Abstract
2,4-Dichlorophenoxyacetic acid (2,4-D) is a synthetic plant growth regulator that is highly toxic to most broad leaved plants and relatively nontoxic to monocotyledonous plants; is frequently used as weed killer. The study aimed to investigate cytogenetic effects of different concentrations of 2,4-D (0.67, 1.34, 2.01, 2.68, 3.35 and 4.02 mg/L) on Allium cepa bulblets’ root tips treated for 24 and 48 h. The results showed six types of structural aberrations: C-mitosis, stickiness, laggards, bridges, fragments and multipolarity that varied numerically compared to control. It significantly affected mitotic index (MI) at 24 and 48 h treatment. In the Allium test, MI increased significantly at three lower concentrations (0.67, 1.34, 2.01 mg/L) after treatment with 2,4-D for 24 h and decreased significantly at higher concentration. Whereas, 2,4-D treatment for 48 h increased MI at all concentrations with significantly decreased MI at the highest concentration. The experiment was extended using comet test that did not reveal significant difference among treatments except for application of 4.02 mg/L 2,4-D for 48 h; where cell damages were verified by comet test. Rest of the concentrations for any duration of time were not damaging and toxic to cells. The results showed, visible mitodepressive action of 4.02 mg/L 2,4-D when treated for 48 h that had tendency to become toxic if the roots had been in touch with 2,4-D for a longer time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4-Dichlorophenoxyacetic acid (2,4-D) is a post-emergence systemic broad leaf herbicide used for selective control of broadleaf plants and as powerful plant growth regulator (auxin) at low concentrations (Kaynak and Memiş 1997; Truta et al. 2011).
Every year number of new studies are reported explaining different mechanisms related to toxic actions of 2,4-D, a common herbicide on plants, animals and human cells (Fantel 1996; Don-Pedro 1996; Coppen and Jepsons 1996; Schrader et al. 1999; Heiden et al. 2006; Hardell 2008; Cogliano et al. 2011; Mortensen et al. 2012; Von Stackelberg 2013; Song 2014). Degradation by soil micro organisms is assumed as main cause of 2,4-D decomposition in soil. 2,4-D half life in soil is 7–10 days depending on environmental conditions like nutrient composition, moisture, oxygenation, temperature etc. (Vogue et al. 2004; Gilbert 2014; Anonymous 2015a). Contrarily, 2,4-D is reported to have relative persistence in anaerobic aquatic environments with low oxygen. Here half-life of 2,4-D ranges 41–333 days (USEPA 2005).
Studies on 2,4-D has pointed out number of long term adverse effects of 2,4-D and its formulations on human health, terrestrial plants, aquatic life and environmental risks with strong indications that these have been underestimated (Anonymous 2015b). At present it is well known that 2,4-D can disturb some chemical reactions by interacting with phospholipids generating free radicals causing lipid peroxidation. It also binds to enzymes resulting in change of their activity (Fantel 1996; Don-Pedro 1996). Increased lipid peroxidation and oxidative stress can affect the activities of protective enzymatic antioxidants that have been shown to be sensitive indicators of increased oxidation reactions (Fantel 1996; Schrader et al. 1999).
Higher plants are perceived as great hereditary models to distinguish environmental mutagens and are regularly utilized for the purpose. Allium is regularly used to assess damages like chromosome aberrations and disturbances in the mitotic cycle since it has large chromosomes. In addition, the Allium cepa test has indicated high affectability and great relationship when compared to other test systems (Morais Leme and Marin-Morales 2009).
Uses of comet test in plants are still restricted compared to their use in animal systems. Comet test analyse cell reaction to genotoxic agents and additionally a few biotic and abiotic stresses that prompt oxidative DNA damage. Comet test can help in evaluating the capability of higher plants as steady sensors in biological communities and source of data on the genotoxic effect of perilous contaminations (Ventura et al. 2013).
No previous report suggests possible actions of 2,4-D on A. cepa L. (2n = 16) roots in agar solidified MS (Murashige and Skoog 1962) medium. This study aimed to distinguish effects of conditioning of different concentrations of 2,4-D for 24 and 48 h in agar solidified MS medium on A. cepa bulblets’ root tips cytogenetically and confirm these by comet test.
Materials and methods
Plant material and surface sterilization
Healthy, unbruised 10–15 mm diameter bulbs of A. cepa L. (2n = 16) were used as the test plant. The bulblets were purchased from the open market. The A. cepa bulblets were surface sterilized with 20, 25, 30 and 35 % commercial bleach (5 % NaOCl) for 10 min. After the surface sterilisation, the bulblets were rinsed 3 × 5 min in double distilled sterilized water.
Media and culture conditions
Bulblets were cultured under aseptic conditions on MS basal medium (Catalogue No. M5519, Sigma-Aldrich Chemie GmbH, STeinheim, Germany) for growth and development of roots that grew to 1.5–2 cm length in 5 days time.
Previous studies reported 0.01–5 mg/L 2.4 D as optimum concentration for regeneration in tissue culture (Smith 1992; George 1993; Franklin and Dixoni 1994; Ateeq et al. 2002). Therefore, growth inhibition test was performed as described by Fiskesjo (1988) with minor modifications using roots induced on 12 bulblets. The roots were treated with 0.67, 1.34, 2.01, 2.68, 3.35 and 4.02 mg/L 2,4-D (PESTANAL®, analytical standard (Fluka, Buchs, Switzerland) 2,4-dichlorophenoxyacetic acid, Empirical Formula, C11H15NO2, Mol. Weight: 193.24, Catalogue No. 31518 from Sigma-Aldrich Chemie GmbH) in MS medium solidified with 0.7 % (w/v) agar (Sigma Agar type A and 30 g/L sucrose) for 24 and 48 h. A control was also planted using untreated roots on MS medium solidified with 0.7 % (w/v) agar and 30 g/L sucrose for 24 and 48 h. The stock solution was prepared by solving 1 mg 2,4-D in 0.2 mL ethanol followed by making 1 mL volume with double distilled bioinised water; which was stored at 4 °C.
Each experimental treatment used 12 bulblets divided into six replications such that each replication contained 2 explants. The experiment was conducted by inducing roots on MS medium containing 2,4-D or 2,4-D added to MS medium after development of roots. The bulblets that induced roots on MS medium were transferred to 2,4-D containing medium after 5 days of root induction. pH of all culture media was adjusted to 5.7 ± 0.1 using 1 M KOH or 1 M HCl before adding agar. All media were autoclaved at 104 kPa pressure at 121 °C for 20 min.
All cultures were incubated in growth chamber at 24 ± 2 °C under 16 h light (42 µMol photons−2 s−1) and 8 h dark photoperiod.
Preparation of root tip materials for cytogenetic analyses
Root tips obtained from all experimental treatments were fixed in 3:1 absolute alcohol:acetic acid at 4 °C for 16 h and then they were transferred to 70 % alcohol. Following all treatments, the root tips were macerated, stained, and squashed as described earlier by Yüzbaşıoğlu et al. (2003). Cytological preparations were made from ten bulblet roots using Feulgen schedule (Darlington and La Cour 1976) and made permanent by mounting in Depex (Catalogue No. 06522, Sigma-Aldrich Chemie GmbH, Riedstrasse).
The mitotic index (MI) was calculated from 1000 cells and counting the stages of mitotic cells and mitotic abnormalities. Slides were prepared from different bulblets; 1000 cells were screened from each of 10 bulblets yielding 10,000 cells for each concentration and duration of time.
Isolation of nuclei and the comet (single cell gel electrophoresis) assay
Firstly A. cepa roots were exposed to MS medium for 5 days. Thereafter, these were subjected to 0.67, 1.34, 2.01, 2.68, 3.35 and 4.02 mg/L of 2,4-D concentrations for 24 and 48 h similar to those used for cytogenetic analysis described above. Slides were prepared and stained as described by Juchimiuk et al. (2006) and Justyna et al. (2012) with some modifications. The roots were cut 5 mm from their ends and about 1–2 mm of root tips were used for the test. These root tips were immersed in 400 µL cold 0.4 M Tris–HCl buffer (pH = 7.5) on Petri dishes placed on ice for 2 min. All treatments were conducted in dim red light. The roots were gently sliced and isolated for collection of root nuclei in the buffer. Microscope slides were pretreated by spreading two fold 1 % normal melting point (NMP) agarose in distilled water evenly over the slide and allowing the agarose to harden. Thereafter, 90 µL suspension of nuclei mixed with 90 µL 0.75 % agarose (1:1) (low-melting point agarose) in Tris–HCl buffer was spread on the slides. Thereafter, the slides were covered with a coverslip, kept on ice for 5 min and then coverslips were removed. Then, 90 µL of 0.75 % agarose (low-melting point agarose) was spread on each of the slides and the slides were covered with a coverslip again. Following coverslips were removed and the slides were placed on horizontal electrophoresis tank filled with freshly prepared cold electrophoresis buffer (300 mM NaOH, 1 mM EDTA pH > 13) kept cold using ice. The slides were incubated in the buffer for 20 min to facilitate DNA unwinding followed by performing electrophoresis for 20 min at 27 V, 300 mA at 4 °C. The slides were washed three times with 0.4 M Tris–HCl buffer (pH = 7.5). Each slide was stained with 80 µl of ethidium bromide (1:4 EtBr:distilled water) and kept at 4 °C until analysis. The comet analysis was performed on randomly selected 25 nuclei by using specialized image analysis system (Comet Assay IV, Perceptive Instruments Ltd., UK).
Statistical analysis
The data obtained were analysed using z test for the MI and, χ2 test for the frequency of mitotic phases and mitotic abnormalities. Comet test values were analysed and subjected to t test to detect differences between the treatment and control groups.
Results and discussions
The results showed contamination of root tips treated with 20 % commercial bleach for 10 min sterilization. No contamination was noted after treatment with all other concentrations. On the other hand, minimum damage to cell walls of bulblet scale cells was noted after treatment with 25 % commercial bleach, as noted under microscope (results not shown). At higher concentrations of commercial bleach, considerable damages were noted on cells. The surviving cells varied slightly, swelled due to commercial bleach interaction and lost their squared shape outlines. Therefore, 25 % commercial bleach treatment for 10 min was preferred for surface sterilization of bulblets throughout the study.
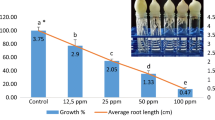
After culturing in 2,4-D containing media, the bulbs formed small and abnormal root structures (Fig. 1a) compared to those cultured in agar solidified MS medium-control (Fig. 1b). It was difficult to take samples for analysis and calculate their root length and EC50 value. The results showed that roots were very sensitive to prolong treatment of 2,4-D. Both genotoxicity and cytotoxicity can be monitored by using cytological parameters such as the number of chromosome breaks and micronuclei, MI, chromosome and mitotic damage. Therefore, this test could be used for easy detection of chemicals having mutagenic effect in the laboratory (Grant 1999) using macroscopic parameters of root growth and root morphology (Fiskesjo 1993).
However, the bulblets that induced roots on MS medium for 5 days followed by their culture on different concentrations of 2,4-D for 24 and 48 h showed variable responses. It is known that changes in DNA can occur during in vitro culture (Cecchini et al. 1992; Zheng 1991; Puolimatka and Karp 1993).
Mitotic index in root tip cells among different concentrations of 2,4-D on chromosomes after 24 h treatment varied significantly (P < 0.001) that ranged from 3.93 ± 0.19 to 10.7 ± 0.31 compared to the control (Table 1). MI of control treatment was 8.01 ± 0.27. Maximum MI was noted in root cells treated with 1.34 mg/L 2,4-D. MI significantly increased at the three lower concentrations (0.67, 1.34, 2.01 mg/L); and decreased at three higher concentrations (2.68, 3.35 and 4.02 mg/L). Similarly, after 48 h treatment of 2,4-D, MI among different concentrations varied significantly that ranged from 9.07 ± 0.28 (at 1.34 mg/L) to 3.10 ± 0.17 (at 4.02 mg/L) compared with control (Table 1). MI of control remained at 4.81 ± 0.21. Maximum MI was noted on 1.34 mg/L treated roots. 2,4-D treatment significantly decreased the MI only at the highest concentration (4.02 mg/L). However, it was interesting to note that MI significantly increased at the 1.34, 2.01, 2.68 and 3.35 mg/L concentrations at 48 h 2,4-D treatment. Fernandes et al. (2007) suggested that the cytotoxicity level of a chemical could be measured by increase or decrease in MI. If MIs are lower than the negative control, they indicate changes due to chemical actions during growth and development of organisms (Hoshina 2002). Whereas, Morais Leme and Marin-Morales (2009), emphasized that if MIs are higher than the negative control, they show accelarated cell division, which can be damaging to the cells, prompting a disarranged cell multiplication and can lead to the development of oncogenic tissues. The reduction in the MI clearly indicates the mitodepressive and cytotoxic effects of 2,4-D in the present experiment. Similarly, Kocaman and Güven (2015) found that 1-naphthaleneacetamide (NAAm) caused statistically significant reduction both in the MI after 48 h treatment period and in the nuclear division index (NDI) after 24 and 48 h treatment periods as compared to the control groups.
Application of 2,4-D for 24 and 48 h resulted in increased rate of prophase cells compared to the control groups. The results are in clear agreement with observations of Mohandas and Grant (1972); Tomkins and Grant (1976), Prasad and Das (1977) and Kumari and Vaidyanath (1989).
Metaphase and anaphase cell rates were lower than the control groups at all (except 0.67 mg/L concentration of the 48 h) treatments. These results show that, mitotic anaphase decreased variably and irregularly with each increase in the concentration of 2,4-D. All rates of cell divisions in telophase increased after 24 h application of 2,4-D (except 3.35 and 4.02 mg/L concentrations). Forty eight (48) h treatment showed different rates of divisions in telophase at different concentrations. However, the rate of changes in different phases was statistically non significant. Similarly, Liman et al. (2011) investigated the genotoxic effects of fenaminosulf, fungicide and micro-biocide, and found that rate of division of prophase cells increased at 24 h treatment at 12.5 and 50 ppm. Furthermore, they found that rate of division of cells decreased at metaphase, anaphase and telophase in all treatment concentrations compared to control at each exposure period. Truta et al. (2011) treated common beans root meristems with 1–10 mg/L 2,4-D concentrations to common beans root meristems and noted prophase > metaphase > anaphase > telophase decreasing order of cell division at mitotic phases. 2,4-D treatment decreased the rate of prophase activity. However, using 1 mg/L 2,4-D an increased metaphase or decreased anaphase and at 10 mg/L a decreased metaphase or increased anaphase activity was noted in root meristems of radish. They also reported that the cell rate in telophase increased in radish root meristems. These results are different from the results reported in this study. This difference may have resulted from the differences of plant materials and concentrations of 2,4-D used in these experiments.
Application of 2,4-D for 24 and 48 h in A. cepa roots increased the frequency of mitotic abnormalities compared to the control. However, this increase was not statistically significant. Six types of structural aberrations were recorded: C-mitosis, stickiness, laggards, bridges, fragments and multipolarity (Fig. 2).
C-mitosis values varied between 0.09 and 0.96 at both treatment periods (Table 2). Zeljezic and Garaj-Vrhovac (2004) reported that 2,4-D caused increase in chromatid and chromosome breaks, number of micronuclei and nuclear buds. Difference of results in this study and those reported in this experiment could be attributed to the use of different methodologies, treatments and protocols used in the two studies. The mechanisms of chromosomal damage due to 2,4-D is poorly understood. Previous reports suggested that 2,4-D affected the frequency of homologous recombination in Arabidopsis, Raphanus sativus L. and Phaseolus vulgaris L. (Filkowski et al. 2003; Truta et al. 2011). Differences in C-mitosis results may have their connection with inactivation of the mitotic spindle subsequent to an unarranged scattering of the condensed chromosomes (Levan 1938).
Lagging chromosomes were noted on treatments with 2.68 and 3.35 mg/L of 2,4-D with the frequency of 0.39 and 0.43, respectively, at 24 h treatment. At 48 h treatment period, 0.67 and 2.68 mg/L concentrations induced lagging chromosomes with the frequency of 0.37 and 0.15, respectively. Lagging chromosomes arise mainly due to abnormal spindle formation and as a result, spindle fibre failed to carry respective chromosomes to the polar regions with appearance of lagging chromosomes (Tarar and Dyansagar 1980; Tartar et al. 2006; Pulate and Tarar 2014).
At 24 h exposure time, chromosome stickiness was noted for concentrations of 2,4-D at 0.67, 2.01 and 2.68 mg/L with the frequency of 0.14, 0.10, 0.92, respectively. At 48 h exposure, 0.67, 1.34, 2.01 and 4.02 mg/mL concentrations induced stickness at the frequency of 0.57, 0.12, 0.22 and 0.32, respectively. The presence of sticky chromosomes might have resulted from uncoiling of chromosomes in abnormal way during anaphase to telophase (Qian et al. 2006). It is assumed that the sticky nature of chromosomes is due to delay in chromosome movement by 2,4-D treatment. Thus the chromosomes could not reach the poles and remained scattered in the cytoplasm and appeared condensed and sticky (Ajay and Sorbhoy 1988).
In this study, bridges were observed at all concentrations of 2,4-D and control groups at both treatment periods. Bridges were found to be the result of stickiness of chromosome (El-Ghamery et al. 2000). Chromosomal bridges mainly arises due to the non disjunction of sticky chromosome or breakage and reunion during separation at anaphase (Koduru and Rao 1981).
Fragments were noted on treatment with 0.67 and 2.68 mg/L 2,4-D treatments with values of 0.10 and 0.13 at 24 h treatment, respectively. At 48 h treatment, fragments were observed at only 2.01 mg/L concentration with value of 0.11. The failure to reunite broken segments to the original chromosomes lead to formation of fragments (Kumar 2010). Multipolars were noted both for 24 and 48 h treatments each except for 1.34, 2.01 mg/L 2,4-D at 24 h and 2.01, 3.35 mg/L 2,4-D at 48 h. These ranged 0.19–0.64. Morais Leme and Marin-Morales (2009) explained that the chromosome bridges and breaks are indicators of a clastogenic action, whereas chromosome losses, delays, adherence, multipolarity and C-metaphase results from aneugenic effects.
All these aberrations including C-mitosis, chromosome stickiness, bridges and the above mentioned other irregularities are in agreement with Ateeq et al. (2002). Ateeq et al. (2002) noted cytological abnormalities in the meristematic mitotic cells of A. cepa. They found that 2,4-D evidently induced morphological changes at higher concentrations. Some changes like crochet hooks, c-tumours and broken roots were unique to 2,4-D at 5–20 ppm (5–20 mg/L) and the chromosome aberrations were statistically significant. The results are also in agreement with Mohandas and Grant (1972). They studied cytological effects of 2,4-D on A. cepa, Triticum aestivum, T. dicoccum, Hordeum vulgare, Secale cereale and found cytological abnormalities induced in root-tip cells. They found that 2,4-D caused chromosome bridges, fragments, lagging chromosomes, and chromatin bodies. 2,4-D also induced chromosome contraction and C-mitosis. Similarly, Kumar (2010) found that 2,4-D exerted a threat on economically important crops by cytological damage to the cells. The researcher found that 50–1200 ppm 2,4-D treatment for 72 h at room temperature had negative effects on chromosomal morphology in mitotic cells of T. aestivum. The researcher also found twelve distinct chromosome structure abnormalities over control. The observed irregularities were stickiness, c-mitosis, multipolar chromosomes with or without spindles, fragments and bridges, lagging chromosomes, unequal distribution of chromosomes, over contracted chromosomes, unoriented chromosomes, star shaped arrangement of the chromosomes, increased cell size and failure of cell plate formation. Truta et al. (2011) reported cytogenetic effects induced by 2,4-D in root meristems of R. sativus L. and P. vulgaris L. on MI, rate and categories of ana-telophase chromosome aberrations. They found that 2,4-D (1 and 10 mg/L) had a slightly cytotoxic effect in radish, but did not show genotoxic potential for P. vulgaris. Kumar and Jagannath (2015) observed that the chromosomal abnormalities increased as the concentration of the butachlor herbicide increased when compared to control in wheat. They observed sticky chromatin, chromosomal bridge, nuclear lesion, scattered chromosome, fragmented metaphase, fragmented anaphase, multipolar chromosome and micronuclei. They explained that butachlor can produce negative effects on mitotic division in somatic cells of wheat.
Results obtained with the comet assay are summarized in Table 3. DNA damage was not significantly high in treatments containing different concentrations of 2,4-D for 24 and 48 h compared to the control (P < 0.05). Mean tail intensity ranged from 39.30 ± 4.60 to 67.42 ± 5.52; which did not show any significant difference compared to control. Mean tail moment ranged from 54.06 ± 6.32 to 120.01 ± 20.53 that also showed significant differences compared to control for both treatment periods. Comet test confirmed presence of undamaged DNA after application of test (Fig. 3a). Although damaged cells were confirmed by comet test (Fig. 3b) after application of 4.02 mg/L 2,4-D for 48 h, their number was insufficient for clearly determining any trend.
Using comet assay Türkoğlu (2012) also confirmed single strand breaks and significant increase in DNA damage of A. cepa roots after 24–48 h treatment with chlorfenvinphos and fenbuconazole. The roots were treated with 10, 20, 40, 60, 80, and 100 ppm concentrations for 24 and 48 h. The researcher noted that DNA damage was significantly high in treatments of both chemicals compared to the control. In addition, the researcher also reported that chlorfenvinphos and fenbuconazole significantly increased the frequency of abnormal cells at all concentrations and treatment periods when compared to their controls. Setha et al. (2008) also confirmed that cytogenetic and DNA damage effects of Cadmium could be tested by comet assay on the root meristem cells of A. cepa that were exposed to 10, 20, and 40 mL for 24 h and also left in water for 24 h recovery. They reported that exposure of Cd revealed significant inhibition of MI, induction of CA, mitotic aberrations (MA), and micronucleus (MN) formation. In addition, they explained that at 40 mM significant increase in the tail length and tail moment was observed as compared to control. However, cells examined at 24 h post-exposure showed concentration-dependent decline in all the endpoints. Findings of their study confirmed that the root meristem cells of A. cepa are a suitable model for detecting both the environmentally induced CA as well as DNA damage analyzed by Comet assay. Yıldız et al. (2009) reported that copper sulphate (CS) and cobalt chloride (CC) increased total chromosome aberrations with an increase in the exposure time (24 and 48 h) and the concentrations of both chemicals (CS 1.5, 3 ppm and CC 5.5, 11 ppm) in A. cepa root tips. They reported that in all concentrations, CS and CC induced a significant increase in DNA damage. Therefore, the researchers confirmed that the chromosome aberration and comet assays are still the preferred plant genotoxicity tests of many researchers in a global, comparative analysis of chromosome abnormality and DNA damage in plants due to environmental pollutions. The results of these assays are suitable for monitoring genotoxicity of environmental pollutants using plants. Liman et al. (2011), using Fenaminosulf, fungicide and micro-biocide also noted a significant increase in DNA damage at a concentration of 25 ppm used for 24 h, 25 and 50 ppm for 96 h by Comet assay in A. cepa root tips.
Conclusion
2,4-D treatment after 48-h, significantly decreased MI at the highest concentrations as confirmed cytologically and by comet test. The results of the present study clearly show negative cytogenetic effects of 4.02 mg/L 2,4-D for 48 h in plant tissue culture studies that could result in undesirable variations affecting genetic purity of treated plant material. The other concentrations for any duration of time were not damaging and toxic to cells. The results of this study also showed that 2,4-D may not have clastogenic and/or mutagenic potential at low concentrations in cultured A. cepa root tips.
References
Ajay KJ, Sorbhoy RK (1988) Cytogenetic studies on the effect of some chlorinated pesticide. Cytologia 53:427–436
Anonymous (2015a) Extension toxicology network. Pesticide information profile—2,4-D. June 1996. Accessed 18 Oct 2015
Anonymous (2015b). https://www.testbiotech.org/sites/default/files/Risks%20of%20herbicide%202_4-D0.pdf. Accessed 18 Oct 2015
Ateeq B, Farah MA, Ali MN, Ahmad V (2002) Clastogeneticity of pentachlorophenol, 2,4-D and butachlor evaluated by Allium root tip test. Mutat Res 514:105–113
Cecchini E, Natali L, Cavallini A, Durante M (1992) DNA variations in regenerated plants of pea (Pisum sativum L.). Theor Appl Genet 84:874–879
Cogliano VJ, Baan R, Straif K et al (2011) Preventable exposures associated with human cancers. J Natl Cancer Inst 103:1827–1839
Coppen GDA, Jepsons PC (1996) The effects of the duration of exposure on the toxicity of diflubenzuron, hexaflumuron and teflubenzuron to various stages of II instar Schistocerca gregaria. Pestic Sci 46:191–197
Darlington CD, La Cour LE (1976) The handing of chromosomes. George Allen and Unwin Ltd., London, p 201
Don-Pedro KN (1996) Fumigant toxicity is the major route of ınsecticidal activity of citruspeel essential oils. Pestic Sci 46:71–78
El-Ghamery AA, El-Nahas AI, Mansour MM (2000) The action of atrazine herbicide as an indicator of cell division on chromosomes and nucleic acids content in root meristems of Allium cepa and Vicia faba. Cytologia 65:277–287
Fantel AG (1996) Reactive oxygen species in developmental toxicity: review and hypothesis. Teratology 53:96–217
Fernandes TCC, Mazzeo DEC, Marin-Morales MA (2007) Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol 88:252–259
Filkowski J, Besplug J, Burke P et al (2003) Genotoxicity of 2,4-D and dicamba revealed by transgenic Arabidopsis thaliana plants harboring recombination and point mutation markers. Mutat Res 542:23–32
Fiskesjo G (1988) The Allium test—an alternative in environmental studies: the relative toxicity of metal ions. Mutat Res 197:243–260
Fiskesjo G (1993) The Allium test in waste-water monitoring. Environ Toxicol Water Qual 8:291–298
Franklin CI, Dixoni RA (1994) Initation and maintenance of callus and cell suspension cultures. In: Dixon RA, Gonzales RA (eds) Plant tissue culture—a pratical approach, 2nd edn. Oxford University Press, Oxford, pp 1–27
George EF (1993) Plant propagation by tissue culture. Part I. The technology, second education. Exegenetics Ltd, England, p 57
Gilbert SG (2014) 2,4-D. In: McKeen S (ed). http://www.toxipedia.org/display/toxipedia/2,4-D. Accessed 18 Oct 2015
Grant WF (1999) Higher plant assays for the detection of chromosomal aberrations and gene mutations–a brief historical background on their use for screening and monitoring environmental chemicals. Mutat Res 426:107–112
Hardell L (2008) Pesticides soft-tissue sarcoma and non-Hodgkin lymphoma—historical aspects on the precautionary principle in cancer prevention. Acta Oncol 47:347–354
Heiden TK, Carvan MJ III, Hutz RJ (2006) Inhibition of follicular development, vitellogenesis, and serum 17 beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 90:490–499
Hoshina MM (2002) Avaliação da possível contaminação das águas do Ribeirão Claro município de Rio Claro, pertencente à bacia do rio Corumbataí, por meio de testes de mutagenicidade em Allium cepa, Trabalho de conclusão (Bacharel e Licenciatura - Ciências Biológicas). Universidade Estadual Paulista, Rio Claro, p 52
Juchimiuk J, Gnys A, Maluszynska J (2006) DNA damage induced by mutagens in plant and human cell nuclei in acellular comet assay. Folia Histochem Cytobiol 44:127–131
Justyna GUZY, Zgórska A, Ziembińska A (2012) Comet assay optimization with Allium cepa as an indicator for ecotoxicological usage. Archit Civ Eng Environ 5:109–116
Kaynak L, Memiş M (1997) Bitki büyüme engelleyici ve geciktiricilerinin etki mekanizmaları. Akd Üniv Zir Fak Dergisi 10:237–248
Kocaman Y, Güven B (2015) In vitro genotoxicity assessment of the synthetic plant growth regulator, 1-naphthaleneacetamide. Cytotechnology 1–10
Koduru PRK, Rao MK (1981) Cytogenetics of synaptic mutants in higher plants. Theor Appl Genet 59:197–214
Kumar S (2010) Effect of 2,4-D and isoproturon on chromosomal disturbances during mitotic division in root tip cells of Triticum aestivum L. Cytol Genet 44:14–21
Kumar NKH, Jagannath S (2015) Cytological effects of herbicide butachlor 50 EC on Somatic cells of Triticum aestivum L. J Appl Biol Biotechnol 3:30–34
Kumari TS, Vaidyanath K (1989) Testing of genotoxic effects of 2,4-dichlorophenoxyacetic acid (2,4-D) using multiple genetic assay systems of plants. Mutat Res 226:235–238
Levan A (1938) The effect of colchicine on root mitosis in Allium. Hereditas 24:471–486
Liman R, Ciğerci IH, Akyıl D et al (2011) Determination of genotoxicity of Fenaminosulf by Allium and Comet tests. Pestic Biochem Physiol 99:61–64
Mohandas T, Grant WF (1972) Cytogenetic effects of 2,4-D and amitrole in relation to nuclear volume and DNA content in some higher plants. Can J Genet Cytol 14:773–783
Morais Leme D, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Mortensen DA, Egan JT, Maxwell BD et al (2012) Navigating a critical juncture for sustainable weed management. Bioscience 62:75–84
Murashige T, Skoog FA (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–479
Prasad G, Das K (1977) Effects of some growth substances on mitosis. Cytologia 42:323–329
Pulate PV, Tarar JL (2014) Cytogenetic effects of tilt on root tip meristem of onion Allium cepa L. Int J Plant Anim Environ Sci 4:53–57
Puolimatka M, Karp A (1993) Effect of genotype on chromosome variation in tissue culture of inbred and outbred rye. Heredity 71:138–144
Qian XW, Luo WH, Zheng OX (2006) Joint effects of microwave and chromium trioxide on root tip cells of Vicia faba. J Zhejiang Univ Sci 7:221–227
Schrader KK, Rimando AM, Tucker CS, Duke SO (1999) Factors affecting toxicity of ferulate towards the cyanobacterium Oscillatoria cf chalybea. Pestic Sci 55:726–732
Setha CS, Misraa V, Chauhanb LKS, Singh RR (2008) Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and comet assay approach. Ecotoxicol Environ Saf 71:711–716
Smith R (1992) Plant tissue culture techniques and experiments. Academic Press Inc, University College Station, pp 1–22
Song Y (2014) Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56:106–113
Tarar JL, Dyansagar VR (1980) Effect of gamma rays and EMS on growth sand branching in Turneria ulmifolia L. J Cytol Genet 14:118–124
Tartar G, Kaymak F, Muranli FDG (2006) Genotoxic effects of avenoxan on Allium cepa L. and Allium sativum L. Caryologia 59:241–247
Tomkins DJ, Grant WF (1976) Monitoring natural vegetation for herbicide induced chromosomal aberrations. Mutat Res 36:73–84
Truta E, Zamfirache MM, Rosu C et al (2011) Cytogenetic effects induced by 2,4-D and kinetin in radish and common bean root meristems. Rom Agric Res 28:207–215
Türkoğlu Ş (2012) Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pestic Biochem Physiol 103:224–230
USEPA (2005) 2,4-D red facts. http://www.epa.gov/oppsrrd1/REDs/factsheets/24d_fs.htm. Accessed 18 Oct 2015
Ventura L, Giovannini A, Savio M et al (2013) Single cell gel electrophoresis (comet) assay with plants: research on DNA repair and ecogenotoxicity testing. Chemosphere 92:1–9
Vogue PA, Kerle EA, Jenkins JJ (2004) 2,4-D technical fact sheet. Osu extension pesticide properties database. Oregon State University, Corvallis
Von Stackelberg K (2013) A systematic review of carcinogenic outcomes and potential mechanisms from exposure to 2,4-D and MCPA in the environment. J Toxicol 2013:1–53
Yıldız M, Ciğerci IH, Konuk M et al (2009) Determination of genotoxic effects of copper sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere 75:934–938
Yüzbaşıoğlu D, Ünal F, Sancak C (2003) Cytological effects of the herbicide racer “flurochloridone” on Allium cepa. Caryologia 56:97–105
Zeljezic D, Garaj-Vrhovac V (2004) Chromosomal aberrations, micronuclei and nuclear buds induced in human lymphocytes by 2,4-dichlorophenoxyacetic acid pesticide formulation. Toxicology 200:39–47
Zheng S (1991) Chromosome variation in callus culture of Gossypium hirsutum L. In: Adams RP (ed) Conservation of plant genes, DNA banking and in vitro biotechnology. Academic Press, San Diego, pp 211–221
Acknowledgments
The authors would like to thank the Gazi University Research Fund for financial support under Grant No. 04/2014-02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özkul, M., Özel, Ç.A., Yüzbaşıoğlu, D. et al. Does 2,4-dichlorophenoxyacetic acid (2,4-D) induce genotoxic effects in tissue cultured Allium roots?. Cytotechnology 68, 2395–2405 (2016). https://doi.org/10.1007/s10616-016-9956-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-9956-3