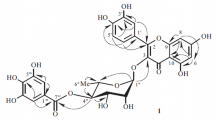
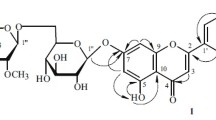
The structure of a new flavone glycoside isolated from twigs and leaves of Juniperus sabina was established as isoscutellarein 7-O-β-D-rhamnopyranosyl-(1→3)-α-L-xylopyranoside by spectroscopic methods, including 1D and 2D (1H–1H COSY, HSQC, and HMBC) NMR and ESI-MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Juniperus sabina (Cupressaceae) is a perennial creeping shrub distributed throughout the northwest region of China [1]. The twigs and leaves of this plant are used to treat various diseases in Uyghur folk medicine such as rheumatoid arthritis, wind-cold headache, and difficulty of urination [2]. Phytochemical studies demonstrated the presence of a wide array of secondary metabolites, including flavonoids [3, 4], lignans [5], and terpenes [6]. However, to our knowledge pharmacological studies on this species have been little reported. Therefore, the active compounds and extracts from this plant were studied to obtain an efficacy material base. In the present investigation on the anti-inflammory constituents of twigs and leaves of this species, one new flavone glycoside has been isolated, isoscutellarein 7-O-β-D-rhamnopyranosyl-(1→3)-α-L-xylopyranoside (1). This paper deals with the isolation and structural elucidation of compound 1.
Compound 1, a yellow powder, has the molecular formula C26H28O14 determined by positive ion ESI-MS (at m/z 565 [M + H]+) as well as 13C NMR and HMQC data. It gave a positive green coloration with 1% FeCl3 reagent and a positive result with the Molisch reaction, suggesting it is a flavone glycoside.
The 1H NMR spectrum of 1 (Table 1) analyzed with the aid of HSQC and HMBC showed three hydroxyl proton signals at δ 12.38, 10.38, and 8.70; two one-proton singlets at δ 6.61 and 6.83, attributed to H-6 and H-3, respectively; and two doublets at δ 8.05 (2H, d, J = 8.8 Hz, H-2′, 6′) and 6.95 (2H, d, J = 8.8 Hz, H-3′, 5′), suggesting that 1 was a 5,7,8,4′-tetrahydroxyflavone derivative. The 1H NMR also showed sugar proton signals at δ 3.65 (1H, m), 3.43 (1H, m), 3.44 (1H, m), 3.35 (1H, m), 3.79 (1H, m), and 5.32 (1H, d, J = 7.2 Hz) attributed to H-3′′ and 2′′, 4′′, 5′′, 1′′, and the anomeric proton H-1′′. The position of attachment of a sugar moiety to the flavone skeleton was determined by HMBC experiments, which showed long-range coupling between the anomeric H-1′′ (δ 5.32) and the C-7 at δ 151.3. The 1H and 13C NMR spectroscopic data (Table 1) of the aglycone and sugar moieties of 1 were similar to those of isoscutellarein 7-O-β-D-xylopyranoside except for one additional sugar unit in 1 [4]. The full connectivity of 1 was deduced from the HMBC correlations (Fig. 1). In particular, the long-range correlations between the proton signals at δ 5.15 (H-1′′′) and the carbon signals at δ 76.9 (C-3′′) suggested that the α-L-rhamnose unit was linked to C-3′′ of β-D-xylopyranoside. The anomeric configuration of D-xylopyranoside was determined to be of the β-form on the basis of the JH–H value (7.2 Hz) in the 1H NMR spectrum, and the configuration of L-rhamnose was determined to be of the α-form from examination of the chemical shifts (100.6, 70.4, 70.5, 72.0, 68.6, 17.9) in the 13C NMR spectrum of 1. Based on the above evidence, the structure of 1 was characterized as the new natural product isoscutellarein 7-O-β-D-rhamnopyranosyl-(1→3)-α-L-xylopyranoside.
Experimental
General Procedures. Melting points were measured on a YRT-3 capillary melting apparatus. NMR spectra were obtained on a Varian NMR spectrometer (400 MHz for 1H, 100 MHz for 13C) in DMSO-d6 solutions with tetramethylsilane as an internal reference. 1H–1H COSY, HMQC, and HMBC NMR spectra were obtained with the usual pulse sequences, and data processing was performed with standard Gemini and Bruker software. ESI-MS data was obtained on a LC-MSD-Trap-SL instrument. UV spectra were taken with a Shimadzu UV 2510 UV vis recording spectrometer. IR spectra were recorded on a Bruker VERTEX 70 instrument using potassium bromide pellets.
Plant Material. Twigs and leaves of Juniperus sabina L. were collected in September 2012 around the Houxia region in Urumqi, China. The plant was identified by Associate Researcher Jiang He, Institute of Materia Medica of Xinjiang, China. A specimen sample of the plant material has been preserved in the Institute of Materia Medica of Xinjiang, China.
Extraction and Isolation. Dried twigs and leaves of Juniperus sabina L. (11.0 kg) were cut into small pieces and extracted with 95% ethanol at reflux three times. The ethanol extract was suspended in water and then partitioned with petroleum ether (5 × 2 L), chloroform (5 × 2 L), EtOAc (5 × 2 L), and n-BuOH (5 × 2 L). Various fractions were condensed under reduced pressure to obtain the petroleum ether, chloroform, EtOAc, and n-BuOH extracts (997 g, 1043 g, 203 g, and 371 g, respectively), and 350 g of the n-BuOH extract was subjected to silica gel CC eluted with solvents of increasing polarity CHCl3–MeOH (20:1 to 1:1) to obtain 21 fractions (BE1–BE21). Fraction BE 4-7 was further purified by column chromatography over silica gel with the CHCl3–MeOH gradient system (10:1 to 1:1) to obtain 19 fractions (BBE1–BBE19). Subfraction BBE 5-9 was further purified by column chromatography over ODS RP-18 using 30–50% MeOH as eluent and finally purified by Sephadex LH-20 eluted with 60% MeOH to afford 213 mg of compound 1 as a yellow amorphous powder.
Isoscutellarein 7- O - β -D-rhamnopyranosyl-(1→3)- α -L-xylopyranoside, yellow amorphous powder, mp 216–218°C. UV (MeOH, λ max, nm): 224, 279, 309, 355. IR (KBr, νmax, cm–1): 3546, 3341, 1662, 1610, 1581, 1503, 1441, 1382, 1332, 1269, 1227, 1062, 949, 838. For 1H, 13C, and HMBC NMR data, see Table 1 and Fig. 1. ESI-MS m/z 565 [M + H]+.
References
Editorial Board of Flora Repub. Sinicae, Flora Republicae Popularis Sinicae, Vol. 7, Beijing, Science Press, 1978, 360 p.
Y. M. Liu, Pharmacography of Uighur, Part One, Xinjiang Science & Technology & Hygiene Publishing House, Urumuqi, China, 1999, 550 p.
W. B. Wang, H. K. Wu, H. Ba, and A. Hajia, Chem. Nat. Compd., 41, 473 (2005).
J. Zhao, M. Yan, Y. Huang, W. Y. He, and Y. Zhao, Chem. Ind. Forest. Prod., 28 (2), 33 (2008).
W. B. Wang, H. Ba, and A. Hajia, Nat. Prod. Res. Dev., 17 (5), 588 (2005).
J. Janar, A. E. Nugroho, C. P. Wong, Y. Hirasawa, T. Kaneda, Q. Shirota, and H. Morita, Chem. Pharm. Bull., 60, 154 (2012).
Acknowledgment
This work was financially supported by the National Nature Science Foundation of China (No. 81160515).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2015, pp. 392–393.
Rights and permissions
About this article
Cite this article
Zhao, J., Xu, F., Ji, T. et al. A New Flavone Glycoside from Twigs and Leaves of Juniperus sabina . Chem Nat Compd 51, 448–450 (2015). https://doi.org/10.1007/s10600-015-1312-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1312-x