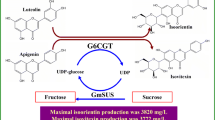
Isoquercitrin exhibits a number of biological properties and has a higher water solubility than quercetin. In this work, an Arabidopsis glycosyltransferase with high activity on quercetin (AtUGT78D2) was coupled with an efficient quercetin-tolerant Glycine max sucrose synthase (GmSUS) to synthesize isoquercitrin by regenerating UDP-glucose from UDP. The important factors for optimal synergistic catalysis were determined. Through the use of a fed-batch operation, the final titer of isoquercitrin increased to 3830 mg/L with a corresponding molar conversion of 94.3% and a maximum number of UDP-glucose regeneration cycles (RCmax) of 33.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The glycosylation of flavonoids is an efficient tool for enhancing the properties of flavonoids. Glycosylation is an important biological mechanism for the structural and functional diversification of natural flavonoids, which can give them special activity, selectivity and pharmacological properties [1]. For example, isoquercitrin (quercetin-3-O-β-D-glucopyranoside) not only exhibits a number of biological properties but also has a higher water solubility than quercetin and better intestinal absorption than rutin [2, 3]. Although some α-L-rhamnosidases have been used to prepare isoquercitrin through the hydrolysis of rutin [3, 4], enzymatic methods for production of isoquercitrin through glycosylation of quercetin have not been reported in vitro.
In plants, glycosyltransferases catalyze the glycosylation of secondary products by transferring a sugar molecule from its activated form to an acceptor. Glycosyltransferases display high regioselectivity and stereochemical control in the glycosylation of secondary products, and they are therefore widely recognized as highly valuable glycosylation catalysts [5]. However, the process requires the consumption of UDP-sugars, which are expensive. Enzymatic methods have been reported for in vitro production of UDP-sugars [6]. Several sucrose synthase genes from different organisms have been reported and used to produce small-molecule glucosides [7,8,9,10]. However, for in vitro isoquercitrin production with synergistic catalysis, sucrose synthase must be tolerant to quercetin, and the characterizations of glycosyltransferase and sucrose synthase must be complementary.
In this paper, a method of synergistic catalysis was determined, in which a glucosyltransfer from UDP-glucose to quercetin, catalyzed by a recombinant Arabidopsis glycosyltransferase (AtUGT78D2), is coupled with the removal of UDP and regeneration of UDP-glucose, catalyzed by a recombinant Glycine max sucrose synthase (GmSUS).
According to the biochemical properties, three sucrose synthases (GmSUS, SUS3, and AcSUS) were regarded as candidate enzymes with AtUGT78D2 for synergistic catalysis [8, 11, 12]. The recombinant enzymes including GmSUS (NP_001237525.1), SUS3 (NP_192137.1), and AcSUS (WP_004872341.1) were successfully expressed in E. coli. Recombinant GmSUS in the cell-free extract was purified to gel electrophoretic homogeneity through a Ni-NTA affinity. Recombinant SUS3 and AcSUS were partially purified to gel electrophoretic homogeneity. Although SUS3 and AcSUS have excellent properties in terms of high specific activities and temperature stability [11, 12], their enzyme activities are strongly inhibited by 1 mM quercetin (Fig. 1a), and further study showed that minimal product was generated by the synergistic catalysis of AtUGT78D2 and SUS3 or AcSUS (Fig. 1b). Thus, SUS3 and AcSUS were not suitable for production of isoquercitrin.
GmSUS was tolerant to quercetin up to a concentration of 1.5 mM (Fig. 1c). A large amount of production was generated by AtUGT78D2 and GmSUS, and no product was produced by using only AtUGT78D2 or GmSUS (Fig. 1b). These results show that there is a synergistic reaction between AtUGT78D2 and GmSUS. A comparison of the m/z values of the molecular ions [M − H]− of the enzyme-catalyzed product (463.0885) showed that differences corresponded to a D-glucose residue of quercetin (301.0353), and it has been reported that AtUGT78D2 can only glycosylate flavonoids at the 3C-O position of quercetin [13]. These results confirmed that the enzyme-catalyzed product was isoquercitrin.
To examine the role of GmSUS in driving the glucosylation of quercetin, the synergistic reaction was performed to compare the AtUGT78D2 conversion of quercetin in the absence and presence of GmSUS. The initial rates and final conversion and space-time yields for the production of 1.2 mM isoquercitrin are summarized in Table 1. Although there was no difference in the initial rates in the absence and presence of GmSUS, the final conversion was much lower in the absence of GmSUS (64.8%) than in the presence of GmSUS (98.4%). These results indicate that for AtUGT78D2, it is necessary to remove the accumulated UDP in situ.
Use of 1.25 mM UDP-glucose instead of 0.25 mM UDP in synergistic reactions caused slight increases in final conversion (about 1.6%) and space-time yield but a more significant improvement in the initial rate by about 250%. However, in application, the improvement of the initial rate and the similar final conversions does not compensate for the additional cost of replacing UDP with a 5-times higher concentration of the more expensive UDP-glucose.
The effects of the ratio of AtUGT78D2 to GmSUS on isoquercitrin production were determined (Table 2). Isoquercitrin production increased eightfold when the amount of GmSUS was raised from 12 mU/mL to 120 mU/mL and slightly increased when the GmSUS continued to increase without changing the amount of AtUGT78D2 (70 mU/mL). Thus, the optimal ratio of AtUGT78D2 to GmSUS was 70:120 (mU/mL).
Temperature and pH are important factors in synergistic catalysis, affecting enzyme specific activities and stabilities. The results in Fig. 2a showed that the optimal temperature for synergistic catalysis was 40°C. However, isoquercitrin production was 88% of the maximum production at 35°C, and the lower temperature is more conducive to maintaining the thermostability of the enzymes. The optimal pH for synergistic catalysis was pH 7.5 with phosphate buffer or Tris-HCl buffer (Fig. 2b).
Optimization of bioconversion conditions for isoquercitrin production by synergistic catalysis. (a) The effects of temperature on isoquercitrin production. (b) The effects of pH on isoquercitrin production. Tris-HCl buffer (filled squares), phosphate buffer (filled diamonds) (c) The effects of DMSO on isoquercitrin production, (d) The effects of quercetin on isoquercitrin production.
The effects of different concentrations of DMSO and quercetin on isoquercitrin production were investigated. The results are shown in Fig. 2c, d. Isoquercitrin production was significantly increased with increasing concentrations of DMSO, and the optimal concentration of DMSO was 10%. Maximal isoquercitrin production reached 0.64 mM with 10% DMSO and 1.5 mM quercetin. Isoquercitrin production was rapidly decreased when the concentration of quercetin exceeded 1.5 mM or the concentration of DMSO exceeded 10%.
To increase the final concentration of isoquercitrin in the synergistic reaction and to avoid the inhibition of a high concentration of quercetin on the activity of GmSUS, we changed the operation mode from batch to fed-batch, adding fresh quercetin to a concentration of 1.25 mM once the acceptor substrate had been consumed. Up to six rounds of quercetin addition was carried out using a highly concentrated stock solution of 300 mM quercetin in pure DMSO to minimize the resulting volume change. A kinetic analysis of quercetin consumption and isoquercitrin production over time is shown in Fig. 3. The specific productivity was 878 mg/L/h during the first hour after the initiation of synergistic catalysis. Although fresh enzymes were supplied at 4 and 8 h, the specific productivity gradually decreased as the reaction continued.
The specific productivities were 305 mg/L/h during the reaction time from 1 to 4 h, 255 mg/L/h during the reaction time from 4 to 8 h, and 127 mg/L/h during the reaction time from 8 to 16 h. Co-addition of enzyme could not alleviate the decrease in specific productivity, which probably resulted from the effect of product inhibition. After 16 h, 3830 mg/L of isoquercitrin was produced with a corresponding molar conversion of 94.3% and the maximum number of UDP-glucose regeneration cycles (RCmax) of 33 (8.25/0.25). Although the AtSUS1 from Arabidopsis thaliana has been used to catalyze the glycosylation of poorly water-soluble quercetin, it has resulted in scarcely more than micromolar concentrations of product, in addition to requiring UDP-glucose rather than UDP in the cascade reactions [14, 15]. In comparisons with results from the literature therefore, the bioconversion process described herein for the glucosylation of quercetin from sucrose and UDP stands out based on its millimolar production of isoquercitrin. In terms of product concentration, the synergistic catalysis of AtUGT78D2 and GmSUS has thus achieved remarkable results.
In this study, AtUGT78D2 and GmSUS were screened for the development of a one-pot two-enzyme system, which provides an efficient method for the synthesis of isoquercitrin involving the cycling and regeneration of costly UDP-glucose in the reaction. This synergistic system provides a widely available approach for structural modification of the plant natural products via a low-cost process.
Experimental
Strains and Plasmids.Escherichia coli JM109 and BL21 (DE3) were used for plasmid propagation and recombinant enzyme production. pACYCDuet-1 was purchased from Novagen (Darmstadt, Germany). pGEX-78D2 harboring the AtUGT78D2 gene was constructed by our labs [5].
Plasmid Construction. GmSUS (NP_001237525.1), SUS3 (NP_192137.1), and AcSUS (WP_004872341.1) were synthesized to incorporate E. coli codons. The NcoI site was added to the 5′ ends of the genes, the EcoRI site was added to the 3′ ends of the genes, and six histidine residues were fused to the C-termini of recombinant enzymes. The synthesized genes (GmSUS, SUS3, and AcSUS) were digested with NcoI and EcoRI and subcloned into the expression vector pACYCDuet-1 at the NcoI and EcoRI sites to create pACYCDuet-GmSUS, pACYCDuet-SUS3, and pACYCDuet-AcSUS, respectively.
Purification and Characterization of Recombinant Enzymes. Recombinant AtUGT78D2 was purified according to methods described previously [5]. Recombinant GmSUS, SUS3, and AcSUS were purified by immobilized metal affinity column (Novagen, USA). Protein was examined by SDS-PAGE, and protein bands were analyzed by density scanning with an image analysis system (Bio-Rad, USA). Glycosyltransferase activity was measured as described previously [5]. Sucrose synthase activity was measured as described previously [11].
Synergistic Catalysis. Standard reaction mixtures containing 1.25 mM quercetin, 200 mM sucrose, 0.25 mM UDP, 50 mM phosphate buffer (pH 7.5), 10% DMSO (v/v), 70 mU/mL glycosyltransferase, and 120 mU/mL sucrose synthase in 100 μL were incubated for 30 min at 35°C. The reaction was stopped by adding 400 μL methanol and assayed via HPLC.
The optimum pH for synergistic catalysis was determined by incubation at 35°C for 30 min in 50 mM Tris-HCl buffer from pH 7.0 to 9.0 or 50 mM phosphate buffer from pH 7.0 to 8.0. The optimum temperature for synergistic catalysis was determined in a standard assay ranging from 25 to 45°C in 50 mM phosphate buffer, pH 7.5. The optimal ratio of AtUGT78D2 to GmSUS was determined in a standard assay by adding different ratios of AtUGT78D2 to GmSUS. For optimizing the conversion conditions, the concentrations of the substrate and DMSO were varied separately: UDP (0−1 mM), sucrose (0−500 mM), quercetin (0.1−5 mM), and DMSO (1−20%, v/v).
Fed-batch Conversions of AtUGT78D2 Coupled with GmSUS. Quercetin concentrations used in the batch conversions were modified for fed-batch experiments. The reaction solution contained 1.25 mM quercetin, 500 mM sucrose, 0.25 mM UDP, 50 mM phosphate buffer (pH 7.5), 8% DMSO (v/v), 70 mU/mL glycosyltransferase, and 120 mU/mL sucrose synthase in 1 mL. The reaction was incubated at 35°C and 150 rpm on a thermomixer. Quercetin (1.25 mM) was added from a stock of 300 mM quercetin in DMSO at 30 min, 2 h, 3 h, 6 h, 8 h, and 12 h. Fresh enzymes (70 mU/mL glycosyltransferase and 120 mU/mL sucrose synthase) were added at 4 h and 8 h.
HPLC and LC/MS Analysis. HPLC analysis of quercetin and isoquercitrin was performed using an HPLC 1200 system (Agilent, USA) and a C18 (250 × 4.6 mm; i.d., 5 μm) column with methanol (A) and distilled water (B) at A/B ratios of 55:45 for 15 min. The flow rate was 0.8 mL/min, and detection was performed by monitoring the absorbance at 368 nm. LC/MS for quercetin and isoquercitrin was analyzed in an LTQ Orbitrap XL LC/MS in negative mode with an ion trap analyzer. The ion spray was operated at 25 Arb N2/min, 3.5 kV, and 300°C.
References
X. Wu, J. Chu, B. Wu, S. Zhang, and B. He, Bioresour. Technol., 129, 659 (2013).
K. Valentova, J. Vrba, M. Bancirova, J. Ulrichova, and V. Kren, Food Chem. Toxicol., 68, 267 (2014).
L. Weignerova, P. Marhol, D. Gerstorferova, and V. Kren, Bioresour. Technol., 115, 222 (2012).
L. Ge, A. Chen, J. Pei, L. Zhao, X. Fang, G. Ding, Z. Wang, W. Xiao, and F. Tang, BMC. Biotechnol., 17, 21 (2017).
J. Pei, P. Dong, T. Wu, L. Zhao, X. Fang, F. Cao, F. Tang, and Y. Yue, J. Agric. Food Chem., 64, 7966 (2016).
S. K. Chung, S. I. Ryu, and S. B. Lee, Bioresour. Technol., 110, 423 (2012).
S. Masada, Y. Kawase, M. Nagatoshi, Y. Oguchi, K. Terasaka, and H. Mizukami, FEBS Lett., 581, 2562 (2007).
L. Bungaruang, A. Gutmann, and B. Nidetzky, Adv. Synth. Catal., 355, 2757 (2013).
A. Gutmann, L. Bungaruang, H. Weber, M. Leypold, R. Breinbauer, and B. Nidetzky, Green Chem., 16, 4417 (2014).
J. Pei, A. Chen, L. Zhao, F. Cao, G. Ding, and W. Xiao, J. Agric. Food Chem., 65, 6042 (2017).
M. Diricks, F. De Bruyn, P. Van Daele, M. Walmagh, and T. Desmet, Appl. Microbiol. Biotechnol., 99, 8465 (2015).
E. Baroja-Fernandez, F. J. Munoz, J. Li, A. Bahaji, G. Almagro, M. Montero, E. Etxeberria, M. Hidalgo, M. T. Sesma, and J. Pozueta-Romero, Proc. Natl. Acad. Sci. USA, 109, 321 (2012).
E. K. Lim, D. A. Ashford, B. Hou, R. G. Jackson, and D. J. Bowles, Biotechnol. Bioeng., 87, 623 (2004).
M. H. Son, B. G. Kim, D. H. Kim, M. Jin, K. Kim, and J. H. Ahn, J. Microbiol. Biotechnol., 19, 709 (2009).
K. Terasaka, Y. Mizutani, A. Nagatsu, and H. Mizukami, FEBS Lett., 586, 4344 (2012).
Acknowledgment
This work was supported by the National Key R & D Program of China (2017YFD0600805), the National Natural Science Foundation of China (31570565), the Open Foundation of Jiangsu Provincial Engineering Laboratory for Biomass Conversion and Process Integration (JPELBCPI2017002), the Qing Lan Project, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May-June, 2019, pp. 390-393.
Rights and permissions
About this article
Cite this article
Pei, Jj., Chen, An., Zhao, Lg. et al. Synergistic Catalysis of Glycosyltransferase and Sucrose Synthase to Produce Isoquercitrin Through Glycosylation of Quercetin. Chem Nat Compd 55, 453–457 (2019). https://doi.org/10.1007/s10600-019-02712-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02712-9