Abstract
A soft coral-derived fungus Penicillium sp. among other isolates e high antibacterial, anti-yeast and cytotoxic activities. The fungus, Penicillium sp. MMA, isolated from Sarcphyton glaucoma, afforded nine diverse compounds (1–9). Their structures were identified by 1D and 2 D NMR and ESI–MS spectroscopic data as two alkaloids: veridicatol (1), aurantiomide C (2); one sesquiterpene, aspterric acid (3); two carboxylic acids, 3,4-dihydroxy-benzoic acid; (4) and linoleic acid (5); three steroids, ergosterol (6), β-Sitosterol (7), β-Sitosterol glucoside (8) along with the sphingolipid, cerebroside A (9). Biologically, the antimicrobial, antioxidant, in vitro cytotoxicity and antibiofilm activities were studied in comparison with the fungal extract. The in silico computational studies were implemented to predict drug and lead likeness properties for 1–4. The fungus was taxonomically characterized by morphological and molecular biology (18srRNA) approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal Endophytes obtained from unique environmental habitats offer a pool of potentially useful medicinal entities and have proven to be rich sources of bioactive natural products (Xia et al. 2018; Hardoim et al. 2015; Kaul et al. 2012; Pimentel et al. 2011; Sarasan et al. 2017). In the recent investigations of bioactive metabolites, the marine-derived fungi from invertebrates (e.g. sponges and soft corals) represent an outstanding source of novel bioactive metabolites having functional potentiality as drugs or drug leads (Haefner 2003; Saleem et al. 2007; Yin and Keller 2011). Such marine hosts are expected, therefore, to harbor marine-derived fungi having the capability to deliver various secondary metabolites (Suryanarayanan 2012). Large numbers of these metabolites have been included in the medical applications (Nicoletti and Trincone 2016; Pejin et al. 2013). Penicillium species represent the major source of antibiotics and mycotoxins (Frisvad et al. 2004), synthesized by different biosynthetic pathways, including terpenes, polyketides and alkaloids (Leitao 2009).
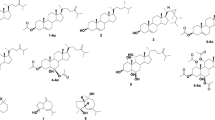
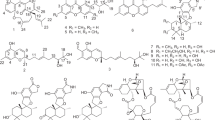
The fungus Penicillium sp. MMA, among others isolated from Sarcphyton glaucom got from Red Sea, exhibited high antibacterial, anti-yeast and cytotoxic activities. The strain was accordingly applied to upscale fermentation to isolate and identify its produced metabolites using different chromatographic techniques. Nine diverse metabolites were obtained: veridicatol (1), aurantiomide C (2), aspterric acid (3), 3,4-dihydroxy benzoic acid (4), linoleic acid (5), ergosterol (6), β-sitosterol (7), β-sitosterol glucoside (8) and cerebroside A (9) (Fig. 1). Their chemical structures were verified by NMR spectroscopic and mass spectrometric means and literature comparison. Biologically, the antimicrobial, antioxidant, antitumor and antibiofilm activities of the produced metabolites were investigated in comparison with the original extract. The computational calculations with respect to the physicochemical properties, ADME, acute oral toxicity were performed for compounds 1–4. Taxonomically, the studied fungus was characterized based on morphological and molecular biology (18srRNA) strategies (Fig. 2).
Materials and methods
General experimental procedure
The NMR spectra were measured on a Bruker AMX 300 (300.135 MHz), a Varian Unity 300 (300.145 MHz) and a Varian Inova 500 (125.820 MHz) spectrometer. ESI MS was recorded on a Finnigan LCQ with quaternary pump Rheos 4000 (Flux Instrument). EI mass spectra were recorded on a Finnigan MAT 95 spectrometer (70 eV) with perfluorkerosine as reference substance for EI HRMS. Flash chromatography was carried out on silica gel (230–400 mesh). Rf-values were measured on Polygram SIL G/UV254 (Macherey–Nagel & Co.). Size exclusion chromatography was done on Sephadex LH-20 (Pharmacia).
Sampling and Isolation of the producing fungus
Collection of Sarcophyton sp. and isolation of the desired endophytic fungus strain were performed as same as done in our recently reported work (El-awady et al. 2019), then kept at 4 °C (Debbab et al. 2009). Four pure isolates were obtained and deposited in the Microbial Biotechnology Department, NRC, Egypt, until investigation.
Pre-screening
The four fungal isolates (F1–F4) obtained were fermented in a small scale on rice-solid media at 30 °C for 7 days. After incubation, their culture media were individually soaked in ethyl acetate, followed by decantation, filtration and in vacuo concentration till dryness. Then extract of the isolates were (a) biologically tested as antimicrobial, antioxidant, antitumor agents (using Ehrlich’s antitumor activities) and (b) chemically screened (during TLC, visualized by UV and spraying reagents). The fungal isolated F4 was the most interesting among the four isolates through the biological and chemical screening criteria and selected, therefore, for full taxonomical characterization and large-scale fermentation to isolate its desired bioactive metabolites.
Genetic identification
The molecular identification of the selected fungal strain F4 was carried out by genomic DNA extraction using Qiagen DNeasy Mini Kit following the manufacturer’s instructions. The PCR amplification was performed using two primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), the reaction mixture was as follows: 1 µg fungal genomic DNA, 1 µL (20 µM of each primer), 10 mM dNTPs mixture, 2 units of Taq DNA polymerase enzyme and 10 µL 5 × reaction buffer) with the following PCR thermal profile: 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 90 s and a final extension step at 72 °C for 5 min. The PCR product was purified using JeneJET purification kit (ThermoFisher Scientific) and shipped for sequencing by Macrogen, South Korea (Blunt et al. 2007). The 18S rRNA gene sequence was aligned using BLAST available at NCBI database (GenBank C, https://www.ncbi.nlm.nih.gov/Genbank/National Institute of Biotechnology Information, Bethesda, Maryland, USA). The phylogenetic tree was constructed using neighbor-joining tree method using the software MEGA7, identifying it as Penicillium sp. MMA.
Large-scale cultivation
Well-grown colonies of Penicillium sp. MMA were inoculated into 100 mL of International System Project (ISP2) medium composition (g L−1): Malt extract (10); Yeast extract (4), glucose (4), 50% natural sea water, pH 6. and applied to cultivation on shaker at 30 °C for 3 days. The grown seed culture was served to inoculate 5 × 1 L Erlenmeyer flasks, each containing 100 g commercial rice and 150 mL 50% natural sea water. The seeded culture medium was then applied to static incubation at 28 °C for 14 days (Bara et al. 2013). After harvesting, the obtained yellowish-brown culture was soaked in ethyl acetate, followed by decantation and filtration. The solid culture residue was further re-soaked in methanol followed by filtration and concentration in vacuo; then the water residue obtained was extracted by ethyl acetate. The organic extracts were combined and concentrated to dryness giving 8.1 g.
Isolation and purification
The fungal extract was fractionated using column chromatography on silica gel (3 × 100 cm), eluted by cyclohexane-CH2Cl2–MeOH gradient. According to TLC visualization using UV (254/366 nm) and consequent spraying with anisaldehyde/sulphuric acid, four fractions were obtained: I (3.73 g), II (1.51 g), III (0.92 g) and FIV (1.11 g). Fraction I was re-purified on silica gel column (2 × 60 cm) and then Sephadex LH-20 (DCM/50% MeOH) to afford three colourless solids: ergosterol (6, 25.0 mg), β-sitosterol (7, 8.2 mg) and linoleic acid (5, 55.2 mg). Like fraction I, Fraction II was fractionated by silica gel column into three sub-fractions F-IIa (0.32 g), F-IIb (0.41 g) and F-IIc (0.21 g). Purification of FIIa on Sephadex LH-20 (DCM/40% MeOH) afforded veridicatol (1, 18.1 mg), while FIIb gave aurantiomide C (2, 18 mg) as further colourless solid. An application of sub-fraction F-IIc to PTLC (DCM/5% MeOH) followed by Sephadex LH-20 (MeOH) delivered a colourless solid of 3,4-dihydroxy-benzoic acid (4, 4.2 mg). Purification of fraction III through silica gel column (2 × 60 cm) and elution with DCM-MeOH gradient afforded aspterric acid (3) as major crude compound (225 mg), which after re-purification on Sephadex LH-20 (MeOH) was afforded (200.1 mg) as colourless solid. The polar fraction IV was fractioned on silica gel column using DCM-MeOH gradient, followed by purification on Sephadex LH-20 (MeOH) to afford two colourless solids of β-sitosterol glucoside (8, 22.1 mg) and cerebroside A (9, 25.0 mg). Spectroscopic data of the isolated compounds (1–9) are found in “Supplementary Data” file.
Biological activity
Antimicrobial activity
40 μL for each of compounds 1–9 (dissolved in CH2Cl2/10% MeOH, 1 mg/mL) were soaked on paper discs (6 mm ∅) and dried under sterilized conditions. Then, they were placed on inoculated agar plats and incubated for 24 h at 37 °C for bacterial and 48–72 h (30 °C) for the fungal isolates. The disc diffusion test has been done according to Bauer et al. (1966). Inhibition zones were measured in mm and recorded. The microbes Bacillus subtilis, Staphylococcus aureus, Pseudomonas areuginosa, Escherichia coli, Candida albicans and Aspergillus niger were served. The isolates were obtained from the Microbial Biotechnology Department, NRC, Egypt. The nutrient agar medium (g/l): Beef extract 3; peptone,10; and agar, 20 (pH 7.2) were served for growing of bacteria and yeasts, while the test fungal strains were grown on Czapek-Dox medium.
Antioxidant assaying
The free radical scavenging activity (RSA) was measured by the decolouration of an ethanolic solution of DPPH radical and evaluated spectrophotometrically at 517 nm according to Brand-Williams et al. (1995).
Antitumor activity assaying against Ehrlich cells
The in vitro antitumor activity testing, based on Ehrlich’s, was performed according to Bennett et al. (1976).
Cytotoxic activity
Five human cancer cell lines were tested using the MTT assay (Mosmann 1983): Hepatocellular (HePG-2), Epitheliod (Hela), Epdermoid (HEP2), Mammary gland (MCF-7) and colorectal (HCT-116) carcinoma. The cell lines were obtained from American Type Culture Collection (ATCC) via Holding company for biological products and vaccines (VACSERA), Cairo, Egypt.
Biofilm inhibitory assaying
To evaluate anti-biofilm efficiency, microtitre plate assay (MTP) was carried out against four clinical microbes (P. aeruginosa, S. aureus, E. coli and B. subtilis) using 96-well flat-bottom polystyrene titre plates according to (Christensen et al. 1985; Hamed et al. 2020).
Study of the physicochemical properties, ADME-parameters, acute oral toxicity and toxicity targets
The physico-chemical properties and ADME parameters of compounds 1–4 were performed according to Daina et al. (2017). Prediction of rodent oral toxicity and indication of possible toxicity targets were estimated by ProTox web server according to Drwal et al. (2014).
Results and discussion
Pre-screening
Four fungal strains (F1–F4) isolated from Sarcophyton sp. were biologically screened. Anti-microbially, using paper-disk diffusion assay, isolates F1–F4 showed high similarity in their potential activity against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Candida albicans. In contrast to the high activity of the fungal isolates F1 and F2 against Escherichia coli, isolates F2 and F3 were exceptionally inactive against the latter. Nevertheless, the four isolates displayed no antifungal activity against A. niger (see supplementary data, Table S1). The organic extracts of the four isolates were further investigated for antioxidant and antitumor by Ehrlich’s activities, revealing that F4 > F2 > F1 > F3 in their antioxidant potentiality,;meanwhile, they were ordered as F4 > F1 > F2 > F3 according to their antitum or potency (see supplementary data, Table S2). Based on this study, the fungal isolate F4 was remarked to be the most active from the antioxidant (79.1%) and antitumor (67.2%) activities point of view in addition to its potential antimicrobial activity as well. Therefore, the strain F4 was selected for full taxonomy, large-scale fermentation and studied for its produced bioactive metabolites in both of chemical assignments and biological activities.
Genetic identification
The genomic DNA of the selected fungal isolate F4 was extracted, amplified and the sequence of 18S rRNA gene was obtained and aligned to identify the similarity score with other known sequences available in the GenBank database using BLAST tool (https://www.blast.ncbi.nlm.nih.gov/Blast). The obtained result confirmed a very close similarity of the 18S rRNA gene sequence obtained with 100% homology of the isolate coded MMA with Penicillium sp. The phylogenetic analysis and tree were constructed using the neighbor-joining method by MEGA 7 program and based on the genetic analysis and similarity score the selected fungus MMA was identified as Penicillium sp. MMA and deposited in GenBank with the accession no. MK026953.
Isolation and Structure identification
The fungus Penicillium sp. MMA was up-scale cultivated on solid rice medium, worked up and its bioactive metabolites produced were purified using different chromatographic techniques (see the experimental section) delivering nine compounds. Structures of the obtained metabolites were assigned by the study of their NMR (1D, 2D) spectroscopy and mass spectrometric means (see supplementary data), identifying them as viridicatol (1) (Shaaban et al. 2016; Hamed et al. 2019), aurantiomide C (2) (Hamed et al. 2019), aspterric acid (3) (Shimada et al 2002), 3,4-dihydroxy benzoic acid (4) (Syafni et al. 2012), linoleic acid (5) (Shaaban 2004; Hamed et al. 2019), ergosterol (6) (Nagia et al. 2012), β-sitosterol (7), β-sitosterol glucoside (8) (Hamed et al. 2019) and cerebroside A (9) (Hamed et al. 2019). According to our searches in literature and different databases, the obtained compounds in this investigation were reported previously either from Penicillium sp. or Aspergillus sp. However, they are reported herein for the first time from Penicillium sp. MMA which has the highest similarity to Penicillium crustosum FRR 1669 (ex-type) (Nicoletti and Trincone 2016; Yu et al 2019) based on our genomic characterization mentioned above. In accordance, originality of the compounds investigated herein has been assured.
Viridicatol (1) belongs to 4-arylquinolin-2(1H)-ones, showing cytotoxicity toward human cervix (KB, KBv200), lung (A549), liver (HEPG2, SMMC7721), breast (MCF7), leukemia (K562) and gastric (SGC7901) tumor cell lines (Hamed et al. 2019; Luckner & Mothes 1962; Austin & Myers 1964). Aurantiomide C (2) was alternatively reported to show a potent anticancer activity against hepatocellular carcinoma (BEL-7402) and leukaemia (P388) cell lines (Xin et al. 2007). Aspterric acid (3) is a carotane-type sesquiterpene, a potent inhibitor of pollen development in Arabidopsis thaliana (Shimada et al. 2002). Aspterric acid (3) reported no considerable cytotoxicity in human cell lines (Yan et al. 2018). Benzoic acid derivatives (e.g. 4) are medicinally served as antiseptic, expectorant, antifungal, antipyretic and keratolytic agents (Shaaban 2004; Chapman & Hall Chemical Database 2018). Linoleic acid and its derivatives represent, on the other hand, the main essential unsaturated fatty acids (EFA), which belong to Omega 6 fatty acids (Abdel-Razek et al. 2017; Gaullier et al. 2005), are necessary to human body physiological processes. EFA have several medicinal applications and in treatment of cardiovascular diseases, skin permeability, insulin resistance, cancer, depression and plasmodial activity (Undurti 2008; Melariri et al. 2012). EFA were reported as well to reduce the nerve and breast pains in addition to their reductive efficiency of blood pressure and rheumatoid arthritis (Abdel-Razek et al. 2017).
Sterols represent the first choice of potential natural preventive dietary products. Ergosterol (6) is a common fungal sterol found displaying broad and significant biological activities so that up to date, 8247 articles have been cited in Scifinder referring to its biological importance (scifinder.cas.org). Particularly, ergosterol was reported to increase vitamin D concentrations in serum and liver of mice (Baur et al. 2019). Ergosterol has anti-inflammatory efficiency through the reduction of nitric oxide formation in LPS-stimulated RAW264.7 cells (Patjana et al. 2019). So, it might be served as novel functional food in prevention of allergic diseases (Kawai et al 2019).
β-Sitosterol is a phytosterol, structurally like cholesterol and is well spread in plants, fungi and animals (Peshin and Kar 2017). As a secondary metabolite, it is used as health-promoting constituent of natural foods. According to European foods and safety authority (EFSA) and US Food and Drug Administration (USFDA), it has been recommended to consume 1.5–4 g/day of phytosterol to reduce blood pressure and the risk of heart attack. β-sitosterol-β-d-glucoside has been proposed as a valuable compound for the improvement of new drugs to cure several inflammations accompanied by nitric oxide overproduction (Peshin and Kar 2017). It powerfully inhibits the activity of interleukin 6 of motivated macrophages (Kontogiorgis et al. 2010; Gautam and Navneet 2012). Cerebrosides (e.g. 9) (sphingolipids) (Barreto-Bergter et al. 2011) are playing an important role in major cellular processes (Youssef et al. 2016) and ecological activities of eukaryotic cells (Koga et al. 1998).
Biological activity studies
Antimicrobial activity
The antimicrobial potentiality of obtained metabolites 1–9 (using paper-disk method), revealed that aspterric acid (3) is the most potent against all studied microorganisms: S. aureus (10 mm), B. Subtilis (18 mm), P. aeruginosa (18 mm), E. coli (10 mm), C. albicans (12 mm) and A. niger (20 mm). Veridicatol (1) is weakly to moderately active against B. Subtilis, P. aeruginosa, E. coli and A. niger; meanwhile. ergosterol (6) was inactive against yeast, fungi and the G positive S. aureus. As expected, linoleic acid (5) was weakly to moderately active against Gram-positive and Gram-negative bacteria and fungi (8–10 mm), while being potentially active against C. albicans (16 mm) (Table 1).
Antioxidant activity
The antioxidant activity of compounds 1–9 based on DPPH assay (200 µg/mL) revealed that aurantiomide C (2) is the most potent antioxidant agent, showing maximum DPPH scavenging activity (75.26%), followed by aspterric acid (9) (50.16%), then veridicatol (1) (42.29%). In contrast, the remaining compounds are of very less antioxidant activity (Table 2).
In vitro cytotoxicity
As there is a matching correlation between the natural compounds’ antioxidant activity and their cytotoxicity (Li et al 2007), veridicatol (1), aurantiomide C (2) and aspterric acid (3) were accordingly selected to investigate their efficiency as anticancer agents. So, they were studied against liver (HePG-2), breast (MCF-7), colon (HCT-116), human epithelial type 2 (HeP2) and cervical (Hela) cancer cell lines, in comparison with doxorubicin as reference control. Accordingly, veridicatol (1) showed the maximum potency against the five cell lines (IC50: 6.27–13.87 µg/mL), followed by aurantiomide C (2) (IC50: 9.51–37.63 µg/mL); meanwhile, aspterric acid (3) showed the lowest cytotoxicity (IC50: 26.71–51.10 µg/mL (Table 3).
Anti-biofilm activity
Recently, bacterial biofilm is considered as one of the major public health problems. This process gives bacteria the ability to maintain their attachment to the living and non-living surface by production of a complex exopolymers formed from proteins, polysaccharides and nucleic acids (Chapman et al. 2002; Branda et al. 2005; Steinberg and Kolodkin-Gal 2015). Biofilm participates in a large task in the persistence of pathogenic bacteria (Rabin et al. 2015) and is considered as a source of pathogenic bacteria that are involved in numerous infectious diseases (Donlan and Costerton 2002; Wingender and Flemming 2011).
Based on MTT assay, the biofilm inhibition action of the fungus extract and corresponding pure compounds was investigated against P. aeruginosa, S. aureus, E. coli and B. subtilis. Preliminary antibiofilm results of the crude extract displayed potent biofilm inhibition against P. aeruginosa up (43%) and low biofilm inhibition against S. aureus (19%). However, it showed no activity against E. coli and B. subtilis. β-Sitosterol (7: C1) and Veridicatol (1: C3) reduced the biofilm formation of B. subtilis up to 28% and 35%, respectively (Fig. 3a).S. aureus biofilm formation was inhibited over 64% by β-Sitosterol (7: C1). In contrast, aurantiomide C (2: C6), ergosterol (6: C8) and aspterric acid (3: C9) have low inhibitory activity (Fig. 3). In case of E coli, the highest percentage of biofilm inhibition was shown by Veridicatol (1: C3), Aurantiomide C (2: C6) and ergosterol (6: C8) followed by (-Sitosterol (7: C1) and (-Sitosterol glucoside (8: C2) (Fig. 3). On the other hand, P. aeruginosa biofilm displayed a very low response to β-Sitosterol (7: C1), aurantiomide C (2: C6) and ergosterol (6: C8), while it did not display any response to other compounds (Fig. 3).
Biofilm inhibition (%) of the fungus extract and pure compounds (β-Sitosterol (7: C1), β-Sitosterol glucoside (8: C2), Veridicatol (1: C3), linoleic acid (5: C5), aurantiomide C (2:C6), ergosterol (6: C8), aspterric acid (3: C9), Crude extract: Cr). Test organisms: P. aeruginosa, S. aureus, E. coli and B. subtilis biofilm were assessed by crystal violet staining. Data presented represents mean ± SD of three independent experiments
Based on our updated search in literature, aspterric acid (3) and linoleic acid were reported previously as antifungal agents. Particularly, they showed significant antifungal activities against crop and plant pathogens (Liang et al. 2019; Walters et al. 2004). Fortunately, aspterric acid (3) is reported herein first as potent antibacterial agent especially against B. subtilis. Moreover, linoleic acid showed a weak activity against Gram-positive and Gram-negative bacteria.
On the other hand, the quinolinone and quinazoline moieties, viridicatol (1) and aurantiomide C (2), respectively, were reported previously to exhibit potent anticancer activities (Xin et al. 2007; Hamed et al. 2019). The potential cytotoxic activity of veridicatol against colon (HCT-116), human epithelial type 2 (HeP2) and cervical (Hela) cancer cell lines is reported herein for the first time; meanwhile, the cytotoxicity of aurantiomide C (2) against the reported five cell lines is reported herein for the first time. It is worthy to refer herein that, viridicatol (1), aurnatiomide C (2) and aspterric acid (3) are reported to first time as potential antioxidant agents, and this is strongly matching with their reported subsequent anticancer activities. Finally, the investigated antibiofilm activity of the afforded compounds against P. aeruginosa, S. aureus, E. coli and B. subtilis has been discussed herein for first time so far.
Physicochemical properties and ADME parameters
Swiss ADME web-based tools (Daina et al 2017) were used to estimate the physicochemical properties and ADME behaviors of compounds 1–4. According to drug-likeness rules, the compounds passed Lipinski, Veber and Ghose rules with zero violation, except compound 4 did not pass the filter of Ghose rule attributable to three violations including MW < 160, MR < 40 and number of atoms < 20. The compounds have oral bioavailability (0.56/0.55) and could be possible oral drugs (Table 4). In the same manner, the Bioavailability Radar plot is adopted for a rapid estimation of drug-likeness, taking into account six physicochemical properties (Fig. 4) (Daina et al. 2017; Vuppala et al. 2013). Evidently, compound 2 from the first glance displayed all the properties in the optimal range (pink area).
Lipophilicity is an important physicochemical characteristic quantified by the partition coefficient Log Po/w between water and n-octanol that gives a good indicator of permeability across the cell wall (Rutkowska et al. 2013; Potts and Guy 1992). Compounds 1–4 demonstrated Log Po/w values below 5, ranging from 0.80 to 2.61, suggesting good permeability and absorption across the cell membrane of infected cells. Based on ESOL topological model, compounds 1–4 are soluble (Daina et al. 2017). For defining lead-likeness, they passed the rule of three (RO3), except compound 4 that has one violation against this rule. So, compounds 1, 2 and 3 could be possible lead compounds. For synthetic accessibility score (SAscore) that estimated on similarity of fragments and complexity penalties, compound 4 is the easiest one (1.07), while compound 3 is the most difficult (5.09).
The pharmacokinetic parameters of compounds 1–4 were visualized by vector machine algorithm (SVM) model (Daina et al. 2017). As shown in Table 5, compounds 2 and 3 are non-inhibitors against all the isoenzymes, while compound 1 display selective inhibitory toward CYP1A2 and CYP3A4. The latter (CYP3A4) is also inhibited by compound 4. Based on BOILED-Egg model (Brain or Intestinal Estimate D permeation method, WLOGP vs TPSA) that adapted according to (Daina et al. 2017 and Daina and Zoete 2016) and illustrated in Fig. 5, compounds 1–4 demonstrated high human gastrointestinal absorption (GI). Compounds 2 and 3 are P-gp substrates (PGP + , blue dots), while the others are none. Compounds 2 and 4 are non-permeant for blood–brain barrier (BBB) and hence they have no adverse effects on central nervous system (CNS), while compounds 1 and 3 are BBB permeant (TPSA < 75 Å2).
Predicting the skin permeability coefficient (Kp) of compounds 1–4 was done as described by Potts and Guy (Potts and Guy 1992; Atta et al 2019), where the more negative the log Kp, the less skin permeant is the compound (2).
Prediction of acute oral toxicity and indication of toxicity targets
Based on the ProTox web server, has been indicated the possible toxicity targets of compounds 1–4 (Drwal et al. 2014). Fortunately, the predicted toxicity classes were ranged from four to five. Compound 3 displayed the highest LD50 value (3520 mg/kg), so it has the lowest toxic effect. Also, all the predicted compounds have not any toxic fragments as manifested in Table 6. Regarding with toxicity targets, compound 3 indicated binding to androgen receptor with zero average pharmacophore fit (Table 7).
It is worthy to refer herein and based on our updated searching in literature that, the presented computational studies for compounds 1–4 have been discussed to first time so far, which can be helpful for future drug administration and exploration.
References
Abdel-Razek AS, El-Awady M, Hassan AZ, Yassin FY, Asker M, Shaaban M (2017) Bioactive compounds from marine Stachybotrys sp. QL23. IJNPR 8:322–328
Atta KFM, Farahat OOM, Al-Shargabi TQ, Marei MG, Ibrahim TM, Bekhit AA, El Ashry EH (2019) Syntheses and in silico pharmacokinetic predictions of glycosylhydrazinyl-pyrazolo[1,5-c]pyrimidines and pyrazolo[1,5-c] triazolo[4,3-a]pyrimidines as anti-proliferative agents. Med Chem Res 28:215–227
Austin DJ, Myers MB (1964) 3-O-methylviridicatin, a new metabolite from Penicillium puberulum. J Chem Soc 1:1197–1198
Bara R, Zerfass I, Aly AH, Goldbach-Gecke H, Vijay R, Sass P, Mandi A, Wray V, Polavarapu PL, Pretsch A, Lin WH, Kurtan T, Debbab A, Broetz-Oesterhelt H, Proksch P (2013) Atropisomeric dihydroanthracenones as inhibitors of multi-resistant Staphylococcus aureus. J Med Cchem 56:3257–3272
Barreto-Bergter E, Sassaki GL, de Souza LM (2011) Structural analysis of fungal cerebrosides. Front Microbiol 5:239
Bauer AW, Kirby WM, Sherris JC, Truck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Baur AC, Kuehn J, Brandsch C, Hirche F, Stangl GI (2019) Intake of ergosterol increases the vitamin D concentrations in serum and liver of mice. J Steroid Biochem 194:105435
Bennett JM, Catovsky D, Danniel MT, Galton DAG, Graanlink HR, Sultan C (1976) Proposal for the classification of the acute leukamias. Br J Haemtol 33:451–458
Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR (2007) Marine natural products. Nat Prod Rep 24:31–86
Branda SS, Vik S, Friedman L, Kolter R (2005) Biofilms: The matrix revisited. Trends Microbiol 13:20–26
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006
Ciegler A, Kadis S, Ajl SJ (1971) Microbial toxins: a comprehensive treatise; fungal toxins. Metabolites of Penicillium viridicatum and closely related species. Copyright by Academic press, INC 111 Fifth Avenue, New York 10003, Vol VI, p 509
Daina A, Zoete V (2016) A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem Med Chem 11:1117–1121
Daina A, Michielin O, Zoete V (2017) Swiss ADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717–42730
Debbab A, Aly AH, Edrada-Ebel R, Müller WEG, Mosaddak M, Hakiki A, Proksch P, Ebel R (2009) Bioactive secondary metabolites from the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco. Biotechnol Agron Soc Environ 13:229–234
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R (2014) ProTox: a web server for the in-silico prediction of rodent oral toxicity. Nucleic Acids Res 42:53–W58
El-awady ME, Boulis AG, Attia AR, Shaaban M (2019) Dimeric Naphtho-gamma-pyrones and further diverse bioactive metabolites from the marine-derived Aspergillus flavus Af/MMA 2018. Egypt Pharmaceut J 18:245–253
Frisvad JC, Smedsgaard JL, Larsen TO, Samson RA (2004) Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol 49:201–241
Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O (2005) Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy overweight humans. J Nutr 135:778–784
Gautam SS, Navneet KS (2012) The antibacterial and phytochemical aspects of Viola odorata Linn. extracts against respiratory tract pathogens. Proc Natl Acad Sci India B Biol Sci 82:567–572
Haefner B (2003) Drugs from the deep: Marine natural products as drug candidates. Drug discov Today 8:536–544
Dictionary of Natural Products on CD-ROM (2018) Chapman & Hall Chemical Database
Hamed A, Ismail M, El-Metwally MM, Frese M, Ibrahim TMA, El-Haddad AF, Sewald N, Shaaban M (2019) Diverse Polyketides and alkaloids from Penicillium sp. KHMM: structure elucidation, biological and molecular docking studies. Z Naturforsch C 74:131–137
Hamed AA, Kabary H, Khedr M, Emam AN (2020) Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC Adv 10:10361–10367
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring G, Sessitsch A (2015) The hidden world with in plants: ecological and evolutionary considerations for defining functioning of microbial endophytesThe hidden world with in plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320. https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf
Kaul S, Gupta S, Ahmed M, Dhar MK (2012) Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem Rev 11:487–505
Kawai J, Mori K, Hirasawa N (2019) Grifola frondosa extract and ergosterol reduce allergic reactions in an allergy mouse model by suppressing the degranulation of mast cells. Biosci Biotechnol Biochem 83:2280–2287
Koga J, Yamauchi T, Shimura M, Ogawa N, Oshimai K, Umemura K, Kikuchi M, Ogasawara N (1998) Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J Biol Chem 273:31985–31991
Kontogiorgis CA, Bompou EM, Ntella M, Berghe WV (2010) Natural products from mediterranean diet: from anti-inflammatory agents to dietary epigenetic modulators. Anti Inflamm Anti-Allergy Agents Med Chem 9:101–124
Leitão AL (2009) Potential of Penicillium species in the bioremediation field. Int J Environ Res Public Health 6:1393–1417
Li W-Y, Chan S-W, Guo D-J, Yu PH-F (2007) Correlation between antioxidative power and anticancer activity in herbs from traditional chinese medicine formulae with anticancer therapeutic effect. Pharm Biol 45:541–546
Liang Z-Y, Shen N-X, Zheng Y-Y, Wu J-T, Miao L, Fu X-M, Chen M, Wang C-Y (2019) Two new unsaturated fatty acids from the mangrove rhizosphere soil-derived fungus Penicillium javanicum HK1-22. Bioorg Chem 93:103331
Luckner M, Mothes K (1962) On the biosynthesis of 2,3-dihydroxy-4-phenyl-quinoline viridicatin. Tetrahedron Lett 3:1035–1039
Melariri P, Campbell W, Etusim P, Smith P (2012) In vitro and in vivo antimalarial activity of linolenic and linoleic acids and their methyl esters. Adv Stud Biol 4:333–349
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nagia MMS, El-Metwally MM, Shaaban M, El-Zalabani SM, Hanna AG (2012) Four butyrolactones and diverse bioactive secondary metabolites from terrestrial Aspergillus flavipes MM2: isolation and structure determination. Org Med Chem Lett 2:1–8
Nicoletti R, Trincone A (2016) Bioactive compounds produced by strains of penicillium and talaromyces of marine origin. Mar Drugs 14:37
Patjana T, Jantaharn P, Katrun P, Mongkolthanaruk W, Suwannasai N, Senawong T, Tontapha S, Amornkitbumrung V, McCloskey S (2019) Anti-inflammatory and cytotoxic agents from Xylaria sp. SWUF09-62 fungus. Nat Prod Res. https://doi.org/10.1080/14786419.2019.1652292
Pejin B, Jovanovi KK, Mojovic M, Savic AG (2013) New and highly potent antitumor natural products from marine-derived fungi: covering the period from 2003 to 2012. Curr Top Med Chem 13:2745–2766
Peshin T, Kar HK (2017) Isolation and characterization of β-sitosterol-3-O-β-D-glucoside from the extract of the flowers of Viola odorata. Br J Pharm Res 16:1–8
Pimentel MR, Molina G, Dionisio AP, Maróstica MR, Pastore GM (2011) Use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol Res Int 576:286
Potts RO, Guy RH (1992) Predicting skin permeability. Pharm Res 9:663–669
Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO (2015) Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem 7:493–512
Rutkowska E, Pajak K, Jozwiak K (2013) Lipophilicity-methods of determination and its role in medicinal chemistry. Acta Pol Pharm 70:3–18
Saleem M, Ali MS, Hussain S, Jabbar A, Ashraf M, Lee YS (2007) Marine natural products of fungal origin. Nat Prod Rep 24:1142–1152
Sarasan M, Puthumana J, Job N, Han J, Lee J-S, Philip R (2017) Marine algicolous endophytic fungi-a promising drug resource of the era. J Microbiol Biotechnol 27:1039–1052
Shaaban M (2004) Bioactive secondary metabolites from marine and terrestrial bacteria: isoquinolinequinones, bacterial compounds with a novel pharmacophor. Ph.D. Thesis, Georg-August University, Göttingen, Germany
Shaaban M, Sohsah GE, El-Metwally MM, Elfedawy MG, Abdel-Mogib M (2016) Bioactive compounds produced by strain of Penicillium sp. IJSEA 5:7560
Shimada A, Kusano M, Takeuchi S, Fujioka S, Inokuchi T, Kimura Y (2002) Aspterric acid and 6-hydroxymellein, inhibitors of pollen development in Arabidopsis thaliana, produced by Aspergillus terreus. Z Naturforsch 57:459–464
Steinberg N, Kolodkin-Gal I (2015) The matrix reloaded: probing the extracellular matrix synchronizes bacterial communities. J Bacteriol 197:2092–2103
Suryanarayanan TS (2012) The diversity and importance of fungi associated with marine sponges. Bot Mar 55:553–564
Syafni N, Putra DP, Arbain D (2012) 3,4-Dihydroxybenoic acid and 3,4-dihydroxybenzaldehyde from the Fern Trichomanes chinense L.; isolation, antimicrobial and antioxidant properties. Indo J Chem 12:273–278
Undurti ND (2008) Can essential fatty acids reduce the burden of disease(s)? Lipids Health Dis 7:1–5
Vuppala PK, Janagam DR, Balabathula P (2013) Importance of ADME and bioanalysis in the drug discovery. J Bioequiv Availab 5:1–2
Walters D, Raynor L, Mitchell A, Walker R, Walker K (2004) Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 157:87–90
Wingender J, Flemming HC (2011) Biofilms in drinking water and their role as reservoir for pathogens. Int J Hyg Environ Health 214:417–423
Xia Y, Amna A, Opiyo SO (2018) The culturable endophytic fungal communities of switchgrass grown on a coalmining site and their effects on plant growth. PLoS ONE 13:1–16
Xin Z, Fang Y, Du L, Zhu T, Duan L, Chen J, Gu Q, Zhu W (2007) Aurantiomides A-C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J Nat Prod 70:853–855
Yan Y, Liu Q, Zang X, Yuan S, Bat-Erdene U, Nguyen C, Gan J, Zhou J, Jacobsen SE, Tang Y (2018) Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 559:415–418
Yin W, Keller NP (2011) Transcriptional regulatory elements in fungal secondary metabolism. J Microbiol 49:329–339
Youssef DTA, Ibrahim SRM, Shaala LA, Mohamed GA, Banjar ZM (2016) New cerebroside and nucleoside derivatives from a red sea strain of the marine cyanobacterium moorea producens. Molecules 21:324
Yu G, Sun Z, Peng J, Zhu M, Che Q, Zhang G, Zhu T, Gu Q, Li D (2019) Secondary metabolites produced by combined culture of Penicillium crustosum and a Xylaria sp. J Nat Prod 82:2013–2017
Acknowledgements
The authors are deeply thankful to Prof. H. Laatsch, Institute of Organic and Biomolecular Chemistry, Göttingen, for his support and lab facilities. We thank Dr. H. Frauendorf and Dr. M. John for MS and NMR measurements. M. Shaaban thanks the German Academic Exchange Service (DAAD) for a Postdoc short-term grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boulis, A.G., Hamed, A.A., El-awady, M.E. et al. Diverse bioactive metabolites from Penicillium sp. MMA derived from the red sea: structure identification and biological activity studies. Arch Microbiol 202, 1985–1996 (2020). https://doi.org/10.1007/s00203-020-01923-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01923-x