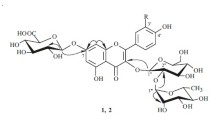
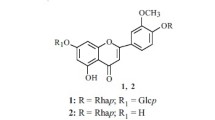
The aerial part of Lespedeza tomentosa (Thunb.) Maxim. growing in southern Primorsky Krai, Russian Federation, yielded 10 identified flavonoid glycosides, eight of which were observed for the first time in this plant. The structures of the two new compounds were elucidated based on NMR spectroscopy and mass spectrometry as morin 3-O-rutinoside and 3-O-(2-O-α-L-rhamnopyranosyl)-β-D-galactopyranoside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plants of the genus Lespedeza (Fabaceae, Leguminosae) are wild bushes including ~50 species. The genus Lespedeza in Russia is represented by five species growing in southern East Siberia, the Far East, Primoryå, and Priamuryå. Most useful biologically active flavonoids in these plants are found in the aerial part of the bush (in runners, flowers, leaves) [1]. However, several species of this genus, e.g., L. bicolor, contain flavonoids in stem and root bark [2, 3].
Previously the aerial part of L. tomentosa collected in China yielded 13 identified flavonoids including isorhamnetin 3-O-rutinoside and 3-O-neohesperidoside; quercetin 3-O-glucoside; kaempferol 3-O-robinobioside and 3-O-rhamnosyl-(1→2)- galactoside [1], trifolin (kaempferol 3-O-β-D-galactopyranoside) [4], apigenin, luteolin, orientin (luteolin 8-C-glucoside), homoorientin (luteolin 6-C-glucoside), saponaretin (isovitexin, apigenin 6-C-glucoside), vitexin (apigenin 8-C-glucoside), and luteolin 7-glucoside [5].
The goal of the present work was to isolate and elucidate the structures of flavonoids from L. tomentosa (Thunb.) Maxim. growing in south Primorsky Krai, Russian Federation.
Chromatography of the EtOH extract of L. tomentosa over various sorbents afforded 10 pure flavonoid glycosides (1–10).

Compounds 1 and 2 according to HPLC-UV–HR-MS had the same formula C27H30O15 but different retention times (RTs) (Table 1). An analysis of 1H and 13C NMR, HSQC, HMBC, and COSY spectra identified 1 and 2 as kaempferol 3-O-rutinoside (nicotiflorin) [6] and kaempferol 3-O-neohesperidoside [7], respectively. Flavonoid 3 had the longest RT (27.58) and formula C28H32O16 and was identified by HPLC-UV–HR-MS (Table 1) as isorhamnetin 3-O-rutinoside, which was isolated earlier from this plant by Japanese researchers [1].
Flavonoids 4 and 5 had identical formulas (C21H20O12) but different HPLC RTs (Table 1). They were quercetin 3-O-β-D-glucoside (4) [8] and morin 3-O-β-D-glucoside (5) [9] according to 1H and 13C NMR spectra and the literature.
Quercetin 3-O-β-D-glucopyranoside (isoquercitrin, hirsutrin) (4) was already isolated earlier from the aerial part of L. tomentosa [1]. Compound 4 is known to possess hepatoprotective properties and to occur in other Lespedeza spp. [10]. Morin 3-O-β-D-glucopyranoside (5), which was isolated earlier from Acridocarpus orientalis and Solena amplexicaulis [9], was observed in L. tomentosa for the first time by us. Compound 5 is known to exhibit significant cytotoxicity for HL60 tumor cells [11] and to inhibit growth of pathogenic fungi [9].
Compounds 6 and 7 gave UV spectra with two absorption maxima and formulas C27H30O16, corresponding to flavonoid diglycosides (Table 1). NMR spectral data for the carbohydrate fragments of 6 and 7 were the same. Compound 7 according to 1H and 13C NMR spectra contained a disaccharide bonded to the C-3 hydroxyl with two anomeric protons at δ 5.31 ppm (d, J = 7.5 Hz) and 4.42 ppm (d, J = 1.1 Hz) that were characteristic of glucose and rhamnose, respectively (Table 2).
However, ring B in 7, in contrast to rutin (6), had hydroxyls bonded to C-2′ and C-4′ C atoms, which was confirmed by an ABX spin system in its PMR spectrum that consisted of three resonances of aromatic protons at δ 7.53 ppm (d, J = 2.0 Hz), 7.64 ppm (dd, J = 2.1, 8.4 Hz), and 6.82 ppm (d, J = 8.5 Hz) (Table 2). Therefore, the structure of 7 was morin 3-O-rutinoside.
Rutin (6) is known to possess antidiabetic; anticoagulant; antiviral; cardio-, nephro-, and hepatoprotective; and other useful pharmacological properties [12].
Flavonoids 8 and 9 had the same formulas (C21H20O12) according to HPLC-UV–HR-MS spectra (Table 1) and different RTs. Flavonoid 8 was identified as quercetin 3-O-(2-O-α-L-rhamnopyranosyl)-β-D-galactopyranoside [13]. The PMR spectrum of 9 contained resonances at δ 5.65 ppm (d, J = 7.8 Hz) and 5.08 ppm (broad singlet) that were attributed to α-rhamnopyranosyl and β-galactopyranosyl. The chemical shifts of the disaccharide resonances in 1H and 13C NMR spectra of 8 and 9 agreed fully (Table 2). This proved that the terminal sugar in 9 was α-rhamnopyranose bonded to the 2-position of β-galactopyranose (Table 2). 1H and 13C NMR spectra of 8 and 9 differed in the positions of hydroxyls on ring B of their aglycons (Table 2).
The ring B OH groups in 9 were located on C-2′ and C-4′, which was confirmed by an ABX spin system in its PMR spectrum consisting of three resonances of aromatic protons at δ 7.52 ppm (d, J = 2.3 Hz), 7.60 ppm (dd, J = 2.3, 8.5 Hz), and 6.82 ppm (d, J = 8.9 Hz) (Table 2). Therefore, the structure of 9 was established as morin 3-O-(2-O-α-L-rhamnopyranosyl)- β-D-galactopyranoside.
Flavonoid 10 had formula C33H40O20 and the shortest RT (21.65) as compared to the other compounds (Table 1). An analysis of 1H and 13C NMR, HSQC, HMBC, and COSY spectra showed that 10 was a quercetin triglycoside. This was confirmed by three anomeric protons in its spectra at δ 5.57 (d, J = 7.8), 4.38 (br.s), and 5.09 (br.s). Glycoside 10 contained two α-L-rhamnopyranose residues bonded to the 2′′- and 6′′-positions of β-D-galactopyranose. Quercetin 3-O-α-Lrhamnopyranosyl-( 1→2)-[α-L-rhamnopyranosyl-(1→6)-β-D-galactopyranoside] was first isolated from the aerial part of Taverniera aegyptiaca [14].
Thus, the chemical composition of flavonoids from L. tomentosa growing in southern Primorsky Krai, Russian Federation, differed considerably from plants of this species from other regions. The aerial part of L. tomentosa yielded 10 flavonol glycosides containing two mono-, seven di-, and one trisaccharide on C-3. Of these, two disaccharides were new. Their structures were established as morin 3-O-rutinoside (7) and morin 3-O-(2-O-α-L-rhamnopyranosyl)-β-Dgalactopyranoside (9).
Experimental
UV spectra were recorded on a UV 1800 spectrophotometer (Shimadzu). 1H and 13C NMR spectra were recorded in DMSO-d6 at 30°C using Bruker Avance III DRX-700 and Avance DRX-500 instruments (Bruker, Karlsruhe, Germany). HMBC spectra were optimized for 5 Hz. Analytical HPLC of fractions and pure compounds used an Agilent Technologies 1100 series chromatograph equipped with a Supelco C-18 column (75 × 3 mm, 3 μm) at 30°C. The solvent flow rate was 0.8 mL/min. Solution A (1% AcOH) and solution B (1% AcOH in MeCN) were used for gradient elution of samples with 10% B (2 min), 20% B (5 min), 30% B (15 min), 40% B (17 min), 50% B (20 min), 90% B (22 min), and 10% B (25 min).
Analytical HPLC-UV–HR-MS (high-resolution mass spectrometry) used a Shimadzu LCMS-IT-TOF LC-MS (Japan) equipped with an LC-20A HPLC, SPD-M20A diode-array detector, and time-of-flight mass spectrometer with an ion trap. Constituents of mixtures were separated over a Discovery HS C-18 column (150 × 2.1 mm, 3 mm) thermostatted at 36°C. UV spectra were recorded in the range λ 200–800 nm. The mobile phase consisted of solutions of formic acid (0.1%) in deionized H2O (A) and MeCN (B). Gradient elution used flow rate 0.2 mL/min with 5% B (5 min), 5–40% B (45 min), 40–95% B (65 min), and 95% B (80 min). Mass spectrometric data were obtained with electrospray ionization and time-of-flight recording of negative and positive ions with resolution 12,000. The range of recorded m/z values was 200–1000. The drying gas (N2) pressure was 150 kPa. The spray-gas flow rate was 1.5 L/min. The ion source potential was –3.8 kV for recording positive ions and 4.5 kV for recording negative ions.
Aerial parts of L. tomentosa were collected in Primorsky Krai (Oktyabr′sky District, in the vicinity of Sinel′nikovo-1) in September 2020. The L. tomentosa plants were 80–100 cm high. Inflorescences of L. tomentosa had a milky tint with lilac bands. The flowers were usually milky- or cream-colored. The stems were covered with reddish-colored fibers. Herbarium specimen (voucher) No. 103616 is preserved in the Herbarium of PIBOC, FEB, RAS.
The dry EtOH extract (15 g) from dried stems and leaves of L. tomentosa was chromatographed over a column packed with polyamide 6 DF (Sigma-Aldrich) using CHCl3 with a gradient of increasing EtOH content up to 100%. Obtained fractions of flavonoids were analyzed by HPLC, HPLC-UV–HR-MS, and subsequently chromatographed over a column packed with Toyopearl HW-10C using H2O–EtOH (40:60). Flavonoid fractions were additionally purified over YMC-GEL ODS-A sorbent using H2O with a gradient of increasing EtOH content from 20 to 100% to give the 10 flavonoid glycosides 1–10 in yields of 2.8, 2.6, 3.0, 4.1, 3.2, 8.0, 2.3, 3.4, 2.5, and 6.0 mg.
Kaempferol 3- O -rutinoside (1), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (700 MHz, DMSO-d6, δ, ppm, J/Hz): 12.48 (1H, br.s, 5-OH), 7.97 (2H, d, J = 8.7, H-2′, 6′), 6.86 (2H, d, J = 8.7, H-3′, 5′), 6.29 (1H, br.s, Í-8), 6.09 (1H, br.s, H-6), 5.26 (1H, d, J = 7.6, H-1′′), 4.39 (1H, d, J = 1.1, H-1′′′), 3.69 (2H, d, J = 10.4, H-6′′), 3.39 (1H, m, H-3′′′), 3.28 (1H, m, H-2′′′), 3.27 (1H, m, H-5′′′), 3.24 (1H, m, H-3′′), 3.22 (1H, m, H-2′′), 3.22 (1H, m, H-5′′), 3.07 (1H, m, H-4′′′), 3.05 (1H, m, H-4′′), 0.99 (3H, d, J = 6.4, H-6′′′). 13C NMR (175 MHz, DMSO-d6, δ, ppm): 176.9 (C-4), 161.1 (C-5), 160.4 (C-7), 160.0 (C-4′), 156.8 (C-9), 156.4 (C-2), 133.3 (C-3), 130.7 (C-2′, 6′), 120.9 (C-1′), 115.1 (C-3′, 5′), 103.1 (C-10), 101.6 (C-1′′), 100.6 (C-1′′′), 99.4 (C-6), 93.8 (C-8), 76.4 (C-5′′), 75.8 (C-3′′), 74.0 (C-2′′), 71.3 (C-4′′′), 70.5 (C-2′′′), 70.3 (C-3′′′), 69.9 (C-4′′), 68.1 (C-5′′′), 66.8 (C-6′′), 17.6 (C-6′′′) [6].
Kaempferol 3- O -neohesperidoside (2), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (700 MHz, DMSO-d6, δ, ppm, J/Hz): 12.48 (1H, br.s, 5-OH), 8.03 (2H, d, J = 8.7, H-2′, 6′), 6.85 (2H, d, J = 8.7, H-3′, 5′), 6.30 (1H, br.s, Í-8), 6.09 (1H, br.s, H-6), 5.42 (1H, d, J = 7.5, H-1′′), 4.42 (1H, d, J = 1.2, H-1′′′), 3.72 (1H, m, H-2′′′), 3.71 (1H, m, H-5′′′), 3.53 (1H, m, H-6′′), 3.46 (1H, m, H-3′′′), 3.42 (1H, m, H-2′′), 3.37 (1H, m, H-3′′), 3.27 (1H, m, H-6′′), 3.12 (1H, m, H-4′′′), 3.08 (1H, m, H-4′′), 3.07 (1H, m, H-5′′), 1.06 (3H, d, J = 6.25, H-6′′′). 13C NMR (175 MHz, DMSO-d6, δ, ppm): 176.9 (C-4), 161.1 (C-5), 160.4 (C-7), 160.0 (C-4′), 156.8 (C-9), 156.3 (C-2), 133.2 (C-3), 130.7 (C-2′, 6′), 120.9 (C-1′), 115.1 (C-3′, 5′), 103.1 (C-10), 101.9 (C-1′′), 99.8 (C-1′′′), 99.4 (C-6), 93.8 (C-8), 78.0 (C-2′′), 77.7 (C-3′′), 77.1 (C-5′′), 71.9 (C-4′′′), 70.6 (C-3′′′), 70.2 (C-2′′′), 69.7 (C-4′′), 68.3 (C-5′′′), 61.0 (C-6′′), 17.5 (C-6′′′) [7].
Isorhamnetin 3- O -rutinoside (3), light-yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (700 MHz, DMSO-d6, δ, ppm, J/Hz): 12.48 (1H, br.s, 5-OH), 7.99 (1H, d, J = 2.3, H-2′), 7.49 (1H, dd, J = 8.8, 2.3, H-6′), 6.89 (1H, d, J = 8.8, H-5′), 6.32 (1H, br.s, Í-8), 6.10 (1H, br.s, H-6), 5.42 (1H, d, J = 7.8, H-1′′), 4.42 (1H, d, J = 0.94, H-1′′′), 3.85 (3H, s, OCH3), 3.71 (2H, d, J = 10.4, H-6′′), 3.38 (1H, m, H-3′′′), 3.28 (1H, m, H-2′′′), 3.26 (1H, m, H-5′′′), 3.24 (1H, m, H-3′′), 3.22 (1H, m, H-2′′), 3.21 (1H, m, H-5′′), 3.07 (1H, m, H-4′′′), 3.05 (1H, m, H-4′′), 1.04 (3H, d, J = 6.0, H-6′′′). 13C NMR (175 MHz, DMSO-d6, δ, ppm): 176.9 (C-4), 161.1 (C-5), 160.4 (C-7), 159.9 (C-4′), 156.8 (C-9), 156.5 (C-2), 147.2 (C-3′), 133.0 (C-3), 121.8 (C-6′), 120.8 (C-1′), 114.7 (C-5′), 113.0 (C-2′), 103.1 (C-10), 101.9 (C-1′′), 99.9 (C-1′′′), 99.4 (C-6), 93.8 (C-8), 76.4 (C-5′′), 75.8 (C-3′′), 74.0 (C-2′′), 71.3 (C-4′′′), 70.5 (C-2′′′), 70.2 (C-3′′′), 69.8 (C-4′′), 68.1 (C-5′′′), 66.8 (C-6′′), 55.8 (OCH3), 17.7 (C-6′′′) [1].
Quercetin 3- O -β-D-glucopyranoside (hirsutrin) (4), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 12.63 (1H, s, 5-OH), 7.58 (1H, d, J = 2.3, H-2′), 7.57 (1H, dd, J = 9.0, 2.3, H-6′), 6.84 (1H, d, J = 9.0, H-5′), 6.40 (1H, d, J = 2.0, H-8), 6.20 (1H, d, J = 2.0, H-6), 5.46 (1H, d, J = 7.5, H-1′′), 3.57 (2H, d, J = 11.0, H-6′′), 3.39 (1H, m, H-5′′), 3.23 (2H, m, H-2′′, 3′′), 3.10 (1H, m, H-4′′). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 177.5 (C-4), 164.2 (C-7), 161.3 (C-5), 156.4 (C-9), 156.3 (C-2), 148.5 (C-3′), 144.9 (C-4′), 133.4 (C-3), 121.6 (C-6′), 121.2 (C-1′), 116.3 (C-2′), 115.3 (C-5′), 104.0 (C-10), 100.9 (C-1′′), 98.7 (C-6), 93.5 (C-8), 77.6 (C-5′′), 76.6 (C-3′′), 73.2 (C-2′′), 70.0 (C-4′′), 61.0 (C-6′′) [8, 10].
Morin 3- O -β-D-glucopyranoside (5), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 12.62 (1H, s, 5-OH), 7.53 (1H, d, J = 2.2, H-3′), 7.66 (1H, dd, J = 8.5, 2.3, H-5′), 6.82 (1H, d, J = 8.5, H-6′), 6.40 (1H, d, J = 2.0, H-8), 6.20 (1H, d, J = 2.0, H-6), 5.36 (1H, d, J = 7.7, H-1′′), 3.56 (2H, m, H-6′′). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 177.5 (C-4), 164.2 (C-7), 161.3 (C-5), 156.4 (C-9), 156.2 (C-2), 148.5 (C-4′), 144.9 (C-2′), 133.5 (C-3), 122.1 (C-5′), 121.15 (C-1′), 121.5 (C-6′), 116.0 (C-3′), 104.0 (C-10), 101.9 (C-1′′), 98.7 (C-6), 93.5 (C-8), 77.6 (C-5′′), 76.6 (C-3′′), 73.2 (C-2′′), 70.0 (C-4′′), 61.0 (C-6′′) [9].
Quercetin 3- O -rutinoside (6), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 12.58 (1H, s, 5-OH), 7.52 (1H, dd, J = 8.4, 1.9, H-6′), 7.50 (1H, d, J = 1.9, H-2′), 6.80 (1H, d, J = 8.4, H-5′), 6.38 (1H, br.s, H-8), 6.19 (1H, br.s, H-6), 5.34 (1H, d, J = 7.5, H-1′′), 4.38 (1H, d, J = 1.1, H-1′′′), 3.97 (1H, dd, J = 12.1, 3.9, H-6′′), 3.63 (1H, dd, J = 12.1, 5.9, H-6′′), 3.39 (1H, m, H-3′′′), 3.28 (1H, m, H-2′′′), 3.27 (1H, m, H-5′′′), 3.24 (1H, m, H-3′′), 3.22 (2H, m, H-2′′, 5′′), 3.07 (1H, m, H-4′′′), 3.05 (1H, m, H-4′′), 1.06 (3H, d, J = 6.9, H-6′′′). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 177.4 (C-4), 164.2 (C-7), 161.3 (C-5), 156.7 (C-9), 156.4 (C-2), 148.5 (C-4′), 144.9 (C-3′), 133.3 (C-3), 121.6 (C-1′), 121.6 (C-6′), 116.1 (C-2′), 115.2 (C-5′), 103.9 (C-10), 101.2 (C-1′′), 100.7 (C-1′′′), 98.8 (C-6), 93.5 (C-8), 76.6 (C-3′′), 76.0 (C-5′′), 74.1 (C-2′′), 71.9 (C-4′′′), 70.6 (C-4′′), 70.4 (C-2′′′), 70.0 (C-3′′′), 68.2 (C-5′′′), 67.0 (C-6′′), 17.9 (C-6′′′) [6, 11].
Morin 3- O -rutinoside (7), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. Table 2 gives the NMR spectral data.
Quercetin 3- O -(2- O -α-L-rhamnopyranosyl)-β-D-galactopyranoside (8), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 12.59 (1H, s, 5-OH), 7.72 (1H, dd, J = 8.4, 2.1, H-6′), 7.48 (1H, d, J = 2.1, H-2′), 6.80 (1H, d, J = 8.4, H-5′), 6.38 (1H, d, J = 2.1, H-8), 6.19 (1H, d, J = 2.1, H-6), 5.63 (1H, d, J = 7.8, H-1′′), 5.06 (1H, br.s, H-1′′′), 3.80 (1H, m, H-5′′), 3.79 (1H, m, H-2′′), 3.75 (1H, m, H-5′′′), 3.73 (1H, m, H-3′′′), 3.63 (1H, m, H-4′′), 3.57 (1H, m, H-3′′), 3.47 (1H, m, H-2′′′), 3.42 (1H, m, H-6′′), 3.27 (1H, m, H-6′′), 3.12 (1H, m, H-4′′′), 0.77 (3H, d, J = 6.1, H-6′′′). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 177.4 (C-4), 164.2 (C-7), 161.3 (C-5), 156.6 (C-2), 156.4 (C-9), 148.1 (C-4′), 144.8 (C-3′), 133.4 (C-3), 122.3 (C-6′), 121.0 (C-1′), 115.7 (C-2′), 115.2 (C-5′), 104.0 (C-10), 100.5 (C-1′′′), 98.8 (C-1′′), 98.7 (C-6), 93.6 (C-8), 75.0 (C-2′′), 75.0 (C-5′′), 74.1 (C-3′′), 71.9 (C-4′′′), 70.7 (C-3′′′), 70.6 (C-2′′′), 68.6 (C-4′′), 68.2 (C-5′′′), 60.0 (C-6′′), 17.2 (C-6′′′) [17].
Morin 3- O -(2- O -α-L-rhamnopyranosyl)-β-D-galactopyranoside (9), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. Table 2 gives the NMR spectral data.
Quercetin 3- O -α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)-β-D-galactopyranoside (10), yellow amorphous compound. Table 1 gives the composition and UV and mass spectral data. 1H NMR (700 MHz, DMSO-d6, δ, ppm, J/Hz): 12.69 (1H, s, 5-OH), 7.67 (1H, dd, J = 8.4, 2.0, H-6′), 7.47 (1H, d, J = 2.0, H-2′), 6.79 (1H, d, J = 8.4, H-5′), 6.32 (1H, br.s, H-8), 6.12 (1H, br.s, H-6), 5.57 (1H, d, J = 7.8, H-1′′), 5.05 (1H, br.s, H-1′′′), 4.38 (1H, br.s, H-1′′′′), 3.80 (1H, m, H-2′′), 3.73 (1H, m, H-2′′′), 3.60 (1H, m, H-3′′), 3.59 (1H, m, H-4′′), 3.57 (2H, m, H-6′′), 3.56 (1H, m, H-5′′), 3.49 (1H, m, H-3′′′), 3.38 (1H, m, H-2′′′′), 3.37 (1H, m, H-5′′′), 3.35 (1H, m, H-5′′′′), 3.29 (1H, m, H-3′′′′), 3.12 (2H, m, H-4′′′, 4′′′′), 1.05 (3H, d, J = 6.1, H-6′′′′), 0.80 (3H, d, J = 6.1, H-6′′′). 13C NMR (175 MHz, DMSO-d6, δ, ppm): 177.0 (C-4), 164.8 (C-7), 161.3 (C-5), 156.4 (C-9), 156.1 (C-2), 148.6 (C-3′), 144.9 (C-4′), 132.8 (C-3), 122.1 (C-6′), 121.1 (C-1′), 115.6 (C-2′), 115.2 (C-5′), 103.5 (C-10), 100.6 (C-1′′′), 100.1 (C-1′′′′), 99.1 (C-1′′), 99.0 (C-6), 93.7 (C-8), 74.9 (C-2′′), 73.9 (C-3′′), 73.2 (C-5′′), 71.97 (C-4′′′, 4′′′′), 70.8 (C-2′′′), 70.7 (C-3′′′, 3′′′′), 70.5 (C-2′′′′), 68.6 (C-4′′), 68.4 (C-5′′′′), 68.2 (C-5′′′′), 65.0 (C-6′′), 17.9 (C-6′′′′), 17.3 (Ñ-6′′′) [18].
References
K. Matsuzaki, Y.-Q. Wang, K. Takahashi, and T. Okuyama, Shoyakugaku Zasshi, 44, 251 (1990).
D. V. Tarbeeva, S. A. Fedoreyev, M. V. Veselova, A. S. Blagodatski, A. M. Klimenko, A. I. Kalinovskiy, V. P. Grigorchuk, D. V. Berdyshev, and P. G. Gorovoy, Fitoterapia, 135, 64 (2019).
S. A. Dyshlovoy, D. Tarbeeva, S. Fedoreyev, T. Busenbender, M. Kaune, M. Veselova, A. Kalinovskiy, J. Hauschild, V. Grigorchuk, N. Kim, C. Bokemeyer, M. Graefen, P. Gorovoy, and G. von Amsberg, Biomolecules, 10, 45 (2020).
G. G. Zapesochnaya and A. I. Bankovskii, Chem. Nat. Compd., 2, 233 (1966).
Plant Resources of Russia: Wild Flowering Plants, Their Constituent Composition and Biological Activity [in Russian], Vol. 3, A. L. Budantsev (Chief Ed.), Tovarishchestvo Nauchnykh Izdanii KMK, St. Petersburg, Moscow, 2010, 601 pp.
Q. Wei, S. Li, and S. Huang, Chem. Nat. Compd., 57, 523 (2021).
J. D. Bacon and T. J. Mabry, Phytochemistry, 14, 295 (1975); T. Iwashina, M. Yamaguchi, M. Nakayama, T. Onozaki, H. Yoshida, S. Kawanobu, H. Ono, and M. Okamura, Nat. Prod. Commun., 5, 1903 (2010).
E. D. Rodrigues, D. B. da Silva, D. C. Rodrigues de Oliveira, and G. V. J. da Silva, Magn. Reson. Chem., 47, 1095 (2009).
J. Hussain, L. Ali, A. L. Khan, N. Ur Rehman, F. Jabeen, J.-S. Kim, and A. Al-Harrasi, Molecules, 19, 17763 (2014).
S. M. Kim, K. Kang, E. H. Jho, Y.-J. Jung, C. W. Nho, B.-H. Um, and C.-H. Pan, Phytother. Res., 25, 1011 (2011).
A. Mondal and T. K. Maity, Nat. Prod. Res., 20, 1 (2019).
P. K. Agrawall, C. Agrawall, and G. B. Blunden, Nat. Prod. Commun., 16, 1 (2021).
M. Olszewska, Acta Pol. Pharm., 62, 127 (2005).
A. R. Hassan, K. F. Amer, S. A. El-Toumy, J. Nielsen, and S. B. Christensen, Nat. Prod. Res., 33, 1135 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2021, pp. 878–882.
Rights and permissions
About this article
Cite this article
Pokhilo, N.D., Fedoreyev, S.A., Tarbeeva, D.V. et al. Flavonoid Glycosides from the Aerial Part of Lespedeza tomentosa. Chem Nat Compd 57, 1023–1028 (2021). https://doi.org/10.1007/s10600-021-03541-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03541-5