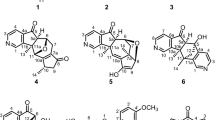
One new anhydride, alpinofficinol (1) was isolated from the rhizomes of Alpinia officinarum Hance (Zingiberaceae). The structure was determined by means of HR-ESI-MS and extensive 1D and 2D NMR spectroscopic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Traditional Chinese medicine consists of a large number of plants with various medicinal and pharmacological importance and hence represents a priceless an invaluable reservoir of new bioactive molecules. Alpinia officinarum Hance (Zingiberaceae), also known as lesser galangal, is indigenous to Southeast China and Indochina, and the plains of West Bengal, Assam, and the Eastern Himalayas in India [1]. It is a perennial herb with thick, creeping reddish-brown rhizomes, lineolate acuminate ornamental leaves, and showy white flowers in racemes. It has been used conventionally both in Ayurvedic and Chinese medicine since very early times and in Europe since the Middle Ages [1]. The rhizome has been used in China to relieve stomach ache, treat colds, invigorate the circulatory system, and reduce swelling. The dry root and rhizome have been used for their antioxidant, antidiarrheal, anti-emetic, analgesic, anti-inflammatory, and anticoagulative effects [2]. In the course of screening for biologically and chemically novel agents from Formosan Zingiberaceous plants [3,4,5,6,7,8,9,10,11,12,13,14,15], A. officinarum was chosen for further phytochemical investigation. In continuation of some studies of chemotaxonomy and biologically active metabolites from this plant, a methanol extraction of the rhizomes of A. officinarum afforded ten compounds, including one new anhydride, two phenylalkanoids, two diarylheptanoids, and five benzenoids [16]. In this paper, we report the isolation and structural elucidation of this new propanoic anhydride.
Compound 1, obtained as yellowish oil, had the molecular formula C18H18O7, as determined by HR-ESI-MS data m/z 369.0955 [M + Na]+ (calcd 369.0950) in combination with its 1H NMR, 13C NMR, and DEPT, requiring 10 degrees of unsaturation. The IR spectrum revealed the presence of an OH stretch band (3400 cm–1), and multiple ester carbonyls C=O (1650 cm–1), one of which was by UV spectrum analysis (λmax, 210 (2.75), 255 (3.55), 280 (2.38) nm) in conjugation with a benzene. Eight of the ten degrees of unsaturation inherent in the formula were accounted for by 13C NMR as two aromatics and the remaining 2 IHD belong to a typical anhydride signal (δ 169.1 and 176.3). Accordingly, the compound 1 contained an anhydride combined with two benzene rings.
The 1H and 13C NMR spectra indicated eight quaternary C atoms, 6 CH, 2 CH2, and 2 OMe groups. In the 1H NMR spectrum, there were typical signals for two OMe groups at δ 3.86 (3H, s, 3-OCH3), and 3.81 (3H, s, 6′-OCH3), signals of α-methylene protons of a ketone at δ 2.53 (2H, t, J = 7.2 Hz, CH2-2′), one β-methylene resonance of a ketone at δ 2.82 (2H, t, J = 7.2 Hz, CH2-3′), one ABX signal at δ 6.69 (1H, d, J = 2.0 Hz), 6.72 (1H, d, J = 8.0 Hz), and 6.62 (1H, dd, J = 8.0, 2.0 Hz) for H-5′, H-8′, and H-9′ as well as another ABX at δ 7.51 (1H, d, J = 2.0 Hz), 6.83 (1H, d, J = 8.4 Hz), and 7.56 (1H, dd, J = 8.4, 2.0 Hz) for H-2, H-5, and H-6, indicating that 1 was probably a propanoic anhydride possessing 4-hydroxy-3-methoxybenzoic and 3-(4-hydroxy-3-methoxyphenyl) groups. The carbons of the propanoic anhydride were assigned, from 13C NMR and DEPT experiments; there were resonances for anhydride functions [δ 169.1 (C-7) and 176.3 (ester C=O groups)], two methoxyl groups [δC 55.9, 56.0], and two aromatic C atoms. The above data of 1 also pointed to a propanoic anhydride moiety.
The above observation accompanied by the 1H–1H COSY (Table 1), and HMBC (Table 1) spectrum of 1 established the presence of the partial three substitutes: fragments, 1a (4-hydroxy-3-methoxybenzoic group), 1b (3-(4-hydroxy-3-methoxyphenyl) group), and 1c (–OCOCH2CH2–, propanoic anhydride group), for anhydride skeleton of 1. The entire skeleton of 1 was constructed with the aid of HMBC spectrum (Table 1).
In the HMBC and NOESY spectra, the significant correlations between δH 7.51 (H-2)/δH 7.56 (H-6) and δC 169.1 (C-7), δH 6.69 (H-5′)/δH 6.62 (H-9′) and δC 30.9 (C-3′), suggested that the substitutes 1a and 1b were connected to C-1 and C-4′ of the propanoic anhydride moiety respectively. The other key HMBC correlations of 1 demonstrated selected cross-peaks as shown in Table 1. The assignments were further verified by significant correlations of H-3′ (δ 2.82)/H-5′ (δ 6.69) and H-9′ (δ 6.62) in the NOESY experiments (Fig. 1) and further supported the positions of each substituent (1a & 1b) on the anhydride moiety.
From the above data, compound 1 was characterized as 4-hydroxy-3-methoxybenzoic 3-(4-hydroxy-3-methoxyphenyl) propanoic anhydride, named alpinofficinol, and its structure was illustrated as 1, which was further confirmed by COSY, NOESY (Fig. 1), HSQC, and HMBC (Table 1) experiments.
Experimental
General. UV spectra were obtained in CH3CN, IR spectra were measured on a Hitachi 260-30 spectrophotometer. 1H NMR (400 MHz), 13C NMR (100 MHz), HSQC, HMBC, COSY, NOESY, and DEPT spectra were obtained on a Varian (Unity Plus) NMR spectrometer. Low-resolution ESI-MS spectra were obtained on an API 3000 (Applied Biosystems) and high-resolution ESI-MS spectra on a Bruker Daltonics APEX II 30e spectrometer. Silica gel 60 (Merck, 70–230 mesh, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254), 0.20 mm and 0.50 mm, were used for analytical TLC and preparative TLC respectively, visualized with 50% H2SO4.
Plant Material. The rhizomes of A. officinarum were collected from Taoyuan County, Taiwan, in June 2017. Plant material was identified by Dr. Fu-Yuan Lu (Department of Forestry and Natural Resources College of Agriculture, National Chiayi University). A voucher specimen was deposited at the Department of Medical Technology, School of Medical and Health Sciences, Fooyin University, Kaohsiung, Taiwan.
Extraction and Isolation. The rhizomes (3.24 kg) of A. officinarum were extracted repeatedly with MeOH (5 L × 3) at room temperature for 24–48 h. The MeOH extract was dried and evaporated to leave a viscous residue (112.4 g). The residue was placed on a silica gel column (3.5 kg, 70–230 mesh) and eluted with CH2Cl2 gradually enriched with MeOH to afford ten fractions. Fraction 2 (25.8 g) was subjected to silica gel chromatography (1.4 kg, 70–230 mesh), by eluting with n-hexane–acetone (80:1), enriched gradually with acetone, to furnish four fractions (2-1–2-4). Fraction 2-4 (7.5 g) was further purified on a silica gel column using n-hexane–acetone (60:1) to obtain alpinofficinol (1) (11 mg).
Alpinofficinol (1), colorless oil. UV (MeCN, λmax, nm) (log ε): 210 (2.75), 255 (3.55), 280 (2.38). IR (νmax, cm–1): 3400 (OH), 1650 (C=O). ESI-MS m/z 369 [M + Na]+; HR-ESI-MS m/z 369.0955 [M + Na]+ (calcd for C18H18O7Na, 369.0950). 1H and 13C NMR data, see Table 1.
References
T. K. Lim, Edible Medicinal and Non-medicinal Plants: Modified Stems, Roots, Bulbs, Vol. 12, Springer, London, 2002, p. 178.
Z. S. Xie, X. J. Xu, C. Y. Xie, J. Y. Huang, M. Yang, and D. P. Yang, J. Pharm. Anal., 3, 215 (2013).
C. Y. Chen, C. L. Kao, H. C. Yeh, P. L. Song, H. T. Li, and W. J. Li, Chem. Nat. Compd., 56, 75 (2020).
H. M. Wang, C. L. Kao, W. J. Li, H. T. Li, and C. Y. Chen, Chem. Nat. Compd., 54, 7 (2018).
C. N. Kuo, C. H. Chen, S. N. Chen, J. C. Huang, L. J. Lai, C. H. Lai, C. H. Hung, C. H. Lee, and C. Y. Chen, Int. Ophthalmol., 38, 747 (2018).
C. Y. Chen, C. L. Kao, and C. M. Liu, Int. J. Mol. Sci., 19, 2729 (2018).
C. M. Liu, C. L. Kao, Y. T. Tseng, Y. C. Lo, and C. Y. Chen, Molecules, 22, 1477 (2017).
Y. L. Hsu, C. Y. Chen, M. F. Hou, E. M. Tsai, Y. J. Jong, C. H. Hung, and P. L. Kuo, Mol. Nutr. Food Res., 54, 1307 (2010).
R. J. Lin, C. Y. Chen, J. D. Lee, C. M. Lu, L. Y. Chung, and C. M. Yen, Planta Med., 76, 1852 (2010).
H. M. Wang, C. Y. Chen, H. A. Chen, W. C. Huang, W. R. Lin, T. C. Chen, C. Y. Lin, H. J. Chien, P. L. Lu, C. M. Lin, and Y. H. Chen, Phytother. Res., 24, 1825 (2010).
Y. L. Hsu, C. Y. Chen, I. P. Lin, E. M. Tsai, P. L. Kuo, and M. F. Hou, J. Agric. Food Chem., 60, 852 (2012).
C. Y. Chen, K. C. Cheng, A. Y. Chang, Y. T. Lin, Y. C. Hsu, and H. M. Wang, Int. J. Mol. Sci., 13, 1762 (2012).
H. P. Dong, R. C. Yang, I. C. Chunag, L. J. Huang, H. T. Li, H. L. Chen, and C. Y. Chen, Nat. Prod. Commun., 7, 883 (2012).
S. H. Huang, C. H. Lee, H. M. Wang, Y. W. Chang, C. Y. Lin, C. Y. Chen, and Y. H. Chen, J. Agric. Food Chem., 62, 9171 (2014).
R. J. Lin, C. Y. Chen, C. M. Lu, Y. H. Ma, L. Y. Chung, J. J. Wang, J. D. Lee, and C. M. Yen, Acta Trop., 140, 50 (2014).
C. Y. Chen, C. L. Lin, C. L. Kao, H. C. Yeh, H. T. Li, and C. T. Chang, Chem. Nat. Compd., 55, 1176 (2019).
Acknowledgment
This investigation was supported by a grant from Fooyin University of the Republic of China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2023, pp. 405–407.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C.Y., Kao, C.L., Yeh, H.C. et al. A New Anhydride of Alpinia officinarum. Chem Nat Compd 59, 478–480 (2023). https://doi.org/10.1007/s10600-023-04028-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-04028-1