Abstract
Cynanchum sarcomedium Meve & Liede is a member of Apocynaceae, seen in dry and rocky areas. The present study highlights the cytotoxic potential of C. sarcomedium mediated by apoptosis on cells of Allium cepa and human red blood cells (RBCs). Cytogenetic changes in A. cepa and in situ visualization of cell death were revealed through acetocarmine and Evans blue staining techniques. Quantitative estimation of cell death was carried out at 600 nm in a spectrophotometer. Membrane characteristics of RBC in response to the treatment were evaluated by May-Grünwald-Giemsa staining and scanning electron microscopy (SEM). Cell membrane damage is a major factor for assessing apoptosis which is observed in the present study (90.91 %). Cell shrinkage, cytoplasmic fragmentation, condensed chromatin and presence of apoptotic bodies were the common cytological changes in A. cepa associated with apoptosis. Blebs in RBC evidenced by SEM revealed the membrane damage potential of the plant. Results obtained hereby suggest that the plant is an effective source to be used in toxicological studies and anti-cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed cell death is a genetically controlled process consisting of initiation, genetic control and execution phases. These culminate in unique morphological changes within cells, known as apoptosis. Apoptosis exhibits several distinct morphological features, including condensation of cytoplasm, fragmentation of nucleosomal DNA, condensation of nuclear chromatin, membrane blabbing and appearance of apoptotic bodies (Golstein and Kroemer 2007). Recently, the relationship between apoptosis and cancer has been emphasized, which hints an effective anti-cancer approach, and many chemotherapeutic agents were reported to exert their anti-tumour effect including apoptosis of cancer cells (Ouyang et al. 2012).
Humans have been using plants for millennia as medicine to treat various diseases. The medicinal value of plants lies in some chemical substances (usually secondary metabolites) that produce a definite physiological action on the human body (Nostro et al. 2000). In the present scenario, researchers focus on herbal preparations to be used as effective medicines with negligible side effects along with nominal probability of developing resistance in the treatment of cancer and other infectious diseases. It is noted that the management of cancer is still not up to the mark and there are always needs to search new drugs for the management of cancer. In this context, the plants still hold the hope for the treatment and prevention of cancer. Hence, an initial research focuses on screening the ability of various plant extracts for their cytotoxic and anti-proliferative potential.
Cynanchum sarcomedium (milky weed) is a perennial leafless, jointed trailing shrub with green, cylindrical, fleshy glabrous, twining branches having milky white latex. Molecular analyses have demonstrated that the genus, Sarcostemma, is deeply nested in the predominantly Madagascan stem-succulent clade of Cynanchum L. Hence, it has been treated as a synonym of Cynanchum. Some of the former Sarcostemma species have been transferred to Cynanchum in the course of various Flora treatments, and some new species belonging to this group have been described under Cynanchum (Meve and Liede-Schumann 2012). It is having bronchospasmolytic, tocolytic action and so many other pharmaceutical activities (Kumar et al. 2006; Kumar et al. 2007). C. sarcomedium is a least explored plant species with regard to its biological activities, especially toxicity studies.
Owing to the aforementioned background, the present study addresses the apoptosis-mediated cytotoxic potential of C. sarcomedium on Allium cepa and human red blood cell (RBC), and these test systems can be correlated with other models like mammals, human lymphocytes and bone marrow cells.
Materials and methods
Plant material
Stem of C. sarcomedium Meve & Liede was collected from Wayanad, Kerala, India (coordinates 11.605° N, 76.083° E). The specimen was authenticated, and voucher specimen (CALI No. 123741) was deposited at Herbarium of the Department of Botany, University of Calicut, Malappuram, Kerala, India.
Test system and chemicals
Onion bulbs (A. cepa L., 2n = 16), free from agricultural pesticides and growth inhibitors (procured from TNAU, Tamil Nadu), were used for the present study. Chemicals used were Evans blue (Himedia) and N,N-dimethylformamide (EMPLURA).
Preparation of aqueous plant extracts
Fresh aqueous plant extract was prepared using sterile distilled water with the aid of pestle and mortar. One percent stock solution was prepared by grinding 1 g stem part and made up to 100 mL. Subsequent lower concentrations were then prepared from this stock (0.005, 0.01, 0.05 and 0.1 %).
Cytogenetic evaluation—A. cepa assay
Prior to initiating the test, the outer dry scales of onion bulbs were removed without destroying the root primordia. They were grown in glass vials at room temperature. Germinated bulbs with healthy roots (1–2 cm) were collected at a period of maximum mitotic activity (between 9 am and 10 am on sunny days) and washed with distilled water. The bases of bulbs were kept in vials containing different concentrations of plant extracts in such a way that only roots were suspended in extracts. Methyl parathion and distilled water were also kept as positive and negative control. Root tips were collected from the different vials at 0.5, 1, 2 and 3 h. The collected samples were washed in distilled water and immediately fixed in Carnoy’s fluid for 1 h. Then, the root tips were subjected to hydrolysis with 1 N HCl for 5–10 min and washed in distilled water. Staining was done using acetocarmine for 4 h, and destaining was done by 45 % acetic acid. Permanent slides were then prepared, and the numbers of damaged cells and total cells were counted in six different fields of view using 40× of light microscope (Olympus CX21FS1, Japan) for cytogenetic effects.
In situ visualisation of cell death
For the assessment of cell death, control and treated bulbs with intact roots were placed in 0.25 % (w/v) aqueous solution of Evans blue for 15 min, followed by washing of the roots in running tap water for 30 min (Baker and Mock 1994). Roots with dead cells that stained blue were macro-photographed. Subsequently, ten root tips measuring equal length (10 mm) from control and the treated groups were excised and soaked in 3 mL of N,N-dimethylformamide for 1 h at room temperature. The absorbance of Evans blue released was measured spectrophotometrically at 600 nm (Shimadzu, Japan).
Red blood cell suspension preparation
Human blood was obtained from a blood bank, Calicut, and collected into tubes containing ethylenediaminetetraacetic acid (EDTA) as anti-coagulant. Samples were centrifuged at 10,000 rpm, for 10 min at 4 °C; the supernatant was discarded and replaced by the same volume of saline solution (0.9 % NaCl, pH 7.4); the whole process was repeated three times. Fractions of this stock of RBC suspension were placed in Eppendorf tubes for further experiments.
Morphological evaluation of red blood cell
Histological preparations were carried out with RBC suspension (500 μL) treated with the equal volume of C. sarcomedium extract (0.1 %) for 60 min at room temperature and with saline solution as control group. Blood smears were prepared, dried and fixed, and staining was done using May-Grünwald-Giemsa method (Junqueira and Carneiro 2004; Maiworm et al. 2008). After that, images of the RBC were acquired from blood smears under ×100 of optical microscope (Olympus CX21FS1, Japan).
Scanning electron microscopy
Treated and control samples were mounted on an aluminium stub using carbon tape and dried by keeping it in a desiccator for 3 h, which were subsequently sputtered with gold for 60 s at 40 mA, and images were captured using a scanning electron microscope (Hitachi SU 6600, Japan).
Statistical analyses
Statistical analyses were performed using the SPSS version 10 software package program. Data obtained were then subjected to one-way ANOVA and Duncan’s multiple range test (DMRT) to confirm the variability of the data and validity of results. All results were expressed as mean ± SE, and differences between corresponding controls and exposure treatments were considered statistically significant at P < 0.05.
Results and discussion
Recently, the use of some herbs has drawn a great deal of attention as one of the alternative cancer therapies from the point of less toxicity and cost benefits, and reports of effects of plant extract on membrane damage of plants and animals are in their infancy. Therefore, an attempt has been made to evaluate the cell membrane-damaging activity of C. sarcomedium on two test systems viz., A. cepa and human RBC. Out of four concentrations of plant extracts tested, highest concentration (0.1 %) was cytotoxic in both test models. Major cytological distortions observed on A. cepa were cytoplasmic shrinkage (Fig. 1c, h, m), cell fragmentation (Fig. 1b, f, m), cell membrane damage, cytoplasmic vacuolation (Fig. 1e), cellular breakage (Fig. 1k), etc. All these cytological perturbations were associated with membrane damage and receding of cell contents. Along with these cytological aberrations, genetic material was also affected viz., micronucleus, chromatin globules, nuclear extrusion, nuclear peak with lesions, nuclear fragmentation, hyperchromasia, pulverised nucleus and heteropycnosis (Fig. 1a, d, g, h–j, l, n). One of the common genetic aberrations, pycnosis—the result of chromatin condensation—is the most characteristic feature of apoptosis (Elmore 2007). Moreover, other chromosomal aberrations like micronucleus, chromatin globules, nuclear peak, etc. would have resulted from the elimination of excess genetic material and could not reestablish to cell viability.
Chromosomal aberrations induced by aqueous extracts of C. sarcomedium on root tip cells of A. cepa. a Micronucleus. b Cell fragmentation and hyperchromasia. c Cytoplasmic shrinkage. d Chromatin globules and microcell formation. e Cytoplasmic vacuolation. f Cell fragmentation and receding of cell contents. g Nuclear extrusion. h Cytoplasmic shrinkage, nuclear peak and lesions. i Nuclear fragmentation. j Hyperchromasia. k Cellular breakage. l Pulverised nucleus. m Cytoplasmic shrinkage and cellular fragmentation. n Cytoplasmic heteropycnosis. o Nuclear, cytoplasmic shrinkage and cell death. Bar = 10 μm
In the present study, most of the cytological deformations were associated with cell membrane damage and vacuolar disentegrations. Significant membrane damage is observed in highest concentration of plant extract at 3 h (Fig. 2). The exposure of A. cepa to plant extracts induced distortions of vacuole development throughout the cell which may severely affect the cellular integrity and physiology. Moreover, shrunken root cells, multiple cytoplasmic protrusions or blebs, marked nuclear chromatin condensation or remodeling and, ultimately, fragmentation clearly indicate the possibilities to tend towards apoptosis. It is important to mention that a cell that suffers a genotoxic stress may select to complete nuclear division or to choose apoptosis (Kirsch-Volders et al. 1997). Along with all these cytogenetic aberrations, marked reduction in mitotic index was also observed in cells treated with plant extract. Plants have evolved death strategy that is mediated by the vacuolar processing enzyme (VPE) system, which activates target proteins concerning the vacuolar collapse and thereby disintegration of cellular components (Hatsugai et al. 2006). The destructive mode of vacuole-mediated cell death is initiated by the collapse of the vacuolar membrane releasing hydrolytic enzymes directly into the cytosol to degrade cytoplasmic components that results in rapid and direct cell death (Hara-Nishimura and Hatsugai 2011).
Cell death, a marker of cytotoxicity, is determined by Evans blue staining method on the basis of its penetration to non-viable cells (Panda et al. 2011). Evans blue staining of treated and control roots of A. cepa points to an indirect evidence of cell death by visualising intensity of Evans blue taken up by roots, suggesting the loss of viability of cells. Dead cells stained deeply and were readily distinguished from viable cells after incubation time of 15 min. Intensity of dye absorbed by root cells was directly proportional to the cell death (Fig. 3e, f); this could be seen within few minutes after the treatment, in corroboration with the result reported by Achary et al. (2008). Spectrophotometric determination of apoptosis suggested that severe cell death was observed in higher concentration of plant extract (Fig. 4).
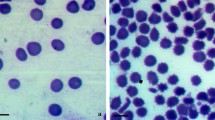
The present study also describes the interaction of aqueous extracts of C. sarcomedium with human RBC or erythrocytes. Despite of it being less specialised, the cell membrane of RBC performs several important functions such as active and passive transport, as well as the production of ionic and electric gradients (Suwalsky et al. 2008), and hence was selected as representative cellular membrane in the study. Figure 5a, b represents photomicrographs of blood smears from samples of blood treated with saline (control) and treated with the C. sarcomedium extract, respectively, indicating that the extract is capable of inducing alterations on the morphology of the RBC. Cellular blebbing seen in RBC occurred as a result of the treatment with the higher concentration of plant extract. In negative control, completely spherical RBC cells with intact membrane were observed (Fig. 5a and c).
Intact human erythrocytes incubated with aqueous extracts of C. sarcomedium show marked changes in membrane morphology, from a normal discoid to an echinocytic form (Fig. 5d), evidenced by scanning electron microscopy (SEM). According to the bilayer couple hypothesis (Lim et al. 2002), the shape changes induced in erythrocytes by foreign molecules are due to differential expansion of the two monolayers of the red cell membrane. It also showed structural perturbations of the RBC attributed to oxidative modifications of the membrane lipids (Zavodnik et al. 2001).
Previous studies reported the cytotoxicity of lavender oil and clove oil on human skin cells leading to membrane death and thereby apoptosis (Prashar et al. 2004; Prashar et al. 2006). Significant morphological changes like decreased cell volume, marked alterations in shape with multiple blebs and protrusion as well as nuclear chromatin clumping were seen in the treatment group. Many phytochemical constituents like flavonoids, terpenoids, polyphenols, etc. have been reported in this plant by GC-MS analysis and have been considered to be responsible for cell death probably through apoptosis (Kumari et al. 2012). Considering the ability of these natural polyphenols especially the tannins to absorb proteins and metal ions, there is a possibility that they can elicit apoptotic signals through various receptors or proteins (Taraphdar 2001).
Through this study, C. sarcomedium was found to have unique apoptotic-mediated cytotoxic property on A. cepa and RBC. Promising findings of the present study can be extended to the characterization of bioactive compounds responsible for apoptosis and their execution of in vivo studies. However, further chemical work and pharmacological evidences at molecular level are required to elucidate the possible mechanism of bioactivities of the extract.
References
Achary VMM, Jena S, Panda KK, Panda BB (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70(2):300–310
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evan’s blue. Plant Cell Tissue Organ Cult 39:7–12
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32(1):37–43
Hara-Nishimura I, Hatsugai N (2011) The role of vacuole in plant cell death. Cell Death Differ 18(8):1298–1304
Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2006) A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis 11(6):905–911
Junqueira LC, Carneiro J (2004) Aparelho respiratório. Histologia Básica 8:285–300
Kirsch-Volders M, Elhajouji A, Cundari E, Van Hummelen P (1997) The in vitro micronucleus test: a multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat Res Genet Toxicol Environ Mutagen 392(1):19–30
Kumar SP, Soni K, Saraf MN (2006) In-vitro tocolytic activity of Sarcostemma brevistigma Wight. Indian J Pharm Sci 68:190–194
Kumar SP, Soni K, Jadhav SR, Doshi NS, Saraf MN (2007) Mechanism of spasmolytic activity of a fraction of Sarcostemma brevistigma Wight. Indian J Exp Biol 45:419–424
Kumari TKS, Muthukumarasamy S, Mohan VR (2012) GC-MS analysis of ethanol extract of Sarcostemma secamone (L.) Bennet (Asclepiadaceae). Sci Res Rep 2:187–191
Lim G, Wortis M, Mukhopadhyay R (2002) Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: evidence for the bilayer-couple hypothesis from membrane mechanics. Proc Natl Acad Sci U S A 99:16766–16769
Maiworm AI, Presta GA, Santos-Filho SD, Paoli SD, Giani TS, Fonseca AS, Bernardo-Filho M (2008) Osmotic and morphological effects on red blood cell membrane: action of an aqueous extract of Lantana camara. Rev Bras Farm 18(1):42–46
Meve U, Liede-Schumann S (2012) Taxonomic dissolution of Sarcostemma (Apocynaceae: Asclepiadoideae). Kew Bull 67(4):751–758
Nostro A, Germano MP, Dángelo V, Cannatelli MA (2000) Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol 30:379–384
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45(6):487–498
Panda KK, Achary VMM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB (2011) In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol In Vitro 25(5):1097–1105
Prashar A, Locke IC, Evans CS (2004) Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif 37(3):221–229
Prashar A, Locke IC, Evans CS (2006) Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif 39(4):241–248
Suwalsky M, Vargas P, Avello M, Villena F, Sotomayor CP (2008) Human erythrocytes are affected in vitro by flavonoids of Aristotelia chilensis (Maqui) leaves. Int J Pharm 363(1):85–90
Taraphdar AK (2001) Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr Sci 80(11):1387–1396
Zavodnik IB, Lapshina EA, Zavodnik LB, Bartosz G, Soszynski M, Bryszewska M (2001) Hypochlorous acid damages erythrocyte membrane proteins and alters lipid bilayer structure and fluidity. Free Radic Biol Med 30:363–369
Acknowledgments
The first author gratefully acknowledges INSPIRE-DST, Government of India (C/2003/1FD/2014-15), for the award of fellowship. It is grateful to acknowledge Anjali Prashar, Hypha Discovery Ltd, UK, for providing supporting literature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Handling Editor: Jan Raoul De Mey
Rights and permissions
About this article
Cite this article
Bhagyanathan, N.K., Thoppil, J.E. Pre-apoptotic activity of aqueous extracts of Cynanchum sarcomedium Meve & Liede on cells of Allium cepa and human erythrocytes. Protoplasma 253, 1433–1438 (2016). https://doi.org/10.1007/s00709-015-0898-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0898-y