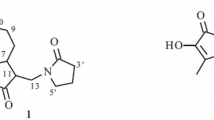
A new sesquiterpene lactone that was called inulonolide was isolated from Inula britannica. Its structure was established as 2-keto-1β,10β-epoxy-5,7,8,11α(H)-guai-3-en-8,12-olide based on spectral data and an X-ray crystal structure analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In continuation of research on Inula britannica (Asteraceae) growing in Uzbekistan [1], a sesquiterpene lactone that was previously unreported in the literature was isolated and called inulonolide by us.
The IR spectrum of 1 had an absorption band at 1742 cm–1 that corresponded to a γ-lactone carbonyl and bands for ketone carbonyl at 1692 cm–1, C=C double bond at 1604 cm–1, and epoxy group at 1269 and 931 cm–1.

The structure of 1 was established based on an analysis of PMR and 13C NMR spectra and a DEPT experiment. The PMR spectrum of 1 showed resonances for tertiary methyl as a singlet at δ 1.72 ppm (H-14), methyl on a double bond as a triplet at δ 2.20 ppm (H-15) with a through-space spin–spin coupling constant (SSCC) (J = 1.3 Hz), and secondary methyl at δ 1.21 ppm (H-13) as a doublet with SSCC J = 7.2 Hz. Furthermore, the strong-field region showed resonances for protons of two methylenes at δ 1.44 (ddd, J = 13.6, 12.7, 10.9 Hz; H-6a), 1.75 (dd, J = 13.6, 5.9 Hz; H-6b), and 2.33 (m, H-9) and for two methines at δ 2.68 (br.d, J = 10.9 Hz, H-5) and 2.81 (quintet, J = 7.1, H-11). The SSCC (J = 10.9 Hz) for H-5 was indicative of its α-configuration [2]. Resonances of γ-lactone protons H-7 and H-8 in the PMR spectrum were located at δ 2.77 and 4.47 ppm as a multiplet and triplet of doublets with SSCC J = 4.6 and 2.6 Hz, respectively. A SSCC of <8 Hz for H-8 was indicative of cis-fusion of the lactone ring. The weak-field region of the PMR spectrum of 1 had only one 1H resonance at δ 6.14 ppm as a doublet of quartets with meta SSCC (J = 2.6 and 1.3 Hz) that was characteristic of an olefinic proton.
An analysis of 13C NMR and DEPT spectra of 1 showed resonances for 15 C atoms as three methyls, two methylenes, five methines, and five quaternary C atoms, including two carbonyl C atoms at δ 201.76 (C-2) and 177.96 ppm (C-12). The aromatic part of the 13C NMR spectrum showed resonances for an olefinic C atom (C-3) at δ 132.41 ppm. Resonances at stronger field were observed for three C atoms bonded to oxygen at δ 78.51 (C-8), 65.53 (C-1), and 64.52 ppm (C-10). Resonances in the strong-field region at 9–45 ppm in the 13C NMR spectrum corresponded to C atoms of three methyls at δ 9.75 (C-13), 18.08 (C-14), and 18.30 (C-15); two methylenes at δ 22.75 (C-6) and 35.16 (C-9); and three methines at δ 41.33 (C-11), 43.81 (C-7), and 44.33 (C-5). The studies allowed the sesquiterpene lactone to be classified as a linear guaiane-type structure that had ketone and epoxy groups and an endomethylene double bond. Table 1 presents detailed PMR and 13C NMR spectral data for 1.
The structure 2-keto-1,10-epoxyguai-3-en-8,12-olide was proposed for lactone 1 based on the obtained data. An X-ray crystal structure analysis (XSA) of 1 was performed for unambiguous proof of its structure. Figure 1 shows the molecular structure of inulonolide.
The molecular structure of 1 according to the XSA (Fig. 1) showed that the compound was a sesquiterpene 7,8-guaiane lactone. The three rings were A/B/C-cis fused. Ring A of inulonolide was planar within ± 0.035 A°. The lactone ring had the 7α-envelope conformation. Seven-membered ring B adopted the chair conformation. The experimental Flack parameter [0.1(2)] defined the absolute configurations of the chiral centers as 1S,5S,7R,8R,10R,11S.
The epoxide ring of inulonolide was situated in the C1-C10 position with the β-orientation. The ketone of ring A was located on C2 [C2=O distance 1.213(4) A° ]; the double bond, C3=C4 [1.341(4) A° ]. The other bond lengths were within 3σ of normal [3]. The distances between inulonolide molecules in the crystal were typical of van-der-Waals interactions.
Thus, the new sesquiterpene lactone inulonolide had the structure 2-keto-1β,10β-epoxy-5,7,8,11α(H)-guai-3-en-8,12-olide.
Experimental
General Comments. The IR spectrum was taken in KBr on a System 2000 Fourier spectrometer (PerkinElmer). NMR spectra were recorded in CDCl3 on a Unity 400plus instrument (Varian) at operating frequency 400 MHz for 1H. The internal standard for PMR spectra was HMDS (0 ppm); for 13C NMR spectra, the solvent chemical shift (CDCl3, 77.16 ppm vs. TMS).
The purity of isolated compounds was monitored by TLC on Silufol UV 254 plates using C6H6–EtOH (9:1). Spots on plates were detected using I2 and NH3 vapors, UV lamps at 254 and 365 nm, and vanillin solution (1%) in conc. H2SO4.
Plant Material. I. britannica was collected in June during flowering in Tashkent Region, Republic of Uzbekistan. The species was determined by Dr. N. Yu. Beshko (Botanical Institute, Academy of Sciences of the Republic of Uzbekistan) by comparing the collected herbarium samples with I. britannica preserved in the Central Herbarium of Uzbekistan (combined herbariums of Tashkent State University and Botanical Institute, AS RUz).
Extraction and Isolation of Inulonolide. Flowers of I. britannica (2 kg) were extracted (5×) with hot (60°C) 93% EtOH with phase contact for 12 h. The combined extracts were condensed in a vacuum evaporation apparatus to 1 L. The extract (100 mL) was evaporated to dryness in a rotary evaporator to afford dry flower extract (38 g) that was dissolved in a small amount of EtOH, mixed with silica gel in a 1:1 ratio (w/w), and placed onto a column of silica gel (50 g) for fractionation by sequential elution by extraction benzine, CHCl3, EtOAc, and MeOH.
The CHCl3 extract (2.650 g) was mixed with KSK silica gel (3 g), placed onto a column of silica gel in a 1:10 (w/w) extract-to-silica gel ratio, and eluted by extraction benzine, its mixtures with EtOAc in a polarity gradient, and EtOH. Elution of the column by benzine–EtOAc (1:1) isolated colorless crystals (12 mg), recrystallization of which from EtOH afforded colorless needle-like crystals with mp 252–254°C. IR spectrum (KBr, νmax, cm–1): 3480, 2925, 1742, 1692, 1604, 1421, 1375, 1324, 1269, 1193, 1167, 1043, 952, 865. Table 1 lists the NMR spectral data.
XSA. Crystals of inulonolide were obtained from EtOH by slow evaporation at room temperature as colorless tetragonal prisms. Unit-cell constants were determined and refined on a CCD Xcalibur Ruby diffractometer (Oxford Diffraction) using Cu Kα-radiation (293 K, graphite monochromator). Absorption corrections were made semi-empirically using the SADABS program [4]. Table 2 presents the main parameters of the XSA.
The structure was solved by direct methods using the SHELXS-97 program suite. The structure was refined using the SHELXL-97 program [5]. All nonhydrogen atoms were refined by anisotropic full-matrix least-squares methods (over F2). Atomic coordinates of H atoms were found geometrically and refined with fixed isotropic shift parameters Uiso = nUeq, where n = 1.2 and Ueq is the equivalent isotropic shift parameter of the corresponding C atoms. The XSA data were deposited in the Cambridge Crystallographic Data Centre (CCDC 1977382).
References
R. F. Mukhamatkhanova, D. E. Dusmatova, V. V. Uzbekov, N. Yu. Beshko, and L. T. Dzhuraeva, Universum: Khim. Biol.: elektron. nauchn. zh., No. 7 (49), 8 (2018).
D. E. Dusmatova, Kh. M. Bobakulov, R. F. Mukhamatkhanova, K. K. Turgunov, E. O. Terenteva, E. A. Tsay, I. D. Shamyanov, B. Tashkhodzhaev, Sh. S. Azimova, and N. D. Abdullaev, Nat. Prod. Res., (2019). DOI: https://doi.org/10.1080/14786419.2019.1647423.
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. J. Taylor, J. Chem. Soc., Perkin Trans. 2, S1 (1987).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr, 64, 112 (2008).
G. M. Sheldrick, Program for Empirical Absorption Correction of Area Detector Data, University of Goettingen, Goettingen, 1996.
Acknowledgment
The work was financially supported by the Basic Scientific Research Program of the AS RUz (Project VA-FA-F-6-010, TA-FA-F7-008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2020, pp. 730–732.
Rights and permissions
About this article
Cite this article
Dusmatova, D.E., Turgunov, K.K., Bobakulov, K.M. et al. New Sesquiterpene Lactone from Inula britannica. Chem Nat Compd 56, 852–854 (2020). https://doi.org/10.1007/s10600-020-03168-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03168-y