses of SY and BB caused decreasing mitotic index values at 72 h. FRAP and TEAC assay showed that antioxidant capacity of roots was decreased by increasing concentrations of SY and BB. The tail DNA% and tail length significantly increased for all exposure times when compared to the control group. 50 and 200 ppm of SY and BB caused a genotoxic effect on genetic material at 72 h according to RAPD–PCR. Increasing the doses of SY and BB resulted in increased toxicity to all studied parameters of A. cepa. In conclusion, the SY and BB tested in this study have cytotoxic and mutagenic potential. Furthermore, SY is more harmful than BB for use in the A. cepa root meristematic cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food additives are used as synthetic or natural chemicals and are added to foods for sweetening, coloring, flavoring and as protecting agent agains bacterial degradation. Microbiological, chemical and enzymatic changes of foods are impeded by food preservatives (Zengin et al. 2011). Apart from natural dyes, synthetic dyes have found much more attention because of their high usage and less sensitivity to light, pH, microbial contamination and reasonably low production cost (Rencüzogullari et al. 2001; Nair 2001). In recent years, many studies have shown that food additives are cytotoxic, carcinogenic, mutagenic and genotoxic (Pandey et al. 2014; Pandir 2016).
Food additives, such as sunset yellow (SY) and brilliant blue (BB) are two of the most used additives in food technology as food preservatives. BB is a water soluble, blue colored, synthetic dye produced by the condensation of 2-formylbenzenesulfonic acid (Fig. 1a) for using in ice cream, packet soups, icings, ice pops, sweets as a coloring agent in various food stuffs (Lucová et al. 2013). SY is a water soluble, yellow-orange, a petroleum-derived orange azo dye (Fig. 1b) and is used in food, cosmetics and drugs (Pandir 2016).
It is widely thought that certain food additives induce genotoxicity in blood and tissues (Türkoglu 2007). Therefore, some food additives have been prohibited because of their toxicity (Rencüzogullari et al. 2001) but it is not known whether all of them are toxic or not. WHO (1974) has shown that benzoate is dangerous for some animals. Sodium sulphite and sodium benzoate break down DNA strands and chromosome structure in roots of Vicia faba (Njagi and Gopalan 1982). Dönbak et al. (2002) demonstrated that boric acid is toxic to Allium cepa and creates mitotic abnormalities and changes mitotic index (MI) of the cells (Türkoglu 2007). Another report has demonstrated that some food additives have harmful effects at only the highest concentration and cause modifications on human male germ cells (Pandir 2016).
Allium cepa has been extensively investigated as a bioindicator for environmental pollution. The reasons for choosing A. cepa as an in vivo model to evaluate the toxic effects of environmental pollutants are: (1) its roots can be affected by chemicals, (2) it possesses a stable number of chromosomes and karyotype, (3) it shows a clear mitotic phase, (4) the occurrence of spontaneous chromosomal damages is rare, (5) it shows rapid response to genotoxic materials, (6) it exhibits diversity of chromosomal morphology and (7) it is considered as a low-cost test material (Grant 1982).
ABTS/TEAC method is the most commonly used antioxidant activity measurement method which involves the oxidation of ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) resulting in ABTS·+ radical solution. Antioxidant-containing samples reduce the radical addition and form blue/green color which is determinated at 600–750 nm wavelength. The reaction result is spent ABTS·+ and its result is calculated as trolox and expressed as equivalent antioxidant capacity (Garcia-Alonso et al. 2004). This method can be applied to both biological fluids and food (Villano et al. 2004). The FRAP method is a relatively simple method and can be used to show changes in the antioxidant activity of plant extracts (Guo et al. 2003).
Numerous xenobiotics and some of free radicals affect DNA of prokaryotic and eukaryotic organisms. This damage can be obtained usually from single stranded and/or double stranded breaks in DNA. This technique is very sensitive and very fast for the estimation of DNA damage at the individual plant and animal cell (Pandır 2015). Agents, which have a genotoxic effect, can cause carcinogenesis or chromosomal aberrations that lead to sister chromatid exchanges, micronuclei, cell death and mutations (Hirose et al. 2006).
The polymerase chain reaction–random amplified polymorphic DNA (PCR–RAPD) is often used as RAPD for genotoxicity assessment as molecular tool. The molecular RAPD approach was used by many researcher, for toxic effect studies, in both plant and animal foods (Tedeschi et al. 2014). Aksakal et al. (2013) showed that the herbicide 2,4-D has genotoxic properties in the root of maize by using the RAPD assay. Tedeschi et al. (2014) used RAPD analyses to characterise Tropea red onion (A. cepa) that is cultivated in specific areas.
However, little is known about the impacts of SY and BB, on vital physiological processes, including root growth of A. cepa. In this paper, eight parameters were used for the first time which are EC50 concentrations, cell death, chromosome aberrations, mitotic index, antioxidant capacity using FRAP and TEAC assay, DNA damage and RAPD–PCR in A. cepa under SY and BB stress. Based on the used parameters, the present study is conducted to evaluate different points of view and their results are supposed to complement with each other. Therefore, the present study is aimed to investigate the genotoxic, cytologic and mutagenic alterations induced by SY and BB toxicity in A. cepa root cells.
Materials and methods
Materials
SY and BB were bought from Sigma–Aldrich. Onions (A. cepa L. 2n = 6) for the Allium test were purchased from a local market (Yozgat, Turkey). Chemicals used Evans blue (Himedia), N,N-dimethylformamide (EMPLURA), DMSO and other chemicals were obtained from Sigma.
Allium test system
Determination of EC50 with inhibition test
Red onions, free from growth inhibitors and agricultural pesticide, were obtained from various local markets (Yozgat/Turkey, A. cepa, 2n = 16) and these onions were standardized with botanical analysis (botanical taxonomy) is free from growth inhibitors and agricultural pesticide. They were cleaned and placed into the test tube filled with distilled water for 24 h. The method of Fiskesjō (1985) was used for the root growth inhibition (inhibition% = changing in growth/total growth × 100) test. After 1 day, SY and BB of different concentrations were added into the test tubes in order to determine the EC50 values at room temperature. After treatment period of 3 days, ten roots from each onion were cut off and the length of each root was measured. It was determined that EC50 concentration decreased the root growth by about 50% compared with the control group. 1/2× EC50, EC50, and 2× EC50 concentrations of SY and BB were determined. These concentrations as well as the 500 ppm concentration of SY and BB used for the Allium test.
Determination of cytogenetic effect and mitotic index (MI)
Healthy six onion bulbs for control and treatment groups were germinated in test tubes for 24 h and 1/2× EC50, EC50, 2× EC50 and 500 ppm were transferred to the treatment groups for 24, 48 and 72 h under same laboratory conditions. 1–2 cm roots were collected at the period of maximum mitotic activity on sunny days and transferred into chilled Carnoy’s fixative in ethanol: glacial acetic acid (3:1) for 24 h and then were hydrolyzed in 1 N HCL at 60 °C for 7 min (Fiskesjō 1985). For cytogenetic evaluation acetocarmine was used for 4 h and destaining was performed using 45% acetic acid. Damaged and total cells were examined, six different slides were observed under a 40× objective of a light microscope (Olympus BX53 DP72, Japan).
About 5000 cells were counted for five slides for each concentration for determination of mitotic index (MI). The MI% was calculated according to the formula published by Fiskesjō (1985).
FRAP (ferric reducing antioxidant power) and TEAC (trolox equivalent antioxidant capacity) assays
The reduction capacity of tissue samples were measured by FRAP assay procedure of Benzie and Strain (1996). FRAP assay determines the alteration of absorbance at 593 nm based on the generation of a blue coloured FeII-tripyridyltriazine compound from colourless oxidised FeIII form via electron donation from antioxidants. Freshly prepared FRAP reagent (1 vol of TPTZ 2,4,6-tripyridyl-s-triazine, with 10 vols of acetate buffer, pH 3.6 in hydrochloride acid and with 1 vol of ferric chloride) was heated to 37 °C. Afterwards, deionized water and the sample was put to the reagent and then the absorbance was measured at 593 nm.
TEAC assay is based upon the inhibition of ABTS·+ absorbance which has a characteristic long wave length absorption spectrum showing a maximum at 734 nm (Re et al. 1999). ABTS·+ was formed by reacting stock solution of ABTS with potassium persulfate and allowing the mixture to stand in the dark for 12–16 h before use at 25 °C. For the present study, solution of ABTS·+ was rarefied with phosphate buffer pH 7.4 to an absorbance of 0.70 at 734 nm. Then, we added biological sample to the diluted ABTS·+ solution and this mixture was incubated for 6 min at 30 °C. The absorbance was observed after initial mixing for tissue samples at 734 nm. These assays were done via a Shimadzu UV 1700 spectrophotometer (Kyoto, Japan).
Determination of cell death in situ conditions
Equal 10 mm length roots of control and treatment groups for cell death were transferred to 3 ml of N,N-dimethylformamide and incubated at room temperature for 1 h. 0.25% (w/v) aqueous solution of Evans blue was added and incubation was done for 15 min. Then roots were washed with tap water for half an hour according to Baker and Mock (1994). Dead and alive cell of roots were stained blue and colorless, respectively according to their vitality. These appearances of roots were macro-photographed.
Determination of DNA damage with comet assay
After 72 h ten roots from each onion were cut with a scalpel into very thin slices. They were centrifuged at 500 rpm for 5 min and then uncubated in PBS for 20 min. Obtained supernatant from control and treatment groups were stirred with low melting point agarose (0.65%), 75 µl of the prepared suspension was immediately layered over slides which were precoated with normal melting point agarose (0.05%). Slides were transferred into cold lysing solution and incubated for 1 h (Özkan et al. 2009) after electrophoresis buffer. Slides were stained with 80 µl of ethidium bromide and scored immediately using BS 200 ProP image analysis software (BS 200 ProP, BAB Imaging System, Ankara, Turkey). A 40× objective on a fluorescent microscope was used in order to observe DNA damage. The tail DNA% (100—Head %DNA), tail length and tail moment of 50 comets were identified and differences between groups were calculated.
DNA extraction
After SY and BB treatment for 72 h, genomic DNA extraction from A. cepa roots was performed using a Qiagen DNeasy plant mini kit (Qiagen, Hilden, Germany). Extraction procedure was performed according to the manufacturer’s instruction.
The optical density (OD) measurements were carried out for sampled DNA at 260 and 280 nm with a spectrophotometer (ACTGene Micro-Spectrophotometer) and OD260/OD280 was used for the purity of DNA. The A260/A280 ratio demonstrate the DNA purity and 1.8–2.0 values suggest “pure DNA” (Per and Sümer-Ercan 2015).
RAPD–PCR
Extraction of all treatment-groups and PCR amplification of genomic DNA were performed according to the study of Per and Sümer-Ercan (2015). 2 µl of DNA template in a 15 µl reaction containing 1.5 μl PCR buffer [10× buffer with (NH4)2 SO4, Fermentas), 0.5 μl dNTPs (10 mM stock solution), 2 μl random primer (10 μM, Opc5; Sequence 5′ → 3′ GATGACCGCC according to Tedeschi et al. (2014), 0.25 μl Taq Polymerase (5 U/μl, Fermentas), 1.5 μl MgCl2 (25 mM stock solution, Fermentas), 1.2 μl BSA (10 mg/ml) and 6.05 μl of sterile distilled water were used. PCR conditions were chosen as follows: one cycle at 94 °C for 2.5 min, followed by 45 cycles of 45 s at 94 °C, 45 s at 35 °C and 45 s at 72 °C, with a final extension step of 5 min at 72 °C. The PCR products were separated by electrophoresis as Tris-Acetic Acid-EDTA buffer by 1% agarose gel for 1.5 h at 80 V and then stained with ethidium bromide. All profiles were photographed under UV light.
Statistics
All obtained data were calculated by one-way analysis of variance (ANOVA) with Turkey test for determination of statistical significance. Standard deviation (SD) was used for mean values. p < 0.05 was chosen for level of significance. All measurements were carried out in triplicates. The results were expressed as mean ± SD and statistically analyzed using MS-Excel software.
Results
The allium test system was used for cytotoxic activity assessment of SY and BB on commercial onion bulbs. Effects of SY and BB were determined with root growth inhibition for about 72 h. Obtained results for 50 and 200 ppm concentration of SY and BB decreased the root length by about 50%, compared with the DMSO (1%) treatment group. It was seen that depending on the increasing doses and time periods, root growth was negatively affected, in other words root growth was decreased. There is a statistical significance between the treatment groups in root growth for the applied concentrations of SY and BB (p < 0.05) (Table 1).
The Evans blue staining method was used for cell death assessment based on its penetration into dead cell. Treated SY and BB and control cell of A. cepa roots were distinguished with this method. Roots were exposed to staining for few minutes and waiting for visualising intensity of roots. Dead cells take up Evans blue staining deeply but living cells do not take up stain (Fig. 2). Proportional of cell death was obtained according to treatment dose of roots. This staining was different in control and treatment groups.
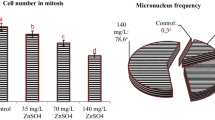
Chromosome aberrations were detected as multiple nuclear lesions, sticky anaphase, disturbed metaphases, C-shaped metaphases, chromosome bridges, chromosome gaps, chromosome mis-segregation, C-mitosis, diagonal anaphase, micronucleus, anaphase bridges, vargant, multipolar anaphase, spindle disturbance in the SY-treatment group. Chromosomal changes were also obtained as anaphase bridge, chromosome break, chromosome mis-segregation, micronucleus, disturbed metaphase, spindle disturbance, chromosome fragments, delayed anaphase, sticky anaphase, clumped chromosome, chromosome loss, multipolar anaphase, C-mitosis, multiple nuclear lesions on root cells in the BB-treatment group for the 72 h treatment groups in comparison to control cells (Figs. 3, 4). While 31.7% anomalies were obtained at the EC50 concentration of SY (50 ppm), 29.6% anomalies were observed at the EC50 concentration of BB (200 ppm) at 72 h (Table 2).
Chromosome abnormalities of SS in root cell. a Normal metaphase, b multiple nuclear lesions, c Sticky anaphase, d disturbed metaphase, e C shaped metaphase, f chromosome bridge, g chromosome gaps, h chromosome mis-segregation, i C-mitosis, j diagonal anaphase, k micronucleus, l anaphase bridge, m vargant, n multipolar anaphase, o spindle disturbance
Chromosome abnormalities of BB in root cell of A. cepa. a Normal metaphase, b anaphase bridge, c chromosome break, d chromosome mis-segregation, e micronucleus, f disturbed metaphase, g spindle disturbance, h chromosome fragments, i delayed anaphase, j sticky anaphase, k clumped chromosome, l chromosome loss, m multipolar anaphase, n C-mitosis, o multiple nuclear lesions
MI (%) of A. cepa root meristem cells were determined in control and experimental groups. MI of the treatment group significantly decreased at different concentrations of SY and BB at each exposure time compared to the control group. The lowest MI% values of SY and BB were obtained from the applications of 500 ppm at 72 h with a score of 6.81 ± 0.09 and 7.31 ± 1.85, respectively (Table 2).
The FRAP and TEAC values of the studied extracts were calculated and the results are presented in Fig. 5. BB and SY treatment represented a decrease in A. cepa roots antioxidant values according to the TEAC and FRAP methods in comparison to control group. When we compared BB with SY treatment group, we observed a higher increment in the values of FRAP and TEAC in the SY group than in the BB group for 72 h (Fig. 5).
Table 3 and Figs. 6 and 7 summarized the tail DNA%, tail length and tail moments in A. cepa root meristem cells exposed to SY and BB for 72 h. DNA damage was significantly (p < 0.05) increased in SY and BB treatment with higher treatment concentrations. The % tail DNA values ranged from 50.09 ± 2.74 to 107.33 ± 22.15 and tail length values ranged from 1.18 ± 0.55 to 85.05 ± 8.75 in the SY-treatment group whereas the % tail DNA values ranged from 47.64 ± 9.65 to 95.65 ± 15.10 and the tail length values ranged from 4.79 ± 0.11 to 69.42 ± 9.42 in the BB-treatment group (p < 0.05).
The structures of RAPD profiles with increasing diazinon concentrations are seen in Fig. 8. Appearance of new bands or/and disappearance of normal bands were obtained from increasing concentrations of the diazinon compared with the control samples.
Discussion
SY and BB are food additives used for controlling microbial organism in fruit and vegetables (Pandir 2016). Effects of SY and BB are closely related to human health risk. Some of the studies clearly demonstrate a statistically significant increase in accumulation and damage to tissue (WHO 1974; Nair 2001). SY and BB were not assessed on A. cepa roots for genotoxicity and cytotoxicity test. For determining the cytotoxic and genotoxic effect of pollutants, A. cepa assay can be used due to its sensitivity and fast system (Panda et al. 2011; Barberio et al. 2011). Also, effects in A. cepa show good correlation with the effects on eukaryotic and prokaryotic cells (Fiskesjō 1985; Yıldız et al. 2009). In this study, SY and BB were selected as toxic chemicals. Particularly, SY and BB could affect A. cepa meristematic activity, root growth, alive test, and changing antioxidant capacity of A. cepa and also possibly lead to DNA damages. The EC50 value is a useful parameter to determine the effective concentration. EC50 (50 and 200 ppm), 2× EC50 (100 and 400) and 1/2× EC50 (25 and 100 ppm) values were obtained according to length of each root for SY and BB, respectively. To determine the possible toxic effects on roots, determined concentrations of SY and BB and control group were used in this study.
Bhagyanathan and Thoppil (2016) used Cynanchum sarcomedium on A. cepa roots for determining viable and non-viable cells. Dead cells stained differently than viable cells according to incubation time and doses. Higher concentration of food additives caused severe cell death and Evans blue staining method showed similar results. In this study, SY and BB appeared to be highly toxic leading to weak or diffused staining in A. cepa roots. Roots excised from six bulbs were processed separately for the determination of cell death. Root staining was significantly enhanced (p < 0.05) at increasing doses of SY and BB for the 72 h-treatments. Furthermore, SY was more effective than BB at the concentrations used.
Some of studies have reported toxic effects of food preservatives on mitotic index in root meristematic cells of A. cepa. Pandey et al. (2014) showed that the effects of food preservatives like butylated hydroxytoluene, butylated hydroxyanisole, sorbic acid, propyl gallate and sodium nitrate on root tips of A. cepa at different concentrations and exposure times. Cytological studies presented a decreased mitotic index and increased cytological abnormalities in the treatment group when compared with the control. Türkoglu (2007) showed that the food preservatives sodium benzoate (SB), boric acid (BA), citric acid (CA), potassium citrate (PC) and sodium citrate (SC) caused a cytotoxic effect which were reduced mitotic division and visible chromosomal changes in A. cepa compared with the respective controls. In this study, genotoxic changes were reported as the possible presence of alterations in the chromosomes of A. cepa root cells because of increasing doses of SY and BB. The effects of SY and BB on the mitotic index (MI) of A. cepa root meristem cells exposed for 24, 48 and 72 h all the test concentrations inhibited MI in a concentration-dependent manner. The total percentages of cytological and chromosomal abnormalities and MI were also altered with increasing concentration and increasing incubation time. The percentage of abnormalities and MI in root system caused by the used preservatives were recorded as BB < SY.
The FRAP and TEAC assay is frequently used by the food industry and agricultural researchers to measure the antioxidant capacities of foods. The FRAP and TEAC assay are simple, fast, inexpensive, and robust methods of measuring the radical scavenging ability of plants (Gliszczynska-Swigło 2006). TEAC methods were regarded as methods using both hydrogen and single electron transfer, as the radicals in these cases can be scavenged by either electron reduction or radical quenching that involve hydrogen transfer (Prior et al. 2005; Jimenez et al. 2004) whereas FRAP is a single electron transfer method (Prior et al. 2005). Thus, one of the objective of the present study was to determine the change in antioxidant activities of A. cepa roots related to the toxicity of SY and BB. The antioxidant capacities are given as Trolox equivalents, in the units of μ mol TE per gram A. cepa roots weight. The FRAP and TEAC assays showed that the roots’ antioxidant capacity was lower in SY and BB exposed A. cepa roots when compared to the control group. It has been found that SY has more effective antioxidant capacity in roots when compared to BB according to the FRAP and TEAC results.
The comet assay was usually used to detect biological effects of various chemical stresses on DNA in living cell (Pandir 2016). Genotoxic effects of 39 chemicals currently in use as food additives were analyzed in the comet assay on 8 mouse organs. Increasing concentrations were applied onto the tissue and DNA damage was demonstrated by comparing the treatment groups to the control group (Sasaki et al. 2002). In another study, lack of genotoxicity of tartrazine and carvacrol were obtained by utilizing our well-established different tissue system with DNA damage by comet assay (Bastaki et al. 2017; Ruiz-Cabello et al. 2016). Tartrazine and carvacrol did not induced DNA damage in a dose-dependent fashion on the rat tissues. In this study, after the exposure period, single and/or double strand DNA breaks were detected by using micro gel electrophoresis (comet assay). Single and/or double strand DNA breaks were measured as tail DNA%, tail length and tail moment of comet. The effects of SY and BB on the root cells of A. cepa with regard to tail DNA% and tail length were determined. It is clearly demonstrated that particularly tail DNA% and tail length increased in the SY and BB treatment groups of SY and BB treated for 72 h. The tail DNA% and tail length values in the SY treatment group were found to be higher than those in the BB treatment group according to EC50 concentration.
For a greater understanding of the genotoxic properties of the test agent, not only the Comet assay, but also RAPD–PCR may be performed. The RAPD markers are used for evidence of various types of DNA damage and mutation (Aksakal et al. 2013). Özakça and Silah (2013) showed that toxic effect of the fungicide flusilazole on somatic cells of A. cepa by using RAPD–PCR method. In the present study, RAPD–PCR assay was used for showing toxic effect of increasing doses of SY and BB on A. cepa root cell. Tretament of SY and BB caused disappearance of DNA bands or variation in band intensities because of severe inhibition of DNA and presence of DNA photoproducts. A genetic distance was seen in most of the samples that were exposed to a concentration of 500 ppm of SY and BB groups for 72 h. According to the RAPD assay, appearance/disappearance of bands and decreases/increases of band intensities between the control and the treatment group sample showed that SY and BB may cause genotoxicity in A. cepa roots and also that SY affects DNA more than BB.
Conclusion
Cytogenetic and mutagenic effects of SY and BB on the A. cepa roots have not previously been described. Our findings indicate that SY and BB administration may have an effect on roots cells depending on the concentration and incubation time. Different chromosomal aberrations and DNA damage were seen in root cells due to the sensitivity of the cells to food additives after increasing exposure time. BB has shown less impact on the root cells when compared with SY according to the studied parameters. However, further investigation is necessary to clarify the toxic effect of SY and BB supplementation on root cells at a cytological to molecular level.
References
Aksakal O, Ertürka FA, Sunar S, Bozari S, Agar G (2013) Assessment of genotoxic effects of 2,4 dichlorophenoxyacetic acid on maize by using RAPD analysis. Ind Crops Prod 42:552–557
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evan’s blue. Plant Cell, Tissue Organ Cult 39:7–12
Barberio A, Voltolini JC, Mello MLS (2011) Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicol 20:927–935
Bastaki M, Farrell T, Bhusari S, Pant K, Kulkarni R (2017) Lack of genotoxicity in vivo for food color additive Tartrazine. Food Chem Toxicol 105:278–284
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Bhagyanathan NK, Thoppil JE (2016) Pre-apoptotic activity of aqueous extracts of Cynanchum sarcomedium Meve & Liede on cells of Allium cepa and human erythrocytes. Protoplasma 253:1433–1438
Dönbak L, Rencüzoğulları E, Topaktaş M (2002) The cytogenetic effects of the food additive boric acid in Allium cepa L. Cytologia 67:153–157
Fiskesjō G (1985) The Allium as a standard in environmental monitoring. Hereditas 102:99–102
Garcia-Alonso M, Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC (2004) Evaluation of the antioxidant properties of fruits. Food Chem 84:13–18
Gliszczynska-Swigło A (2006) Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem 96:131–136
Grant WF (1982) Chromosome aberration assays in Allium. A report of the U.S. Environmental Protection Agency gene-tox program. Mutat Res, Fundam Mol Mech Mutagen 99:273–291
Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y (2003) Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res 23:1719–1726
Hirose H, Sakuma N, Kaji N, Suhara T, Sekijima M, Nojima T, Miyakoshi J (2006) Phosphorylation and gene expression of p53 are not affected in human cells exposed to 2.1425 GHz band CW or W-CDMA modulated radiation allocated to mobile radio base stations. Bioelectromagnetics 27:494–504
Jimenez A, Selga A, Torres JL, Julià L (2004) Reducing activity of polyphenols with stable radicals of the TTM series. Electron transfer versus H-abstraction reactions in flavan-3-ols. Org Lett 6:4583–4586
Lucová M, Hojerová J, Pazoureková S, Klimová Z (2013) Absorption of triphenylmethane dyes brilliant blue and patent blue through intact skin, shaven skin and lingual mucosa from daily life products. Food Chem Toxicol 52:19–27
Nair B (2001) Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol 20:23–50
Njagi GDE, Gopalan HNB (1982) Cytogenetic effects of the food preservatives—sodium benzoate and sodium sulphite on Vicia faba root meristems. Mutat Res 102:213–219
Özakça DU, Silah H (2013) Genotoxicity effects of flusilazole on the somatic cells of Allium cepa. Pestic Biochem Physiol 107:38–43
Özkan D, Yüzbaşıoğlu D, Ünal F, Yılmaz S, Aksoy H (2009) Evaluation of the cytogenetic damage induced by the organophosphorous insecticide acephate. Cytotechnology 59:73–80
Panda KK, Achary VMM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB (2011) In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol In Vitro 25:1097–1105
Pandey H, Kumar V, Roy BK (2014) Assessment of genotoxicity of some common food preservatives using Allium cepa L. as a test plant. Toxicol Rep 1:300–308
Pandır D (2015) Protective effect of (−)-epigallocatechin-3-gallate on capsaicin-induced DNA damage and oxidative stress in human erythrocytes and leucocytes in vitro. Cytotechnology 67:367–377
Pandır D (2016) DNA damage in human germ cell exposed to the some food additives in vitro. Cytotechnology 68:725–733
Per S, Sümer-Ercan F (2015) Comparison of three methods of DNA extraction from Parachipteria willmanni (Acari: Oribatida) collected in Turkey. J Biotechnol Res 1:16–20
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rencüzoğulları E, İla HB, Kayraldız A, Topaktaş M (2001) Chromosome aberrations and sister chromatid exchanges in cultured human lymphocytes treated with sodium metabisulfite a food preservative. Mutat Res Gen Toxicol Environ Mutagen 490:107–112
Ruiz-Cabello ML, Maisanaba S, Puerto M, Prieto AI, Pichardo S, Moyano R, Gonzalez-Perez JA, Camean AM (2016) Genotoxicity evaluation of carvacrol in rats using a combined micronucleus and comet assay. Food Chem Toxicol 98:240–250
Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S (2002) The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res 519:103–119
Tedeschi P, Bonetti G, Maietti A, Brandolini V (2014) Random amplified polymorphic DNA (RAPD) fingerprint and antioxidants profile as markers for Tropea red onion (Allium cepa L.) authenticity. J Food Compos Anal 36:98–103
Türkoğlu S (2007) Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res 10:4–14
Villano D, Fernandez-Pachon MS, Troncoso AM, Garcia-Parrilla MC (2004) The antioxidant activity of wines determined by the ABTS+ method: influence of sample dilution and time. Talanta 64:501–509
WHO (1974) Food additive series. Wld Hlth Org. techn. Rep. Ser., No. 539; and FAO Nutrition Meetings Report Series, No. 53
Yıldız M, Cigerci IH, Konuk M, Fidan AF, Terzi H (2009) Determination of genotoxic effects of copper sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere 75:934
Zengin N, Yüzbaşıoğlu D, Ünal F, Yılmaz S, Aksoy H (2011) The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chem Toxicol 49:763–769
Acknowledgements
The authors would like to thank to Fatih Oğuz Bekdemir for helping us to prepare this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koç, K., Pandir, D. All aspect of toxic effect of brilliant blue and sunset yellow in Allium cepa roots. Cytotechnology 70, 449–463 (2018). https://doi.org/10.1007/s10616-017-0161-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0161-9