A new fatty acid, (2E,4E,6S)-6-hydroxydeca-2,4-dienoic acid (1), along with six known compounds (2–7), were isolated from the gorgonian-derived fungus Xylaria sp. C-2, collected from the South China Sea. The structure of 1 was elucidated by 1D, 2D NMR, and MS spectrometry. The absolute configuration of 1 was determined by using the modified Mosher’s method. All of the isolated compounds were evaluated for their cytotoxic and antibacterial activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fungi of the genus Xylaria, which is the largest genus of the family Xylariaceae, have been shown to be a potential source of novel secondary metabolites relevant for drug discovery. In recent years, secondary metabolites have been reported from marine-derived fungus Xylaria sp., such as cytochalasins with cytotoxic activity [1], sesquiterpenes with antifouling activity [2], and polyketides with enzyme-inhibitory activity [3]. In the course of our search for new bioactive secondary metabolites from gorgonian-derived fungi in the South China Sea [4,5,6], the chemical investigation of a gorgonian-derived fungus Xylaria sp. C-2 led to the isolation of a new fatty acid (1), together with six known compounds (2–7). Herein we report the isolation, structure elucidation, and bioactivity of these metabolites.

Compound 1 was obtained as a light yellowish, amorphous solid. Its molecular formula was determined as C10H16O3 by extensive analysis of its 1D and 2D NMR data (Table 1) and HR-ESI-MS, which required three degrees of unsaturation. The IR absorptions at 3420 and 1697 cm–1 indicated the presence of hydroxy (OH) and ester carbonyl (C=O) groups, respectively. The 13C and DEPT NMR spectra displayed one methyl (δ 14.3), three methylenes (δ 23.3–37.7), five methines, including four olefinic carbons (δ 121.4–147.7) and one oxygenated methylene (δ 71.6), and an ester carbonyl carbon (δ 167.9) (Table 1).
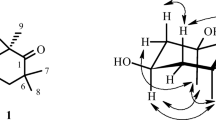
The 1H–1H COSY spectrum of 1 revealed one spin system starting from H-2 (δ 5.87) and ending at H3-10 (δ 0.89) (Fig. 1). An α, β, γ, δ-unsaturated carbonyl moiety was established by HMBC as H-2 (δ 5.87) and H-4 (δ 6.44), which show long-range correlations with C-1 (δ 167.9) and C-2 (δ 121.4), respectively (Fig. 1). The HMBC correlations from H-6 (δ 4.20) to C-4 (δ 127.2) and C-8 (δ 28.3) indicated that the hydroxy was located at C-6. The E geometries of the C-2/C-3 and C-4/C-5 double bonds were assigned on the basis of the larger coupling constants between H-2 and H-3 (J = 15.3 Hz), and between H-4 and H-5 (J = 15.3 Hz). The UV spectrum of 1 exhibited an absorption band at λmax 255 nm, indicating a conjugated diene in 1. The absolute configuration of C-6 in 1 was established using the modified Mosher’s method; each mono-(S)- and (R)-MTPA ester was separately prepared and subjected to 1H NMR data analysis. The absolute configuration at C-6 was readily assigned as S based on the ΔδH(S–R) values (Fig. 2) following the MTPA rules [7]. Thus, the structure of 1 was conclusively determined to be (2E,4E,6S)-6-hydroxydeca-2,4-dienoic acid.
The structures of the known compounds 2–7 were elucidated by comparison of their 1H and 13C NMR spectroscopic data with those reported in the literature as anodendroic acid (2) [8], 7-chloro-5-hydroxymellein (3) [9], methyl indole-3-carboxylate (4) [10], 5-carboxylmellein (5) [11], ergosta-5,7,22-trien-3β-ol (6) [12], and 5α,8α-epidioxy-ergosta-6,22E-dien-3β-ol (7) [13].
Although compound 1 is a simple fatty acid, the structure of a C10 unsaturated fatty acid with one hydroxyl group is uncommon in nature. A literature survey revealed that there has been only one report on (2Z,4E)-C10 unsaturated hydroxy fatty acid isolated from the sea fan (gorgonian)-derived fungus Curvularia sp. [14]. In the present study, 1 was the first example of (2E,4E)-C10 unsaturated hydroxy fatty acid from the gorgonian-derived fungus. Compounds 2–4 were isolated from the genus Xylaria for the first time.
The antibacterial activity was evaluated for all of the isolated compounds against a panel of pathogenic bacteria in vitro. Only 7 showed antibacterial activity against Escherichia coli, Pseudomonas putida, and Kocuria rhizophila with MIC values of 3.13, 1.56, and 6.25 μM, respectively. Ciprofloxacin was used as standard positive control and showed antibacterial activity with MIC values of 0.39, 0.78, and 1.56 μM, respectively. According to the results of Mondol et al. [15], the (2E,4E,9R)-C10 unsaturated fatty acid ieodomycin D bearing a 9-OH showed antimicrobial activities against Bacillus subtilis and Escherichia coli. However, in our research, 1 which bears 6-OH displayed no antimicrobial activity. These results revealed that the substituted positions of the hydroxy groups or the absolute configurations might influence the antimicrobial activity.
The cytotoxicities were also evaluated for 1–7 against human tumor cell lines (HeLa, Hep-2, RD, and A549). Only 6 exhibited weak cytotoxicity towards the HeLa, Hep-2, and RD cell lines with IC50 values of 20.1, 37.0, and 40.2 μM, respectively.
Experimental
General. Optical rotations were measured on a JASCO P-1020 digital polarimeter (Tokyo, Japan) at room temperature. UV spectra were recorded on a UV-2501PC spectrophotometer (Kyoto, Japan). IR spectra were determined on a Nicolet Nexus 470 spectrometer (Thermo Nicolet Corporation, USA). 1H and 13C NMR spectra were acquired on an Agilent DD2 NMR spectrometer (500 MHz, California, USA) and a JEOL JNM-ECP NMR spectrometer (600 MHz, Tokyo, Japan). ESI-MS and HR-ESI-MS were recorded on a Q-TOF Ultima Global GAA076 LC mass spectrometer (Massachusetts, USA) in m/z. HPLC separations were performed using a Hitachi L-2000 prep-HPLC system coupled with a Hitachi L-2455 photodiode array detector (Tokyo, Japan). A Kromasil C18 preparative HPLC column (250 × 10 mm, 5 μm) was used. Silica gel (200–300 mesh; Qingdao Marine Chemical Group Co., Qingdao, People’s Republic of China), octadecylsilyl (ODS) silica gel (45–60 mm; Merck KGaA, Darmstadt, Germany), and Sephadex LH-20 (Amersham Biosciences Inc., Piscataway, NJ, USA) were used for column chromatography. Precoated silica gel GF254 plates (Yantai Zifu Chemical Group Co., Yantai, People’s Republic of China) were used for analytical TLC analyses.
Fungal Material. The fungal strain Xylaria sp. C-2 was isolated from a piece of fresh tissue from the inner part of an unidentified gorgonian (XS-2009-03) collected from the Xisha Islands coral reefs in the South China Sea in December 2009. The strain was deposited in the Key Laboratory of Marine Drugs, Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao, P. R. China, with the GenBank (NCBI) accession number KP901303.
Extraction and Isolation. The fungal strain was cultivated in 25 L of liquid medium (200 g/L cooked and sliced potatoes, 20 g/L glucose in artificial seawater, in 1 L Erlenmeyer flasks each containing 400 mL of culture broth) at 25.0°C without shaking for 9 weeks. The culture was filtered to separate the culture broth from the mycelia. The fermentation broth was extracted three times with an equal volume of EtOAc, and the fungal mycelia were extracted three times with CHCl3–MeOH (1:1). The organic extracts from broth and mycelia were combined and concentrated under vacuum to afford a dry extract (25.2 g). The resulting extract was subjected to silica gel column chromatography (CC) (petroleum ether–EtOAc, gradient) to afford five fractions (Fr.1–5). Fraction 2 was isolated by silica gel CC (petroleum ether–EtOAc, 9:1) and separated by Sephadex LH-20 CC (petroleum ether–CHCl3–MeOH, 2:1:1), then further purified on semipreparative HPLC with 60% MeOH–H2O to yield 3 (5 mg) and 4 (7 mg), and with 55% MeOH–H2O to give 5 (3 mg). Fraction 3 was fractionated on silica gel CC (petroleum ether–EtOAc, 8:2) to obtain two subfractions (Subfr.3-1 and 3-2). Subfraction 3-1 was separated by Sephadex LH-20 CC (petroleum ether–CHCl3–MeOH, 2:1:1) to yield 6 (15.6 mg). Subfraction 3-2 was purified with 55% MeOH–H2O to yield 7 (14.8 mg). Fraction 4 was subjected to repeated Sephadex LH-20 CC (petroleum ether–EtOAc, 7:3) and further purified on semipreparative HPLC with 40% MeOH–H2O to afford 1 (8.0 mg) and 2 (8 mg).
Antimicrobial Bioassay. Antibacterial activity was evaluated by the conventional broth dilution assay [16]. Nine bacterial strains, Bacillus subtilis, Tetragenococcus halophilus, Staphylococcus albus, Staphylococcus aureus, Escherichia coli, Pseudomonas putida, Nocardia brasiliensis, Vibrio parahaemolyticus, and Kocuria rhizophila were used, and ciprofloxacin was used as a positive control.
Cytotoxicity Test. The cytotoxicity against four human tumor cell lines, A549 (lung carcinoma epithelium), HeLa (cervical epithelium), Hep-2 (epider-moid carcinoma-2), and RD (rhabdomyosarcoma cells), was evaluated using the SRB method [17]. Adriamycin was used as a positive control.
Preparation of the ( S )- and ( R )-MTPA Ester Derivatives of 1. To a stirred solution of 1 (1.0 mg) in pyridine (500 μL) was added 4-(dimethylamino)pyridine (2 mg) and (R)-(+)-α-methoxy-α-(trifluoromethyl) phenylacetyl chloride (MTPA-Cl, 10 μL). The mixture was stirred at room temperature for 12 h. The reaction mixture was then passed through a disposable pipet packed with silica gel and eluted with petroleum ether–EtOAc (3:1) to give the (S)-Mosher ester 1s. Treatment of 1 (1.0 mg) with (S)-MTPA-Cl (10 μL) as described above yielded the corresponding (R)-Mosher ester 1r.
1-( S )-MTPA Ester (1s). 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.27 (1H, dd, J = 15.3, 11.1, H-3), 6.45 (1H, dd, J = 15.3, 11.1, H-4), 6.15 (1H, dd, J = 15.3, 7.5, H-5), 5.95 (1H, d, J = 15.3, H-2), 5.55 (1H, dt, J = 12.0, 6.0, H-6), 3.73 (3H, s, MTPA-OCH3), 3.52 (3H, s, MTPA-OCH3), 1.67 (2H, m, H-7), 1.28 (2H, m, H-9), 1.19 (2H, m, H-8), 0.84 (3H, t, J = 7.2, H-10). ESI-MS m/z 437 [M + Na]+.
1-( R )-MTPA Ester (1r). 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.20 (1H, dd, J = 15.3, 11.1, H-3), 6.21 (1H, dd, J = 15.3, 11.1, H-4), 6.05 (1H, dd, J = 15.3, 7.5, H-5), 5.82 (1H, d, J = 15.3, H-2), 5.55 (1H, dt, J = 12.0, 6.0, H-6), 3.72 (3H, s, MTPA-OCH3), 3.55 (3H, s, MTPA-OCH3), 1.74 (2H, m, H-7), 1.36 (2H, m, H-9), 1.34 (2H, m, H-8), 0.90 (3H, t, J = 7.2, H-10). ESI-MS m/z 437 [M + Na]+.
(2 E ,4 E ,6 S )-6-Hydroxydeca-2,4-dienoic Acid (1). C10H16O3, light yellowish, amorphous solid. \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) +12.5°(c 0.20, CH3OH). UV (MeOH, λmax, nm) (log ε): 255 (2.89). IR (KBr, νmax, cm–1): 3420 (br), 2959, 1697, 1249, 1001. 1H (600 MHz, acetone-d6) and 13C NMR (150 MHz, acetone-d6), see Table 1. HR-ESI-MS m/z 183.1018 [M – H]–, (calcd for C10H15O3, 183.1016).
Anodendroic Acid (2). C12H14O4, white amorphous powder. \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) +59.0°(c 0.10, CH3OH). 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 7.74 (1H, s, H-4), 7.71 (1H, d, J = 8.2, H-6), 6.79 (1H, d, J = 8.2, H-7), 4.64 (1H, m, H-2), 3.17 (2H, m, H-3), 1.13 (3H, s, H-3′), 1.12 (3H, s, H-2′). ESI-MS m/z 223 [M + H]+.
7-Chloro-5-hydroxymellein (3). C10H9ClO4, White amorphous powder. \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) +15.0°(c 0.25, CH3OH). 1H NMR (500 MHz, acetone-d6, δ, ppm, J/Hz): 7.24 (1H, s, H-6), 4.82 (1H, m, H-3), 3.23 (1H, dd, J = 17.0, 3.2, H-4a), 2.70 (1H, dd, J = 17.0, 11.5, H-4b), 1.51 (3H, d, J = 6.3, H-9). ESI-MS m/z 229 [M + H]+.
Methyl Indole-3-carboxylate (4). C10H9NO2, white amorphous powder. 1H NMR (500 MHz, acetone-d6, δ, ppm, J/Hz): 8.13 (1H, s, H-2), 8.04 (1H, dd, J = 8.0, 2.5, H-4), 7.50 (1H, dd, J = 8.0, 2.5, H-7), 7.21 (2H, m, H-5, 6), 3.84 (3H, s, OCH3). ESI-MS m/z 176 [M + H]+.
5-Carboxylmellein (5). C11H10O5, white amorphous solid. \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) –189.0°(c 0.15, CH3OH). 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 8.14 (1H, d, J = 8.8, H-6), 6.93 (1H, d, J = 8.8, H-7), 4.71 (1H, m, H-3), 3.91 (1H, dd, J = 17.2, 3.0, H-4a), 3.01 (1H, dd, J = 17.2, 3.0, H-4b), 1.51 (3H, d, J = 6.0, CH3). ESI-MS m/z 245 [M + Na]+.
Ergosta-5,7,22-trien-3 β -ol (6). C28H44O, white amorphous powder. 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 5.56 (1H, d, J = 5.6, H-6), 5.38 (1H, d, J = 5.6, H-7), 5.22 (1H, dd, J = 15.3, 7.6, H-23), 5.16 (1H, dd, J = 15.3, 7.1, H-22), 3.63 (1H, m, H-3), 1.03 (3H, d, J = 6.6, H-21), 0.94 (3H, s, H-19), 0.91 (3H, d, J = 6.8, H-28), 0.84 (3H, d, J = 6.4, H-27), 0.82 (3H, d, J = 6.5, H-26), 0.62 (3H, s, H-18). ESI-MS m/z 397 [M + H]+.
5 α ,8 α -Epidioxy-ergosta-6,22 E -dien-3 β -ol (7). C28H44O3, white amorphous powder. 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 6.51 (1H, d, J = 7.6, H-7), 6.24 (1H, d, J = 7.6, H-6), 5.22 (1H, dd, J = 15.4, 7.6, H-23), 5.15 (1H, dd, J = 15.3, 7.2, H-22), 3.96 (1H, m, H-3), 1.00 (3H, d, J = 6.6, H-21), 0.91 (3H, d, J = 6.6, H-28), 0.88 (3H, s, H-19), 0.84 (3H, s, H-18), 0.82 (3H, d, J = 6.6, H-27), 0.81 (3H, d, J = 6.6, H-26). ESI-MS m/z 451 [M + Na]+.
References
Z. M. Chen, H. B Huang, Y. C. Chen, Z. W. Wang, J. Y. Ma, B. Wang, W. M. Zhang, C. S. Zhang, and J. H. Ju, Helv. Chim. Acta, 94, 1671 (2011).
L. A. McDonald, L. R. Barbieri, V. S. Bernan, J. Janso, P. Lassota, and G. T. Carter, J. Nat. Prod., 67, 1565 (2004).
X. S. Huang, X. F. Sun, S. E. Lin, Z. E. Xiao, H. X. Li, D. Bo, and Z. G. She, Nat. Prod. Res., 28, 111 (2014).
M. Chen, C. L. Shao, C. J. Kong, Z. G. She, and C. Y. Wang, Chem. Nat. Compd., 50, 617 (2014).
M. Chen, C. L. Shao, H. Meng, Z. G. She, and C. Y. Wang, J. Nat. Prod., 77, 2720 (2014).
M. Chen, C. L. Shao, K. L. Wang, Y. Xu, Z. G. She, and C. Y. Wang, Tetrahedron, 70, 9132 (2014).
I. Ohtani, T. Kusumi, Y. Kashman, and H. Kakisawa, J. Am. Chem. Soc., 113, 4092 (1991).
F. J. Arriaga-Giner, E. Wollenweber, I. Schober, and G. Yatskievych, Z. Naturforsch. C, 43, 337 (1988).
C. H. Lu, S. S. Liu, J. Y. Wang, M. Z. Wang, and Y. M. Shen, Helv. Chim. Acta, 97, 334 (2014).
S. C. Hu, R. X. Tan, K. Hong, Z. N. Yu, and H. L. Zhu, Acta Crystallogr. E, 61, 1654 (2005).
T. Okuno, S. Oikawa, T. Goto, K. Sawai, H. Shirahama, and T. Matsumoto, Agric. Biol. Chem., 50, 997 (1986).
D. M. Que, H. F. Dai, Y. B. Zeng, J. Wu, and W. L. Mei, Chin. J. Med. Chem., 3, 200 (2009).
M. Zhang, X. L. Tang, and G. Q. Li, J. Ocean. Univ. China, 5, 89 (2010).
K. Trisuwan, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon, and J. Sakayaroj, Arch. Pharm. Res., 34, 709 (2011).
M. A. M. Mondol, J. H. Kim, M. Lee, F. S. Tareq, H. S. Lee, Y. J. Lee, and H. J. Shin, J. Nat. Prod., 74, 1606 (2011).
G. Appendio, S. Gibbons, A. Giana, A. Pagani, G. Grassi, M. Stavri, E. Smith, and M. M. Rahman, J. Nat. Prod., 71, 1427 (2008).
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T Warren, H. Bokesch, S. Kenney, and M. R. Boyd, J. Natl. Cancer Inst., 82, 1107 (1990).
Acknowledgment
We thank Dr. Chang-Lun Shao, School of Medicine and Pharmacy, Ocean University of China, for sample collection. This work was supported by the National Natural Science Foundation of China (Nos. 81172977; U1406402) and the Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (No. GD2012-D01-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 195–198.
Rights and permissions
About this article
Cite this article
Sun, DW., Cao, F., Liu, M. et al. New Fatty Acid From a Gorgonian-Derived Xylaria sp. Fungus. Chem Nat Compd 53, 227–230 (2017). https://doi.org/10.1007/s10600-017-1958-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1958-7