A new anthraquinone, 7-(γ,γ)-dimethylallyloxymacrosporin (1), along with five known analogues, macrosporin (2), 7-methoxymacrosporin (3), tetrahydroaltersolanol B (4), altersolanol L (5), and ampelanol (6), were isolated from the mangrove endophytic fungus Phoma sp. L28. All of them were first found in Phoma sp. Their structures were established by comprehensive spectroscopic analyses and comparison with the published data. These compounds displayed in vitro antifungal activities against Colletotrichum musae (Berk. & M. A. Curtis) Arx., Colletotrichum gloeosporioides (Penz) Sacc., Fusarium graminearum Schw., Penicillium italicum Wehme, Fusarium oxysporum Schlecht. f. sp. lycopersici (Sacc.) W.C. Snyder et H. N. Hansen, and Rhizoctonia solani Kuhn at different levels. Notably, compound 2 exhibited potent antifungal activity against Fusarium graminearum Schw. as compared with the positive control carbendazim.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Phoma is considered to be one of the largest fungal genera, with more than 3000 infrageneric taxa described, many species of which are recognized as relevant phytopathogenic fungi that may be the main cause of leaf and stem spots [1]. Chemical investigations of Phoma sp. have led to a great diversity of metabolites. Moreover, most of the metabolites exhibited specific bioactivity, such as cytotoxic [2], antiviral [3], and antimicrobial [4] effects.
As part of our ongoing investigation on natural antifungal products from marine fungi [5], a mangrove endophytic fungus, Phoma sp. L28, isolated from the roots of Myoporum bontioides A. Gray attracted our attention for its antifungal activity against plant pathogens [6], which prompted us to investigate the corresponding metabolites. As a result, one new anthraquinone derivative, 7-(γ,γ)-dimethylallyloxymacrosporin (1), and five known analogues, macrosporin (2) [7], 7-methoxymacrosporin (3) [8], tetrahydroaltersolanol B (4) [9], altersolanol L (5) [10], and ampelanol (6) [11] were isolated. There are few reports on the biological activities of the above isolated compounds. Only macrosporin (2) has been reported to have antibacterial activity against Vibrio anguillarum [12], Escherichia coli, Vibrio parahemolyticus, and Staphylococcus albus [13]. The isolation, structural elucidation, and antifungal activity of the metabolites against several plant pathogenic fungi are reported herein.

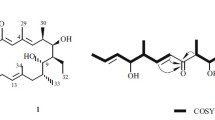
Compound 1 was isolated as yellow needles, and the HR-ESI-MS of 1 showed a molecular ion peak at m/z 353.1380 [M + H]+ (calcd 353.1384) corresponding to the molecular formula C21H20O5, which indicated that compound 1 had 12 degrees of unsaturation. The 13C NMR spectrum of 1 (Table 1) revealed 21 carbon signals. It also suggested that nine of the 12 elements of unsaturation in 1 were due to the 14 olefinic carbons and two carbonyl groups (δ 187.0, 181.7). The other three degrees of unsaturation implied that the molecule was a tricyclic compound. The 1H NMR spectrum of 1 (Table 1) displayed a chelated hydroxyl group at δ 12.87, a pair of meta-coupled aromatic protons at δ 6.65 (1H, d, J = 2.4 Hz) and 7.34 (1H, d, J = 2.4 Hz), a pair of para-coupled aromatic protons at δ 8.02 (1H, d, J = 1.2 Hz) and 7.62 (1H, d, J = 1.2 Hz), a methoxyl at δ 3.93, a methyl at δ 2.34, and a set of characteristic prenyloxy moiety [14] at δ 5.51 (1H, tqq, J = 6.6, 1.2, 1.2 Hz), 4.73 (2H, d, J = 6.6 Hz), 1.82 (3H, d, J = 1.2 Hz), and 1.83 (3H, d, J = 1.2 Hz). The NMR spectral data of 1 were very similar to those of compound 2 [9]. The major difference between them was that one prenyl group of the former replaced one active hydrogen proton of the latter. The molecular formula of 1 was higher by 68 amu (atomic mass units) than that of 2, also confirming that 1 was an O-prenyl ether of 2. The prenyloxy moiety was determined to be attached to the C-7 position of the anthraquinone nucleus by the HMBC correlation (Table 1) from H-1′ (δ 4.73) to C-7 (δ 162.1). Thus the structure of 1 was elucidated as 7-(γ,γ)-dimethylallyloxymacrosporin. The structures of 2–6 were elucidated by comparison with the published data. They were all isolated for the first time from Phoma sp.
The antifungal activity of the isolated compounds was assessed quantitatively by the broth dilution method to determine the minimum inhibitory concentration (MIC) [15]. The results are summarized in Table 2. Among the anthraquinones (compounds 1–3), macrosporin (2) showed broad-spectrum antifungal activity, with MIC values ranging from 3.75 to 100 μg mL–1 on the tested fungi. Notably, its inhibitory activity against F. oxysporum (MIC 3.75 μg mL–1) was even better than that of the positive control carbendazim (MIC 6.25 μg mL–1). In contrast, compounds 1 and 3 only exhibited moderate to weak (MICs 80–200 μg mL–1) or no (MICs > 200 μg mL–1) activity against the six tested fungi. Although additional data will be required, inspection of the in vitro activity of the three compounds would be helpful in the generalization of their structure–activity relationships. Compound 2 bearing an OH group at C-7 exhibited much greater antifungal activity than compound 3 (or compound 1) bearing a methoxy (or prenyloxy) group at the same position, indicating that the etherification of the OH group at C-7 would significantly decrease the antifungal activity, and the OH group at C-7 probably was the important antifungal pharmacophore against the tested pathogens. As for the hydroanthraquinones (compounds 4 and 5), compound 5 showed moderate antifungal activity against P. italicum and R. solani with MIC values of 35 and 50 μg mL–1, respectively, while compound 4 was inactive against all the test pathogens (MICs > 200 μg mL–1) except P. italicum (MIC 80 μg mL–1).
Additionally, compound 5 also exhibited weak activity toward F. graminearum and C. gloeosporioides with MIC values of 100 and 200 μg mL–1, respectively, and was inactive against F. oxysporum and C. musae (MICs > 200 μg mL–1). The activity of compound 6 was not detected due to its small amounts. These results suggested that the isolated compounds, especially compounds 2 and 5, could be valuable for the development of new fungicides or lead compounds for the treatment of plant fungal infections.
Experimental
General. IR spectra were obtained with a Nicolet 5DX-Fourier transform infrared spectrophotometer (Thermo Electron Corporation, Madison, USA). All NMR experiments were recorded in a Bruker AV III 600 MHz NMR spectrometer (Bruker BioSpin GmbH Company, Rheinstetten, Germany) using deuterated dimethyl sulfoxide, acetone, or chloroform as solvent and the residual solvent resonance as internal standard; coupling constants (J) were in Hz. HR-ESI-MS and MS were performed on an LCMS-IT-TOF (Shimadzu, Japan) mass spectrometer. Chromatography was carried out on a silica gel column (200–300 mesh; Qingdao Haiyang Chemicals Co., Ltd., Qingdao, China). All other reagents used were of analytical grade.
Fungus Material and Culture Conditions. The strain of Phoma sp. (collection No. L28) was isolated from the leaves of the mangrove plant Myoporum bontioides A. Gray, collected from the mangrove in Leizhou peninsula, Guangdong Province, China. This fungus was deposited in the College of Materials and Energy (Formerly College of Science), South China Agricultural University, Guangzhou, China. This fungus did not produce any conidia or spores, so it was identified by analysis of DNA sequence of the internal transcribed spacer regions as previously described [15]. Its ITS sequence was the same (100%) as those of three Phoma sp. sequences from NCBI BLAST, including KM259932.1, HQ630963.1, and JQ388280.1, indicating that the strain belongs to Phoma sp.
A small scrap of agar slice with mycelium was added into a 500 mL Erlenmeyer flask containing 250 mL of GYT medium (1% glucose, 0.1% yeast extract, 0.2% peptone, 0.2% crude sea salt) aseptically and the contents incubated at 28°C and 180 rpm for 6 days as seed culture. The fermentation was achieved using 100 Erlenmeyer flasks (size of each flask 1 L), each containing rice medium (100 mL water, 100 g rice, 0.3 g crude sea salt). Seed culture (4 mL) was added into each flask, and the contents cultivated at room temperature for 30 days under static conditions.
Extraction and Separation of Metabolites. Methanol (200 mL) was added into each of the flasks and the contents extracted for 72 h at room temperature. The solvent was concentrated to 3 L with a rotary evaporator in vacuo in a 50°C water bath and extracted three times in sequence with an equal volume of ethyl acetate and n-butyl alcohol. The extracts were evaporated to dryness in vacuo and combined. The combined extract was chromatographed repeatedly on a silica gel column using gradient elution to obtain 7-(γ,γ)-dimethylallyloxymacrosporin (1), macrosporin (2), and 7-methoxymacrosporin (3) from the petroleum ether–ethyl acetate [90:10, 87.5:12.5, and 85:15] fraction, respectively, and tetrahydroaltersolanol B (4), altersolanol L (5), and ampelanol (6) from the ethyl acetate–methanol [90:10, 85:15, and 80:20] fraction, respectively.
7-( γ , γ )-Dimethylallyloxymacrosporin (1). C21H20O5, yellow needles, mp 172–174_C. HR-ESI-MS m/z 353.1380 [M + H]+; calcd 353.1384. For 1H NMR (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Table 1.
Antifungal Acivity in vitro. The following six phytopathogenic fungi were used for bioassay: Colletotrichum musae (Berk.&M. A. Curtis) Arx., Colletotrichum gloeosporioides (Penz) Sacc., Fusarium graminearum Schw., Penicillium italicum Wehme, Fusarium oxysporum Schlecht. f. sp. lycopersici (Sacc.) W. C. Snyder et H. N. Hansen, and Rhizoctonia solani Kuhn. They were obtained from the College of Agriculture, South China Agricultural University. The quantitative tests of the antimicrobial activities of the pure compounds were performed by the broth dilution method as described in the previous report [16] to determine the minimum inhibitory concentration (MIC). Carbendazim was used as the positive control of growth inhibition, and the negative control of solvent influence was detected in parallel. The results are presented as mean values of three measurements.
References
E. Montel, P. D. Bridge, and B. C. Sutton, Mycopathologia, 115, 89 (1991).
E. L. Kim, J. L. Li, H. T. Dang, J. Hong, C. O. Lee, D. K. Kim, W. D. Yoon, E. Kim, Y. H. Liu, and J. H. Jung, Bioorg. Med. Chem. Lett., 22, 3126 (2012).
Y. Kanai, D. Ishiyama, H. Senda, W. Iwatani, H. Takahashi, H. Konno, S. Tokumasu, and S. Kanazawa, J. Antibiot., 53, 863 (2000).
K. Herath, G. Harris, H. Jayasuriya, D. Zink, S. Smith, F. Vicente, G. Bills, J. Collado, A. Gonzalez, B. Jiang, J. N. Kahn, S. Galuska, R. Giacobbe, G. Abruzzo, E. Hickey, P. Liberateor, D. Xu, T. Roemer, and S. B. Singh, Bioorg. Med. Chem., 17, 1361 (2009).
S. Huang, W. J. Ding, C. Y. Li, and D. G. Cox, Pharmacogn. Mag., 10, 410 (2014).
W. J. Ding, S. Q. Zhang, B. Gong, C. Y. Li, and X. F. Wang, Guangdong Agric. Sci., 41, 74 (2014).
A. H. Aly, R. A. E. Ebel, V. Wary, W. E. G. Muller, S. Kozytska, U. Hentschel, P. Proksch, and R. Ebel, Phytochemistry, 69, 1716 (2008).
A. Evidente, R. Rodeva, A. Andolfi, Z. Stoyanova, C. Perrone, and A. Motta, Eur. J. Plant Pathol., 130, 173 (2011).
N. Okamura, K. Mimura, H. Haraguchi, K. Shingu, K. Miyahara, and A. Yagi, Phytochemistry, 42, 77 (1996).
A. Debbab, A. H. Aly, R. A. E. Ebel, V. Wray, W. E. G. Muller, F. Totzke, U. Zirrgiebel, C. Schachtele, M. H. G. Kubbutat, W. H. Lin, M. Mosaddak, A. Hakiki, P. Proksch, and R. Ebel, J. Nat. Prod., 72, 626 (2009).
X. M. Zhou, C. J. Zheng, G. Y. Chen, X. P. Song, C. R. Han, G. N. Li, Y. H. Fu, W. H. Chen, and Z. G. Niu, J. Nat. Prod., 77, 2021 (2014).
Y. N. Wang, C. J. Zheng, C. L. Shao, and C. Y. Wang, Chin. J. Mar. Drugs, 34, 10 (2015).
C. J. Zheng, C. L. Shao, Z. Y. Guo, J. F. Chen, D. S. Deng, K. L. Yang, Y. Y. Chen, X. M. Fu, Z. G. She, Y. C. Lin, and C. Y. Wang, J. Nat. Prod., 75, 189 (2012).
J. H. Wang, S. Huang, C. Y. Li, W. J. Ding, Z. G. She, and C. L. Li, Chem. Nat. Compd., 51, 239 (2015).
S. Prachya, S. Wiyakrutta, N. Sriubolmas, N. Ngamrojanavanich, C. Mahidol, S. Ruchirawat, and P. Kittakoop, Planta Med., 73, 1418 (2007).
J. H. Wang, W. J. Ding, R. M. Wang, Y. P. Du, H. L. Liu, X. H. Kong, and C. Y. Li, Mar. Drugs, 13, 4492 (2015).
Acknowledgment
This work was supported by the National Natural Science Foundation of China (21102049), the Natural Science Foundation of Guangdong Province of China (2015A030313405, 9451064201003751), the Science and Technology Project of Guangdong Province (2016A020222019), the Scientific Research Foundation for Returning Overseas Chinese Scholars, State Education Ministry 2015-311, and the Innovation Experiment Program for University students of Guangdong Province (201510564196).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 204–206.
Rights and permissions
About this article
Cite this article
Huang, S., Xu, J., Li, F. et al. Identification and Antifungal Activity of Metabolites from the Mangrove Fungus Phoma sp. L28. Chem Nat Compd 53, 237–240 (2017). https://doi.org/10.1007/s10600-017-1961-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1961-z