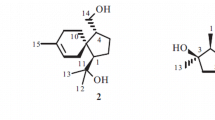
An icetexane diterpenoid, 9(10→20)-abeo-abietane diterpenoid, annlomultialaskoside (1), has been isolated from the endophytic fungus Annulohypoxylon multiforme var. alaskense, derived from the medicinal plant Alnus formosana. The structure was determined by spectroscopic methods, mainly 1D and 2D NMR, and comparison of the spectroscopic data with those reported for structurally related compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The term endophyte refers to a fungal or bacterial microorganism that colonizes interior organs of plants but does not have pathogenic effects on its hosts [1, 2]. Living in special ecoenvironments, endophytic fungi are especially fruitful producers of biologically active secondary metabolites with which they probably regulate their interactions with their hosts [3,4,5,6,7,8]. Many endophytic fungi have proved to be a rich source of bioactive secondary metabolites that are of interest for medicinal or agrochemical applications. An endophytic fungal strain was collected from Tsui-fong, Nan-tou, Taiwan. This strain was determined to be Annulohypoxylon multiforme var. alaskense (Xylariaceace) based on their cultural and anamorphic data [9]. Since 2002, we have started a survey of the diversity of fungal endophytes distributed in Taiwan and its secondary metabolites that possess antifungal, anti-inflammatory, and antitumor activities. We have isolated many bioactive metabolites, including a new azaphilone metabolite with a new substitution pattern, annulohypoxylin, from endophytic Annulohypoxylon boveri var. microspora BCRC 34012 of Cinnamomum sp. (which was collected from Fu-Shan Botanical Garden, I-lan County, Taiwan, during August of 2001) [10], and a total of seven new ones, including 10-hydroxythujopsene, akotriol, xylaritriol, cubentriol, akoenic acid, akodionine, and akolitserin, from endophytic Xylaria cubensis of Litsea akoensis Hayata [11], etc.
In the course of screening for biologically and chemically novel agents from Formosan fungal materials, Annulohypoxylon multiforme var. alaskense was chosen for further phytochemical investigation. This fungus is also being studied and published for the first time. The culture broth of the fungus was subjected to solvent partitioning and chromatographic separation to give the new compound. Their structures were determined by spectroscopic methods, mainly 1D and 2D NMR.
Compound 1 was obtained as an optically active colorless gum; \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) –60.4° (c 0.01, MeOH). It mainly appears in water-soluble compounds. It is separated and purified by column chromatography. Using methanol and chloroform as solvents, a colloidal substance can be obtained. The molecular formula was deduced as C32H50O14 through the analysis of HR-EI-MS m/z 658.7457 [M]+ (calcd for C32H50O14, 658.7425), requiring eight degrees of unsaturation, in accordance with the 1H NMR, 13C NMR, and DEPT data.
The IR absorption bands are due to the presence of the hydroxyl (3445 cm–1) and benzene ring (1624 cm–1). From the 13C NMR, there are signals of 12 carbons between δ 70–80, 108.2, and 107.1, indicating the presence of two glucose moieties. The carbon chemical shifts of the two groups of sugars are C-1′ (108.2), C-2′ (76.1), C-3′ (79.0), C-4′ (71.3), C-5′ (79.2), and C-6′ (62.5), and C-1′′ (107.1), C-2′′ (75.8), C-3′′ (78.5), C-4′′ (72.0), C-5′′ (78.9), and C-6′′ (63.2). The glucose in the literature [12] is connected to C-16 of the triterpenoids, and its carbon chemical shifts are C-1 (δ 106.6), C-2 (δ 75.8), C-3 (δ 78.8), C-4 (δ 71.8), C-5 (δ 78.1), and C-6 (δ 63.0). Comparing the carbon resonances of the two sugars in 1, it can be concluded that the two sugars connected to compound 1 are also glucose.
The molecular weight of compound 1 measured by mass spectrometer is 658, and there are seven quaternary carbons, 31 methine groups, seven methylene groups, and four methyl groups in the carbon spectrum, so the molecular formula is inferred to be C32H50O14. Subtracting the carbon number of the sugar, it can be seen that the unsaturation of the compound remains at six. There are three rings, one of which is a benzene ring, which conforms to a degree of unsaturation of six. The signals of the four methyl groups in the high magnetic field of 1H NMR are at δ 1.37, 1.45, 1.38, and 1.12, and the coupling constants of the two methyl signals at δ 1.38 and 1.12 are both 7.2 Hz. Therefore, it is a typical isopropyl Me group signal. Based on these inferences, it is speculated that this compound may have a rearranged skeleton of abietane with 20 carbons. In addition, 1H NMR has only one δ 6.80 signal in the low magnetic field region, so it shows that the benzene ring has five substituents. Therefore, it is speculated that a hydroxyl group is attached to C-11. In addition, the 12-OH group is connected with glucose via O-linkage. The HMBC (Fig. 1) correlations of 1 from H-1′ to C-12 and from H-1′′ to C-3 suggest the linking position of the two β-glucose moieties. Regarding the relative configuration of the methyl groups, if H-5 is occupied at the α position, the NOE correlations of H-5/H-3/H-1 must show that the orientations of these protons remain α-oriented. The NOESY correlation between CH3-18 and H-3 indicate that CH3-18 is also α-oriented. Therefore, the structure of 1 was identified as shown in Fig. 1, and was named annlomultialaskoside.
EXPERIMENTAL
General. Optical rotations were measured on a Jasco P-1020 digital polarimeter, UV spectra were obtained on a Jasco UV-240 spectrophotometer in MeOH, and IR spectra (KBr or neat) were taken on a Perkin-Elmer System 2000 FT-IR spectrometer. 1D (1H, 13C, DEPT) and 2D (COSY, NOESY, HSQC, HMBC) NMR spectra using CDCl3 as solvent were recorded on a Varian Mercury-400 (400 MHz for 1H NMR, 100 MHz for 13C NMR) spectrometer. Chemical shifts were internally referenced to the solvent signals in C5D5N (1H, δ 7.19; 13C, δ 123.5) with TMS as internal standard. Low-resolution ESI-MS spectra were obtained on an API 3000 instrument (Applied Biosystems) and high-resolution ESI-MS spectra on a Bruker Daltonics APEX II 30e spectrometer. Low-resolution EI-MS spectra were recorded on a Quattro GC/MS spectrometer having a direct inlet system. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography, and silica gel 60 F-254 (Merck) was used for TLC and preparative TLC.
Fungus Material. Annulohypoxylon multiforme var. alaskense was used throughout this study, and specimens were deposited at the Bioresource Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (FIRDI).
Fermentation and Extraction. The research material was maintained on potato dextrose agar (PDA), and the strain was cultured on potato dextrose agar slants at 25°C for 7 days and the spores then harvested by sterile water. The spores (5 × 105) were seeded into 300 mL shake flasks containing 50 mL RGY medium (3% rice starch, 7% glycerol, 1.1% polypeptone, 3% soybean powder, 0.1% MgSO4, 0.2% NaNO3) and cultivated with shaking (150 rpm) at 25°C for 7 days. The culture was filtered to separate broth and mycelia. The culture broth was extracted with n-BuOH (6 × 10 L) six times. The combined organic layer was concentrated under vacuum to afford 3.2 g of residue.
Extraction and Isolation. The crude extract was separated into five fractions (1–5) by column chromatography on RP-18 silica gel and eluted by methanol–H2O (0:100, 30:70, 50:50, 70:30, and 100:0). Fraction 2 (100 mg) was subjected to silica gel CC (step gradient, elution with 0–10% MeOH in CH2Cl2) to afford 1 (1.4 mg).
Annlomultialaskoside (1), gum; \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) –60.4° (c 0.01, MeOH). IR (neat, νmax, cm–1): 3445 (OH), 1624 (aromatic). EI-MS (m/z, Irel, %): 659 ([M + H]+, 100), 577 (58), 551 (62), 522 (37). HR-EI-MS m/z 658.7457 [M]+ (calcd for C32H50O14, 658.7425). 1H NMR (600 MHz, Py-d5, δ, ppm, J/Hz): 1.12 (3H, d, J = 7.0, H-17), 1.37 (3H, s, H-19), 1.38 (1H, d, J = 7.0, H-16), 1.40 (1H, br.d, J = 12.0, H-5), 1.45 (3H, s, H-18), 1.73 (1H, t, J = 11.2, Hβ-1), 1.85 (1H, br.d, J = 12.0, Hβ-6), 2.00 (1H, m, Hα-1), 2.06 (1H, m, Hα-6), 2.30 (1H, br.d, J = 11.6, Hb-2), 2.45 (1H, m, Ha-2), 2.70 (1H, d, J = 13.6, Hβ-20), 2.83 (1H, t, J = 12.0, Hα-7), 2.96 (1H, dd, J = 12.0, 7.0, Hβ-7), 3.01 (1H, overlap, Hα-20), 3.56 (1H, br.d, J = 9.6, H-3), 3.74 (1H, m, H-3′′), 3.75 (1H, m, H-3′), 3.98 (1H, m, H-5′′), 4.05 (1H, t, J = 7.6, H-2′), 4.15 (1H, m, H-15), 4.20 (1H, m, H-4′′), 4.22 (1H, m, H-5′), 4.25 (1H, m, H-2′′), 4.34 (1H, m, H-4′), 4.37 (2H, m, H-6′), 4.53 (2H, br.d, J = 7.2, H-6′′), 4.98 (1H, d, J = 7.6, H-1′), 4.99 (1H, d, J = 7.2, H-1′′), 6.80 (1H, s, H-14). 13C NMR (150 MHz, Py-d5, δ, ppm, J/Hz): 16.9 (C-18), 23.8 (C-6), 24.5 (C-17), 25.0 (C-16), 26.6 (C-15), 27.5 (C-2), 27.7 (C-19), 37.2 (C-7), 41.1 (C-1), 41.3 (C-4), 42.1 (C-20), 58.3 (C-5), 62.5 (C-6′), 63.2 (C-6′′), 71.0 (C-10), 71.3 (C-4′), 72.0 (C-4′′), 76.1 (C-2′), 75.8 (C-2′′), 78.5 (C-3′′), 78.9 (C-5′′), 79.0 (C-3′), 79.2 (C-5′), 88.9 (C-3), 107.1 (C-1′′), 108.2 (C-1′), 116.3 (C-14), 122.9 (C-9), 140.5 (C-13), 142.3 (C-12), 142.6 (C-8), 149.5 (C-11).
References
G. A. Strobel and D. M. Long, ASM News, 64, 263 (1998).
G. A. Strobel, Crit. Rev. Biotechnol., 22, 315 (2002).
G. Strobel, B. Daisy, U. Castillo, and J. Harper, J. Nat. Prod., 67, 257 (2004).
L. P. Bush, H. H. Wilkinson, and C. L. Schardl, Plant Physiol., 114, 1 (1997).
R. X. Tan and W. X. Zou, Nat. Prod. Rep., 18, 448 (2001).
F. B. Sean, M. B. Shana, and C. Jon, J. Am. Chem. Soc., 123, 9900 (2001).
C. Lu and Y. Shen, J. Antibiot., 56, 415 (2003).
R. X. Tan and W. X. Zou, Nat. Prod. Rep., 18, 448 (2001).
H. M. Hsieh, Y. M. Ju, and J. D. Rogers, Mycol., 97, 844 (2005).
M. J. Cheng, M. D. Wu, S. Y. Hsieh, M. T. Hsieh, I. S. Chen, and G. F. Yuan, Helv. Chim. Acta, 94, 1108 (2011).
N. W. Fan, H. S. Chang, M. J. Cheng, S. Y. Hsieh, T. W. Liu, G. F. Yuan, and I. S. Chen, Helv. Chem. Acta, 97, 1689 (2014).
M. Toshio, H. Chizuru, and Y. Sachiyo, Chem. Pharm. Bull., 45, 2029 (1997).
Acknowledgment
This work was partial kindly supported by the Food Industry Research and Development Institute (FIRDI) and supported by Ministry of Science and Technology, R.O.C. (MOST-108-2320-B-080-002- & 110-2320-B-080-001-). The authors also thank Senior Technician Mrs. Chyi Jia Wang of the Center for Resources, Research, and Development (CRRD) of Kaohsiung Medical University for measuring the 2D NMR data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2022, pp. 404–406.
Rights and permissions
About this article
Cite this article
Cheng, MJ., Wu, MD., Hsieh, SY. et al. A New Icetexane Diterpenoid from the Endophytic Fungus Annulohypoxylon multiforme var. alaskense. Chem Nat Compd 58, 471–473 (2022). https://doi.org/10.1007/s10600-022-03713-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03713-x