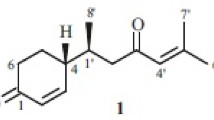
A new sesquiterpenoid, ar-tumerdiol (1) as well as ar-tumerone (2), α-tumerone (3), β-tumerone (4), 4-(1′ ,5′ -dimethyl-3′-oxo-4′-hexenyl)-2-cyclohexen-1-one (5), turmeronol (6), curcumin (7), and hexahydrocurcumin (8), were isolated from the rhizomes of Curcuma longa (Zingiberaceae). The structure of the new sesquiterpenoid was elucidated by chemical and physical evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Traditional system of medicine consists of large number of plants with various medicinal and pharmacological importance and hence represents a valuable source of new bioactive molecules. Curcuma (family Zingiberaceae) is a genus containing 70 known species that has been historically used as a spice, food preservative, and coloring material. Curcuma longa L. is distributed throughout the tropical and subtropical regions of the world. It is used in traditional medicine as a household remedy for various diseases. Also, C. longa has been reported to possess multiple pharmacological activities, including antioxidant, antimicrobial, anti-inflammatory, anticarcinogenic, anticoagulant, antidiabetic, and immunological [1, 2]. In continuation of studies of chemotaxonomy and biologically active metabolites from Zingiberaceous plants [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20], a methanol extraction of the rhizomes of C. longa afforded one new sesquiterpenoid, ar-tumerdiol (1). As part of our continuing investigation of the phytochemical and bioactive compounds of Zingiberaceous plants, ar-tumerdiol (1), ar-tumerone (2) [21], α-tumerone (3) [22], β-tumerone (4) [23], 4-(1′,5′-dimethyl-3′-oxo-4′-hexenyl)-2-cyclohexen-1-one (5) [24], turmeronol (6), curcumin (7) [25], and hexahydrocurcumin (8) [26], were obtained by systematic extraction and isolation from the rhizomes of C. longa. In this paper, we report the isolation and structural elucidation of this new compound (1).
ar-Tumerdiol (1), a yellow oil, was deduced as C15H24O2 by HR-MS-ESI (m/z 259.1671 [M + Na]+; calcd 259.1674). The IR spectrum showed an absorption band for a hydroxyl group at 3450 cm–1. The 1H NMR spectrum of 1 showed four aromatic protons at δ 7.09 (4H, m), two methylene groups at δ 1.62/1.89 (H-4′) and 1.23 (H-2′), two methine protons at δ 3.35 (1H, m, H-3′) and 2.68 (1H, m, H-1′), and three methyl protons at δ 2.32 (3H, s, H-7), 1.24 (3H, s, H-6′), and 1.23 (3H, s, H-7′) and one doublet one at 1.13 (3H, d, J = 4.8 Hz, H-8′), indicating that 1 is probably a bisabolane-type sesquiterpene. The carbons of 1 were assigned, from 13C NMR and DEPT experiments, four methyl at δ 20.9 (C-7), 22.8 (C-8′) 23.1 (C-7′), and 26.5 (C-6′), two methylene at δ 29.9 (C-2′) and 35.5 (C-4′), six methines at δ 39.7 (C-1′), 78.8 (C-3′), 126.8 (C-3, 5), and 129.1 (C-2, 6), and three quaternary carbons at δ 73.1 (C-5′), 135.3 (C-1), and 144.1 (C-4). Complete unambiguous assignments for the 1H and 13C NMR signals were made by a combination of COSY, HSQC, HMBC (Table 1), and NOESY spectra. The HETCOR experiment showed that the carbon/proton signals at δ 20.9/2.32 for C-7, 22.8/1.13 for C-8′, 23.1/1.23 for C-7′, 26.5/1.24 for C-6′, 29.9/1.23 for C-2′, 35.5/1.62, 1.89 for C-4′, 39.7/2.68 for C-1′, 78.8/3.35 for C-3′, 126.8/7.09 for C-3 and C-5 and 129.1/7.09 for C-2 and C-6, respectively. The observation of the NOESY correlations between H-6′, H-7′, and H-4′, between H-2′, H-3′, and H-4′, between H-1′, H-2′, H-8′, H-3, and H-5 and between H-7, H-2, and H-6 established the connective site as shown in structure 1. Thus, the structure of this compound was further confirmed by HMBC experiments (Table 1).

The compound is dehydrogenated to obtain the analogue (+)-ar-curcumone. The measured optical rotation value is close to zero. It can be inferred that the compound is racemic. The compound was determined to be an unrecorded substance after searching the SciFinder database.
Experimental
General. UV spectra were obtained in MeCN, IR spectra were measured on a Hitachi 260-30 spectrophotometer. 1H NMR (400 MHz), 13C NMR (100 MHz), HETCOR, HMBC, COSY, and NOESY spectra were obtained on a Varian (Unity Plus) NMR spectrometer. Low-resolution ESI-MS spectra were obtained on an API 3000 (Applied Biosystems) and high-resolution ESI-MS spectra on a Bruker Daltonics APEX II 30e spectrometer. Silica gel 60 (Merck, 70–230 mesh, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254), 0.20 mm and 0.50 mm, were used for analytical TLC and preparative TLC respectively, visualized with 50% H2SO4.
Plant Material. The rhizomes of C. longa were collected from Chiayi County, Taiwan, in April 2017. Plant material was identified by Dr. Fu-Yuan Lu (Department of Forestry and Natural Resources College of Agriculture, National Chiayi University). A voucher specimen was deposited at the Department of Medical Technology, School of Medical and Health Sciences, Fooyin University, Kaohsiung, Taiwan.
Extraction and Isolation. The rhizomes (0.1 kg) of C. longa were extracted repeatedly with MeOH (3 L × 2) at room temperature for 24–48 h. The MeOH extract was dried and evaporated to leave a viscous residue (21.2 g). The residue was placed on a silica gel column (4.8 kg, 70–230 mesh) and eluted with CH2Cl2 gradually enriched with MeOH to afford five fractions. Part of Fr. 1 (7.6 g) was subjected to silica gel chromatography (1.5 kg, 70–230 mesh) by eluting with n-hexane–acetone (200:1), enriched with acetone to furnish five fractions (1-1–1-5). Part of Fr.1-3 (2.5 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain ar-tumerone (2) (37 mg). Part of Fr. 1-5 (2.5 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain α-tumerone (3) (11 mg) and β-tumerone (4) (14 mg). Part of Fr. 2 (2.1 g) was subjected to silica gel chromatography (0.9 kg, 70–230 mesh) by eluting with n-hexane–acetone (100:1), enriched with acetone to furnish five fractions (2-1–2-5). Part of Fr. 2-2 (0.4 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain 4-(1′,5′-dimethyl-3′-oxo-4′-hexenyl)-2-cyclohexen-1-one (5) (12 mg). Part of Fr. 2-5 (0.5 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain ar-tumerdiol (1) (8 mg). Part of Fr. 3 (2.2 g) was subjected to silica gel chromatography (0.9 kg, 70–230 mesh) by eluting with n-hexane–acetone (90:1), enriched with acetone to furnish three fractions (3-1–3-3). Part of Fr. 3-2 (0.6 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain turmeronol A (6) (13 mg). Part of Fr. 4 (5.3 g) was subjected to silica gel chromatography (0.9 kg, 70–230 mesh) by eluting with n-hexane–acetone (80:1), enriched with acetone to furnish three fractions (4-1–4-3). Part of Fr. 4-2 (2.4 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain curcumin (7) (48 mg). Part of Fr. 5 (4.8 g) was subjected to silica gel chromatography (1.8 kg, 70–230 mesh) by eluting with n-hexane–acetone (70:1), enriched with acetone, to furnish three fractions (5-1–5-3). Part of Fr. 5-2 (1.8 g) was further purified on a silica gel column using n-hexane–acetone mixtures to obtain hexahydrocurcumin (8) (48 mg).
ar-Tumerdiol (1), yellow oil, \({\left[\mathrm{\alpha }\right]}_{\mathrm{D}}^{25}\) –0.38° (c 0.55, CHCl3). UV (CH3CN, λmax, nm) (log ε): 232 (3.94), 248 (3.52). IR (neat, νmax, cm–1): 3450 (OH), 870. ESI-MS m/z 259 [M + Na]+; HR-ESI-MS m/z 259.1671 [M + Na]+ (calcd for C15H24O2Na, 259.1674). 1H and 13C NMR, see Table 1.
ar-Tumerone (2). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 1.25 (3H, d, J = 6.8, H-14), 1.86 (3H, d, J = 1.2, H-13), 2.10 (3H, d, J = 1.2, H-12), 2.31 (3H, s, H-15), 2.62 (1H, dd, J = 15.6, 8.4, H-8a), 2.72 (1H, dd, J = 15.6, 6.0, H-8b), 3.29 (1H, m, H-7), 6.02 (1H, br.s, H-10), 7.10 (4H, d, J = 1.2, H-2, 3, 5, 6). 13C NMR (100 MHz, CDCl3, δ, ppm): 20.6 (C-12), 20.8 (C-15), 21.8 (C-14), 27.5 (C-13), 35.2 (C-7), 52.5 (C-8), 123.9 (C-10), 126.5 (C-2, 6), 129.0 (C-3, 5), 135.4 (C-4), 143.5 (C-1), 155.1 (C-11), 199.8 (C-9) [21].
α-Tumerone (3). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 0.89 (3H, d, J = 6.5, H-14), 1.71 (3H, s, H-13), 1.88 (3H, s, H-12), 2.14 (3H, s, H-15), 2.00–2.30 (5H, m, H-1, 6b,7,8), 2.50 (1H, dd, J = 14.5, 3.8, H-6a), 5.43 (1H, br.s, H-10), 5.65 (1H, dd, J = 9.8, 3.1, H-3), 5.80 (1H, ddd, J = 9.8, 1.9, 1.6, H-2), 6.06 (1H, t, J = 1.3, H-5). 13C NMR (100 MHz, CDCl3, δ, ppm): 17.1 (C-14), 21.0 (C-12), 21.1 (C-15), 25.2 (C-6), 27.6 (C-13), 33.0 (C-7), 38.0 (C-1), 48.7 (C-8), 120.4 (C-5), 124.2 (C-10), 128.4 (C-2), 129.5 (C-3), 131.4 (C-4), 155.1 (C-11), 200.8 (C-9) [22].
β-Tumerone (4). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 0.85 (3H, d, J = 6.5, H-14), 1.38 (1H, m, H-6a), 1.64 (1H, m, H-6b), 1.88 (3H, s, H-13), 2.14 (3H, s, H-12), 2.22 (2H, m, H-1, 5a), 2.25 (1H, m, H-7), 2.40 (1H, m, H-5b), 2.45 (2H, m, H-8), 4.76 (2H, br.s, H-15), 5.66 (1H, d, J = 10.0, H-2), 6.07 (1H, br.s, H-10), 6.16 (1H, dd, J = 10.0, 2.3, H-3). 13C NMR (100 MHz, CDCl3, δ, ppm): 16.5 (C-14), 20.7 (C-12), 24.9 (C-6), 27.5 (C-13), 30.1 (C-5), 33.4 (C-7), 40.5 (C-1), 48.6 (C-8), 110.3 (C-15), 124.1 (C-10), 130.1 (C-2), 133.8 (C-3), 143.4 (C-4), 155.0 (C-11), 200.9 (C-9) [23].
4-(1′,5′-Dimethyl-3′-oxo-4′-hexenyl)-2-cyclohexen-1-one (5). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 0.92 (3H, d, J = 6.8, H-8′), 1.78 (1H, m, H-5a), 1.90 (3H, s, H-6′), 1.96 (1H, m, H-5b), 2.15 (3H, s, H-7′), 2.31 (1H, m, H-2′a), 2.32 (1H, m, H-6a), 2.36 (1H, m, H-1′), 2.48 (1H, m, H-2′b), 2.50 (1H, m, H-4), 2.51 (1H, m, H-6b), 6.03 (1H, ddd, J = 10.0, 2.0, 1.2, H-3), 6.07 (1H, s, H-4′), 6.83 (1H, dt, J = 10.0, 2.0, H-2). 13C NMR (100 MHz, CDCl3, δ, ppm): 16.5 (C-8′), 20.8 (C-7′), 24.2 (C-5), 27.7 (C-6′), 32.7 (C-1′), 37.4 (C-6), 40.6 (C-4), 48.2 (C-2′), 123.8 (C-4′), 130.0 (C-3), 154.4 (C-2), 156.2 (C-5′), 199.7 (C-3′), 199.9 (C-1) [24].
Turmeronol A (6). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 1.21 (3H, d, J = 7.2, H-14), 2.10 (3H, s, H-13), 2.19 (3H, s, H-12), 2.30 (3H, s, H-15), 2.12 (2H, dd, J = 16.9, 8.4, H-8), 3.24 (1H, m, H-7), 5.83 (1H, br.s, H-10), 6.01 (1H, d, J = 1.6, H-2), 6.67 (1H, dd, J = 7.6, 1.6, H-6), 7.00 (1H, d, J = 7.6, H-5) [25].
Curcumin (7). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 3.68 (6H, s, 7, 7′-OCH3), 5.80 (2H, br.s, H-1, 1′), 6.47 (2H, d, J = 15.6, H-4, 4′), 6.93 (2H, d, J = 8.4, H-9, 9′), 7.04 (2H, d, J = 1.6, H-6, 6′), 7.11 (2H, dd, J = 8.4, 1.6, H-10, 10′), 7.60 (2H, d, J = 15.6, H-3, 3′) [26].
Hexahydrocurcumin (8). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 1.63 (1H, dddd, J = 14.0, 10.1, 6.6, 4.3, H-6a), 1.78 (1H, dddd, J = 14.0, 9.2, 9.2, 5.5, H-6b), 2.51 (1H, dd, J = 17.4, 7.9, H-4a), 2.56 (1H, dd, J = 17.4, 3.0, H-4b), 2.60 (1H, ddd, J = 13.0, 9.2, 6.7, H-7a), 2.70 (2H, t, J = 7.2, H-2), 2.71 (1H, ddd, J = 13.0, 10.1, 5.5, H-7b), 2.82 (2H, t, J = 7.2, H-1), 3.85 (3H, s, 3′-OCH3), 3.86 (3H, s, 3′′-OCH3), 4.03 (1H, dddd, J = 9.2, 7.9, 4.3, 3.1, H-5), 5.53 (1H, s, OH), 5.55 (1H, s, OH), 6.64 (1H, dd, J = 8.0, 2.0, H-6′), 6.66 (1H, d, J = 2.0, H-2′), 6.67 (1H, dd, J = 8.0, 2.0, H-6′′), 6.70 (1H, d, J = 2.0, H-2′′), 6.81 (2H, d, J = 8.0, H-5′, 5′′) [27].
References
S. Mehrotra, G. Agnihotri, S. Singh, and F. Jamal, South Asian J. Exp. Biol., 3, 299 (2013).
L. Labban, Int. J. Pharm. Biomed. Sci., 5, 17 (2014).
C. Y. Chen, C. H. Chen, C. H. Kung, S. H. Kuo, and S. Y. Kuo, J. Nat. Prod., 71, 137 (2008).
B. H. Chen, P. Y. Wu, K. M. Chen, T. F. Fu, H. M. Wang, and C. Y. Chen, J. Nat. Prod., 72, 950 (2009).
C. Y. Chen, T. Z. Liu, Y. W. Liu, W. C. Tseng, R. H. Liu, F. J. Lu, Y. S. Lin, S. H. Kuo, and C. H. Chen, J. Agric. Food Chem., 55, 948 (2007).
C. Y. Chen, Y. W. Li, and S. Y. Kuo, Molecules, 14, 959 (2009).
C. Y. Chen, C. J. Tai, J. T. Cheng, J. J. Zheng, Y. Z. Chen, T. Z. Liu, S. J. Yiin, and C. L. Chern, J. Agric. Food Chem., 58, 5604 (2010).
C. Y. Chen, Y. H. Yang, and S. Y. Kuo, J. Nat. Prod., 73, 1370 (2010).
Y. L. Hsu, C. Y. Chen, M. F. Hou, E. M. Tsai, Y. J. Jong, C. H. Hung, and P. L. Kuo, Mol. Nutr. Food Res., 54, 1307 (2010).
R. J. Lin, C. Y. Chen, L. Y. Chung, and C. M. Yen, Acta Trop., 115, 69 (2010).
R. J. Lin, C. Y. Chen, J. D. Lee, C. M. Lu, L. Y. Chung, and C. M. Yen, Planta Med., 76, 1852 (2010).
H. M. Wang, C. Y. Chen, H. A. Chen, W. C. Huang, W. R. Lin, T. C. Chen, C. Y. Lin, H. J. Chien, P. L. Lu, C. M. Lin, and Y. H. Chen, Phytother. Res., 24, 1825 (2010).
C. Y. Chen, W. L. Yang, and S. Y. Kuo, Nat. Prod. Commun., 6, 1671 (2011).
Y. L. Hsu, C. Y. Chen, I. P. Lin, E. M. Tsai, P. L. Kuo, and M. F. Hou, J. Agric. Food Chem., 60, 852 (2012).
C. Y. Chen, K. C. Cheng, A. Y. Chang, Y. T. Lin, Y. C. Hsu, and H. M. Wang, Int. J. Mol. Sci., 13, 1762 (2012).
C. Y. Chen, C. M. Liu, H. C. Yeh, W. J. Li, H. T. Li, and C. H. Chuang, Chem. Nat. Compd., 59, 367 (2023).
C. Y. Chen, C. L. Kao, H. C. Yeh, H. T. Li, M. J. Cheng, W. J. Li, and M. D. Wu, Chem. Nat. Compd., 59, 478 (2023).
C. Y. Chen, C. L. Kao, W. J. Li, H. C. Yeh, H. T. Li, M. J. Cheng, and S. L. Liu, Chem. Nat. Compd., 59, 484 (2023).
S. F. Lin, C. L. Kao, C. E. Kuo, H. C. Yeh, M. J. Cheng, H. T. Li, and C. Y. Chen, Chem. Nat. Compd., 59, 683 (2023).
S. J. Wang, C. L. Kao, H. C. Yeh, H. T. Li, M. J. Cheng, M. D. Wu, and C. Y. Chen, Chem. Nat. Compd., 59, 852 (2023).
G. G. Yue, B. C. Chan, P. M. Hon, M. Y. Lee, K. P. Fung, P. C. Leung, and C. B. Lau, Food Chem. Toxicol., 48, 2011 (2010).
Y. Sasaki, H. Goto, C. Tohda, F. Hatanaka, N. Shibahara, Y. Shimada, K. Terasawa, and K. Komatsu, Biol. Pharm. Bull., 26, 1135 (2003).
S. Uehara, I. Yasuda, K. Takeya, and H. Itokawa, Chem. Pharm. Bull., 37, 237 (1989).
C. Y. Chen, C. L. Kao, H. C. Yeh, P. L. Song, H. T. Li, and W. J. Li, Chem. Nat. Compd., 56, 75 (2020).
M. G. U. Khan, K. Nahar, M. S. Rahman, C. M. Hasan, and M. A. Rashid, Dhaka Univ. J. Pharm. Sci., 8, 39 (2008).
F. Kiuchi, Y. Goto, N. Sugimoto, N. Akao, K. Kondo, and Y. Tsuda, Chem. Pharm. Bull., 41, 1640 (1993).
C. Y. Chen, C. M. Liu, H. C. Yeh, W. J. Li, H. T. Li, and C. H. Chuang, Chem. Nat. Compd., 59, 367 (2023).
Acknowledgment
This investigation was supported by a grant from the Yuan's General Hospital (YGH-23-017).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2024, pp. 560–562.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S.J., Kao, C.L., Li, W.J. et al. A New Sesquiterpenoid of Curcuma longa. Chem Nat Compd 60, 645–648 (2024). https://doi.org/10.1007/s10600-024-04404-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-024-04404-5