Abstract
Two new chlorinated bis-indole alkaloids, dionemycin (1) and 6-OMe-7′,7″-dichorochromopyrrolic acid (2), along with seven known analogs 3–9, were isolated from the deep-sea derived Streptomyces sp. SCSIO 11791. Their structures were elucidated by extensive HRESIMS, and 1D and 2D NMR data analysis. In vitro antibacterial and cytotoxic assays revealed that, compound 1, shows anti-staphylococcal activity with an MIC range of 1–2 μg/mL against six clinic strains of methicillin-resistant Staphylococcus aureus (MRSA) isolated from human and pig. Additionally, compound 1 displayed cytotoxic activity against human cancer cell lines NCI-H460, MDA-MB-231, HCT-116, HepG2, and noncancerous MCF10A with an IC50 range of 3.1–11.2 μM. Analysis of the structure-activity relationship reveals that the chlorine atom at C-6″ could be pivotal for conferring their bioactivities, thus providing hints on chemical modifications on bis-indole alkaloid scaffold in drug design.
Similar content being viewed by others
Introduction
bis-Indole natural products are structurally defined by two monomeric indole alkaloids that contribute to their diverse biological activities. Compounds in the bis-indole family are ubiquitously distributed in plants [1] and microorganisms [2]. Since the early 1960s, this class of compounds has been successfully utilized in combination therapies for cancer [3, 4]. The first anti-cancer bis-indole alkaloids being introduced into clinical use were vinblastine and vincristine. Thereafter, subsequent progress has inspired enduring interest in the discovery of new bis-indole alkaloids from natural sources [5, 6]. Indolocarbazoles are a type of bis-indoles that possess inhibitory activities against protein kinases or DNA topoisomerases, as exemplified by K-252a to d [7], rebeccamycin [8], and staurosporine [9]. Moreover, compounds similarly classified in this class such as UCN-01 and BMY-27557 had been explored in clinical trials for cancer chemotherapy [10, 11]. Marine-derived bis-indole compounds typically contain halogen atoms in their structures. Such halogenated bis-indole alkaloids generally display potent cytotoxic and/or antibacterial activities, and are thus considered as promising anti-cancer or antibacterial leads [12]. The marine-derived brominated bis-indole alkaloids BrBIn is a potential inducer apoptosis in human cancer cells [13, 14]. A series of marine-derived chlorinated bis-indoles was shown to inhibit methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase significantly, with their halogenated indole ring being implicated as a critical pharmacophore [15].
Marine-derived Actinomycetes have been valued as an important reservoir of drug resources of interest to the treatment of various cancers and multi-drug resistant bacteria in clinic [16]. Previously, the cytotoxic properties of the chlorinated bisindole alkaloids spiroindimicins A–D [17] and indimicins A–E [18] were reported from a marine-derived Actinomycetes, Streptomyces sp. SCSIO 03032. As part of our effort to identify bioactive natural products, the deep-sea derived strain Streptomyces sp. SCSIO 11791 was subjected to intensive screening. Chemical investigation on this strain led to identification of nine chlorinated bis-indole alkaloids (1–9), including two new compounds dionemycin (1) and 6-OMe-7′,7′′-dichorochromopyrrolic acid (2) (Fig. 1). Herein, we describe the isolation, structure elucidation, cytotoxic and antibacterial activities of these compounds, and the discussion of their structure-activity relationship (SAR).
Results and discussion
Isolation and structure elucidation
The strain SCSIO 11791 was isolated from a sediment sample collected from the South China Sea. It was fermented scale-up to 21 L. The subsequent extraction, extensive isolation by silica gel chromatography, medium pressure liquid chromatography (MPLC) and HPLC purification led to the isolation of compounds 1–9. Compound 3, as a symmetric structure, was identified as an inhibitor of protein kinase C in a German patent [19]. Compounds 4–6, 8, and 9 were identified as lynamicins A (4), B (5) and D (6) [20], dichlorochromopyrrolic acid (8) [21], and spiroindimicin B (9) [17], respectively. Compound 7 was reported in a PCT patent [22] (Fig. 1).
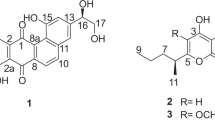
Compound 1 was isolated as an orange amorphous powder. Its molecular formula (C20H10Cl3N3O2) was determined upon analysis of the HRESIMS peak at m/z 429.9912 [M + H]+, requiring 16 degrees of unsaturation. The isotope pattern was consistent with the presence of three chlorines. 1H NMR spectra showed seven aromatic hydrogens, while 13C NMR spectra showed twenty carbon signals from δC 107.0 to δC 174.5 (Table 1). Detailed analysis on 2D NMR spectra confirmed the presence of twenty aromatic carbon signals, including seven methine carbons and thirteen non-hydrogen bearing carbons. HMBC correlations from H-2′ (δH 7.84, s) to C-3′, C-3a′, and C-7a′, from H-4′ to C-5′, C-6′, C-7a′, and C-3′, from H-6′ to C-5′, C-4′, and C-7a′, and from H-7′ to C-3a′ and C-5′ determined the presence of the indole moiety (A). HMBC correlations from H-4′, H-6′, and H-7′ to the carbon C-5′, as well as the coupling constants of H-4′ (d, J = 2.0 Hz), H-6′ (dd, J = 8.5, 2.0 Hz), and H-7′ (d, J = 8.5 Hz) in 1H NMR data revealed the presence of a 5′-substituted indole. Similarly, another indole moiety (B) was elucidated according to the HMBC correlations. However, the ABX system (H-4′, 6′, and 7′) in moiety-A was replaced by two aromatic singlet signals at δH 6.81 (H-4″) and 7.53 (H-7″), indicating that H-6″ was replaced by one chlorine atom in moiety-B. In addition, HMBC correlations from H-2′ to C-3 and the carbonyl at C-2, and from H-2″ to C-4 and the carbonyl at C-5 were observed. Thus, a 3,4-bis-indole-pyrrole-2,5-dione moiety was established. Accordingly, the two remaining chlorine atoms were placed at C-5′ and C-5″ in indole rings. Therefore, the structure of compound 1 was proposed as that in Fig. 2, and named dionemycin.
Compound 2 was purified as a pale-yellow amorphous powder. Its molecular formula (C23H15Cl2N3O4) was acquired by the HRESIMS peak at m/z 468.0502 [M + H]+, which contained 17 unsaturated degrees, and the isotope pattern confirmed the presence of two chlorines. 1D and 2D NMR spectra revealed two carbonyl carbons (C-6, 8), twelve aromatic non-hydrogen bearing carbons, eight aromatic methines, and one methoxy (-OCH3(C-7)) groups. Detailed comparison showed that 1H and 13C NMR data were similar to those bis-indole compounds 6 [20] and 7′,7″-dichorochromopyrrolic acid (CCA) [23] (Table S1). Coupling constants of H-4′/H-4″ (dd, J = 8.0 Hz), H-5′/H-5″ (t, J = 8.0 Hz), and H-6′/H-6″ (dd, J = 8.0, 2.0 Hz) in 1H NMR data indicated the presence of two 1,2,3-trisubstituted benzene rings in 2. The position of the methoxy group at C-6 (-OCH3(C-7), δH 3.71, 3 H, s; δC 51.9) was determined by HMBC correlation of H3-7 to C-6. Moreover, HMBC correlations (Fig. 2), especially with respect to H-4′/4″ to C-3′/3″, H-5′/5″ to C-3a′/3a″, C-7′/7″, and H-6′/6″ to C-4′/4″ and C-7a′/7a″ confirmed that the two chlorine atoms were attached to C-7′ and C-7″ in the two indole rings, respectively. The carboxy group was placed at C-5 as the only leaving substitution position. Therefore, the structure of compound 2 was finally elucidated as 6-OMe-7′,7″-dichorochromopyrrolic acid.
Cytotoxic activities
Compounds 1–9 were tested for cytotoxicity against MDA-MB-435 human breast cancer cells across a concentration gradient of up to 50 μM. Compounds 1, 2, 5, 7, 9 showed moderate cytotoxicity with IC50 values of 3.9, 19.4, 6.9, 13.8 and 3.0 μM, respectively. These five compounds were further evaluated against MDA-MB-231 human breast adenocarcinoma cells, NCI-H460 human non-small-cell lung cancer cells, HCT-116 colon cancer cells, HepG2 liver cancer cells, and the non-cancerous MCF10A human breast epithelial cells. Our results indicated that these five compounds exhibited moderate cytotoxicity against the above-mentioned cells with IC50 values ranging from 2.2 to 30.4 μM (Table 2). Compounds 1, 5 and 7 possessing a chlorine atom at C-6″ produced greater cytotoxicity than compounds 3, 4, 6, 8, (IC50 > 50 μM against MDA-MB-435), which could be potentially useful in rational drug design.
Antibacterial activities
Preliminary screens of compounds 1–9 for antibacterial activities were conducted by using the disk diffusion method against Gram-positive bacteria including Micrococcus luteus ML01, Staphylococcus aureus ATCC 29213, and a panel of MRSA isolated from human patients (MRSA 991, MRSA 1862, MRSA 669 A and MRSA A2) and pig (MRSA GDQ6P012P and MRSA GDE4P037P), and Gram-negative bacteria including Acinetobacter baumannii ATCC 19606, Vibrio coralliilyticus ATCC BAA-450 and Vibrio alginolyticus XSBZ14. Our results show that 1–9 could inhibit the growth of Gram-positive bacteria, but not Gram-negative bacteria at 10 μg per filter paper. Subsequently, compounds 1–6 were further validated for their MIC values by using the broth microdilution method for SAR. Our results (Table 3) suggest that all the tested compounds, except compound 2, demonstrated antibacterial activities against Micrococcus luteus ML01 and Staphyloccocus aureus ATCC 29213 with MIC values ranging between 0.2 and 4 μg/mL. Also, these compounds showed antibacterial activities against MRSA strains with MIC values of 1–16 μg/mL. We found that compounds 1 and 5, which contained a chlorine atom at C-6″, showed more potent antibacterial activities than compounds 3 and 4, supporting the conclusion that the chlorine atom at C-6″ is functionally significant.
Conclusion
Chemical investigation on deep-sea derived Streptomyces sp. SCSIO 11791 led to the identification of nine chlorinated bis-indole alkaloids 1–9, including two new compounds 1 and 2. These chlorinated bis-indole alkaloids showed cytotoxic activities and antibacterial activities in bioactive tests. Notably, compound 1 showed moderate cytotoxicity against a panel of human cancer cell lines with an IC50 range of 3.1–11.2 μM. In addition, 1 showed significant antibacterial activity against Gram-positive pathogenic bacteria, including clinical MRSA strains with MIC values of 0.5–2 μg/mL. SAR analysis of these compounds indicates that the chlorine atom at C-6″ is a key functional atom. This finding may prove information to SAR studies carrying the purpose of developing novel cytotoxic or antibacterial drugs.
Materials and methods
General experimental procedures
NMR spectra were collected with an Avance 500 spectrometer (Bruker) at 500 MHz for the 1H nucleus and 125 MHz for the 13C nucleus. HRESIMS data were operated by using a MaXis quadrupole-time-of-flight mass spectrometer (Bruker). CC (column chromatography) was performed on 100-200 mesh silica gel (Yantai Jiangyou Silica Gel Development Co., Ltd.). RP-MPLC was carried out using CHEETAHTM MP200 (Agela Technologies) and Claricep Flash S Silica columns. RP-HPLC analyses were carried out using a Hitachi HPLC with YMC-Pack ODS-A column (250 × 10 mm, 5 μm).
Strains and fermentation
Strains
The strain SCSIO 11791 was isolated from a sediment sample collected from the South China Sea at a depth of 1,765 m using HRA medium after incubation at 28 °C for 4 weeks [24]. It was preserved at the RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences. According to the morphological characteristics and phylogenetic analysis, it was identified as previously described [24]. Its 16 S rRNA gene sequence was deposited in GenBank with accession no. MK764932, which demonstrated the highest similarity value to Streptomyces specialis GW41-1564T (97.70%).
Fermentation
The strain was inoculated using a modified ISP-2 agar plate (glucose 0.4%, yeast extract 0.4%, malt extract 1%, crude sea salt 3%, pH 7.4) for 5 days at 28 °C. The growth mycelia and spores were transferred into 250-mL Erlenmeyer flasks containing 50 mL modified ISP-4 medium (0.5% soluble starch, 0.05% yeast extract, 0.1% peptone, 0.1% K2HPO4, 0.1% MgSO4·7H2O, 0.2% (NH4)2SO4, 0.4% NaCl, 3% crude sea salt, 0.2% CaCO3, pH 7.4) and incubated for 36 h at 28 °C on rotary shakers (200 rpm) as seed cultures. Then, each flask of seed culture was transferred as an inoculum into a 1-L flask containing 200 mL modified ISP-4 medium and incubated for 9 days at 28 °C on rotary shakers (200 rpm). In total, 21 L liquid culture was obtained.
Extraction and isolation
The culture was harvested and centrifuged at 3600 rpm for 10 min to yield the supernatant and mycelia cake, which was extracted with butanone and acetone, respectively [25]. The extracted residues were combined for purification after HPLC analyses.
The combined extract was first subjected to silica gel CC (column A) by gradient elution with CHCl3/MeOH (100/0, 98/2, 96/4, 94/6, 92/8, 90/10, 80/20, 50/50, v/v, 200 mL for each gradient) to obtain fractions A1–A8. The fractions A1 and A2 were combined and isolated with silica gel CC (column B) eluting with gradient ratios of petroleum ether/EtOAc (100/0, 90/10, 85/15, 80/20, 75/25, 72/28, 70/30, 60/40, v/v, 200 mL for each gradient) to afford fractions B1-B8. The fractions B4-B6 were combined and further subjected to MPLC purification using an ODS column (column C), eluting with a linear gradient of MeCN/H2O from 25/75 to 80/20 for 60 min, and then holding at 100% MeCN for 30 min at a flow rate of 10 mL/min to get fractions C1-C11. Fraction C4 was purified by a semi-preparative HPLC system, eluting with CH3CN/H2O from 40/60 to 100/0 over 20 min and then holding at 100% MeCN for 5 min at a flow rate of 2.5 mL/min to yield compounds 5 (tR 23.3 min, 103.6 mg) and 6 (tR 21.2 min, 28.4 mg). Fraction C6 was subjected to MPLC purification by using an ODS column eluting by MeCN/H2O mixture from 40/60 to 80/20 over 60 min to yield compound 9 (tR 45.8 min, 21.0 mg). Fractions A4-A8 were combined and isolated by MPLC using an ODS column (column E), eluting with a linear gradient of MeCN/H2O from 10/90 to 100/0 over 90 min, and then holding at 100% MeCN for 30 min at a flow rate of 10 mL/min to get fractions E1-E11. Fraction E6 was repeatedly eluted with a linear gradient of MeCN/H2O/HAc from 10/90/0.1 to 60/40/0.1 over 60 min by ODS MPLC to obtain compounds 4 (tR 35.6 min, 21.6 mg) and 8 (tR 49.3 min, 3.4 mg). Fraction E7 was eluted with CHCl3/MeOH (v/v, 50 mL for each gradient, increasing 0.5% MeOH) from 100/0 to 96/4 to get compounds 1 (3.6 mg) and 7 (12.0 mg) at 99/1 and 98.5/1.5, respectively. Fraction E8 was purified by semi-preparative HPLC with CH3CN/H2O from 35/60 to 100/0 over 20 min at a flow rate of 2.5 mL/min to give compounds 3 (tR 16.4 min 5.2 mg) and 2 (tR 17.8 min 16.7 mg).
dionemycin (1): orange amorphous powder; UV (MeOH) λmax (log ε) 442 (3.72), 357 (3.67), 287 (3.99), 217 (4.55) nm; IR (ATR) νmax 1701, 1526 cm−1; see Table 1 for 1H and 13C NMR spectroscopic data; (+)-HRESIMS m/z 429.9912 [M + H]+ (calcd for C20H11Cl3N3O2, 429.9917).
6-OMe-7′,7′′-dichorochromopyrrolic acid (2): pale-yellow amorphous powder; UV (MeOH) λmax (log ε) 261 (4.4), 230 (4.73) nm; IR (ATR) νmax 3418, 1694, 1458, 1238 cm−1; see Table 1 for 1H and 13C NMR spectroscopic data; (+)-HRESIMS m/z 468.0502 [M + H]+ (calcd for C23H16Cl2N3O4, 468.0528).
Assays for cytotoxicity and antibacterial activities
Cytotoxic activities were evaluated by a previously reported MTT method [26]. MDA-MB-435 human breast cancer cells, MDA-MB-231 human breast adenocarcinoma cells, NCI-H460 human non-small-cell lung cancer cells, HCT-116 human colon cancer cells, HepG2 human liver cancer cells, and the non-cancerous MCF10A human breast epithelial cells were used.
The antibacterial activities were evaluated by using filter paper agar plates and broth microdilution-based antimicrobial susceptibility tests for aerobic bacteria [27]. Gram-positive bacteria including Micrococcus luteus ML01, Staphylococcus aureus ATCC 29213, and a panel of clinical MRSA strains isolated from human and pig, as well as Gram-negative bacteria including Acinetobacter baumannii ATCC 19606, Vibrio coralliilyticus ATCC BAA-450, and Vibrio alginolyticus XSBZ14 were used. Minimal inhibitory concentrations (MIC values) of antimicrobial agents that maximally inhibited cell growth in microdilution wells were determined by visual inspection and absorption measurements.
References
Kam TS, Choo YM. Bisindole alkaloids. Alkaloids Chem Biol. 2006;63:181–337.
Veale CG, Davies-Coleman MT. Marine bi-, bis-, and trisindole alkaloids. Alkaloids Chem Biol. 2014;73:1–64.
Cordell GA. The bisindole alkaloids. In: Saxton JE editor. chemistry of heterocyclic compounds: indoles, part four, the monoterpenoid indole alkaloids. Vol 25. New York, NY: John Wiley & Sons, Inc.; 1983.
Rahman MT, Tiruveedhula VV, Cook JM. Synthesis of bisindole alkaloids from the apocynaceae which contain a macroline or sarpagine unit: a review. Molecules. 2016;21:1525–65.
Neuss N, Gorman M, Svoboda G, Maciak G, Beer C. Vinca alkaloids. III. 1. Characterization of leurosine and vincaleukoblastine, new alkaloids from Vinca rosea. J Am Chem Soc. 1959;81:4754–5.
Neuss N, Neuss MN. Therapeutic use of bisindole alkaloids from catharanthus. Alkaloids Chem Pharmacol. 1990;37:229–40.
Yasuzawa T, et al. The structures of the novel protein kinase C inhibitors K-252a, b, c and d. J Antibiot. 1986;39:1072–8.
Bush JA, Long BH, Catino JJ, Bradner WT, Tomita K. Production and biological activity of rebeccamycin, a novel antitumor agent. J Antibiot. 1987;40:668–78.
Omura S, Sasaki Y, Iwai Y, Takeshima H. Staurosporine, a potentially important gift from a microorganism. J Antibiot. 1995;48:535–48.
Long BH, Rose WC, Vyas DM, Matson JA, Forenza S. Discovery of antitumor indolocarbazoles: rebeccamycin, NSC 655649, and fluoroindolocarbazoles. Curr Med Chem Anticancer Agents. 2002;2:255–66.
Lien WC, et al. 7-hydroxy-staurosporine, UCN-01, induces DNA damage response, and autophagy in human osteosarcoma U2-OS cells. J Cell Biochem. 2018;119:4729–41.
Gribble GW. In: Knöelker H-J, editor. Occurrence of halogenated alkaloids. The alkaloids; Academic Press: London, 2012. pp. 1–165.
Salucci S, et al. Marine bisindole alkaloid: a potential apoptotic inducer in human cancer cells. Eur J Histochem. 2018;62:2881.
Bifulco G, Bruno I, Riccio R, Lavayre J, Bourdy G. Further brominated bis-Indole and bris-Indole alkaloids from the deep-water new-caledonian marine sponge Orina sp. J Nat Prod. 1995;58:1254–60.
Veale CG, et al. Synthetic analogues of the marine bisindole deoxytopsentin: potent selective inhibitors of MRSA pyruvate kinase. J Nat Prod. 2015;78:355–62.
Manivasagan P, Kang KH, Sivakumar K, Li-Chan EC, Oh HM, Kim SK. Marine actinobacteria: an important source of bioactive natural products. Environ Toxicol Pharm. 2014;38:172–88.
Zhang W, et al. Spiroindimicins A-D: new bisindole alkaloids from a deep-sea-derived actinomycete. Org Lett. 2012;14:3364–7.
Zhang W, et al. Indimicins A-E, bisindole alkaloids from the deep-sea-derived Streptomyces sp. SCSIO 03032. J Nat Prod. 2014;77:1887–92.
Barth H, et al. Preparation of 3,4-bis(indol-3-yl) maleimides as protein kinase C inhibitors. Germany, Patent 90108468.1, 1990.
McArthur KA, et al. Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J Nat Prod. 2008;71:1732–7.
Du YL, Ryan KS. Expansion of bisindole biosynthetic pathways by combinatorial construction. Acs Synth Biol. 2015;4:682–8.
Mitchell SS, et al. Nereus Pharmaceuticals, Inc., USA. Bis-indole pyrroles useful as antimicrobial agents. USA, PTC 002039, 2005.
Onaka H, Taniguchi S, Igarashi Y, Furumai T. Characterization of the biosynthetic gene cluster of rebeccamycin from Lechevalieria aerocolonigenes ATCC 39243. Biosci Biotechnol Biochem. 2003;67:127–38.
Song Y, et al. Cytotoxic and antibacterial angucycline- and prodigiosin-analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar Drugs. 2015;13:1304–16.
Song Y, et al. Cytotoxic and antibacterial marfuraquinocins from the deep South China Sea-derived Streptomyces niveus SCSIO 3406. J Nat Prod. 2013;76:2263–8.
Ding B, et al. New dimeric members of the phomoxanthone family: phomolactonexanthones A, B and deacetylphomoxanthone C isolated from the fungus Phomopsis sp. Mar Drugs. 2013;11:4961–72.
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. CLSI document M07-A9. Wayne, PA: clinical and laboratory standards institute; 2012.
Acknowledgements
This study was supported, in part, by the Program of Guangzhou Science and Technology Plan (201707010454), the National Natural Science Foundation of China (41676151), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0406), the National Key Research and Development Program of China (2017YFD0201401) and the Special Fund for Strategic Pilot Technology of Chinese Academy of Sciences (XDA13020302-2). We thank the analytical facility center of the South China Sea Institute of Oceanology, Dr. Zhihui Xiao and Mr. Chuanrong Li for recording NMR data and Ms. Aijun Sun, and Ms. Yun Zhang for acquisition of ESIMS and HRESIMS data. Additionally, we appreciate Professor Jianhua Liu from South China Agricultural University and our colleague Professor Chang Chen for their gift of pathogenic bacteria MRSA GDQ6P012P and GDE4P037P and four Vibrio sp. strains, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedicated to Professor William Fenical in recognition of his contributions to marine derived secondary metabolites.
Supplementary information
Rights and permissions
About this article
Cite this article
Song, Y., Yang, J., Yu, J. et al. Chlorinated bis-indole alkaloids from deep-sea derived Streptomyces sp. SCSIO 11791 with antibacterial and cytotoxic activities. J Antibiot 73, 542–547 (2020). https://doi.org/10.1038/s41429-020-0307-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0307-4
- Springer Japan KK
This article is cited by
-
Repurposing of Strychnine as the Potential Inhibitors of Aldo–keto Reductase Family 1 Members B1 and B10: Computational Modeling and Pharmacokinetic Analysis
The Protein Journal (2024)
-
Sulfoxanthicillin from the deep-sea derived Penicillium sp. SCSIO sof101: an antimicrobial compound against Gram-positive and -negative pathogens
The Journal of Antibiotics (2023)
-
Bioactive Microbial Metabolites in Cancer Therapeutics: Mining, Repurposing, and Their Molecular Targets
Current Microbiology (2022)