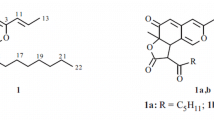
A chemical investigation on the n-BuOH-soluble fraction of the 95% EtOH extract of red mold rice fermented with the fungus Monascus purpureus BCRC 31499 (Eurotiaceae) has resulted in the isolation of one new isochroman derivative, designated as monascupurpurin (1). The structure was elucidated as rel-(2S)-4,6-dimethyl-2-hexyl-7-O-α-L-rhamnosylisochroman-5-ol (1) on the basis of extensive 1D and 2D NMR techniques, including COSY, HMBC, HMQC, and NOESY correlations, as well as HR-ESI-MS analysis and comparison of the spectroscopic data with those reported for structurally related compounds. This is the first report on Monascus metabolites with an isochroman glycoside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Monascus species (family Monascaceae, class Ascomycetes) has been utilized for making fermented food and preserving meat for hundreds of years in Asia [1, 2]. Various secondary metabolites useful as food additives and/or pharmaceuticals have been reported to be produced by Monascus spp. [1]. They comprise five representative species: M. pilosus, M. purpureus, M. ruber, M. kaoliang, and M. anka [1, 2]. Red yeast rice, which is also known as red fermented rice and red mold rice, is produced by growing Monascus sp. on rice to produce a red-colored product. Fungi of the genus Monascus are known to produce several bioactive (e.g., antihypercholesterolemic, hypotensive, and antibacterial) secondary metabolites [3–9]. Careful examination on the n-BuOH-soluble fraction of a 95% EtOH extract of the red mold rice produced by Monascus purpureus led to the isolation of one new isochroman analog, monascupurpurin (1). We herein reported on the isolation and structural elucidation of the new constituent.
Compound 1 was obtained as an optically active oil with [α] 25D –125.4° (c 0.09, MeOH). The ESI-MS and HR-ESI-MS were used to establish the molecular formula of 1 as C23H36O7 with an IHD (index of hydrogen deficiency) of 6. The absorption bands of its IR spectrum suggested the presence of hydroxy groups (3410 cm–1) and an aromatic ring (1590, 1456 cm–1). The UV absorptions (λmax 280 nm) and the exhibition of a bathochromic shift in alkaline solution suggested the presence of a phenolic isochroman skeleton [10]. The 1H NMR spectrum (Table 1) showed an n-hexyl group at δH 0.92 (3H, t, J = 7.5 Hz, CH3-14), 1.36–1.40 (6H, br.s, CH2-11–13), 1.48 (1H, m, H-10b), 1.62 (1H, m, H-10a), 1.62 (2H, m, CH2-9); δC 13.8 (C-14), 24.2 (C-13), 29.7 (C-12), 32.8 (C-11), 26.3 (C-10), 37.8 (C-9), two methyls attached to benzene ring at δH 2.06 (3H, s, CH3-15), 2.18 (3H, s, CH3-16); δC 10.2 (C-16), 11.8 (C-15); one nonequivalent CH2 proton at δH 2.40 (1H, dd, J = 16.0, 11.8 Hz, H-3β), 2.66 (1H, dd, J = 16.0, 2.8 Hz, H-3α); δC 33.8 (C-3), one oxymethine at δH 3.62 (1H, m, H-2); δC 75.7 (C-2), and one nonequivalent OCH2 at δH 4.58, 5.03 (each 1H, each d, J = 13.8 Hz, CH2-8); δC 65.8 (C-8). Individual sugar protons (rhamnosyl unit) were identified by means of COSY (Fig. 1a) and HSQC experiments at δH 1.30 (3H, d, J = 6.4 Hz, CH3-6′), 3.45 (1H, t, J = 9.2 Hz, H-4′), 3.80 (1H, dd, J = 9.2, 3.6 Hz, H-3′), 4.01 (1H, dq, J = 9.2, 6.4 Hz, H-5′), 4.20 (1H, dd, J = 3.6, 2.0 Hz, H-2′), 4.80 (1H, d, J = 2.0 Hz, H-1′); δC 18.0 (C-6′), 72.5 (C-5′), 73.4 (C-4′), 73.3 (C-3′), 72.8 (C-2′), 108.2 (C-1′). The glycosidic bond was α-oriented according to the small coupling constant (J = 2.0) of the anomeric proton at δH 4.80. The 1H, 13C NMR (Table 1), and DEPT spectra displayed signals for four primary carbons at δC 10.2 (C-16), 11.8 (C-15), 13.8 (C-14), 18.0 (C-6′), seven secondary carbons including one O-bearing at δC 65.8 (C-8), 37.8 (C-9), 33.8 (C-3), 32.8 (C-11), 26.3 (C-10), 29.7 (C-12), 24.2 (C-13), six tertiary methines including one hemiacetal at δC 108.2 (C-1′), 75.7 (C-2), 72.8 (C-2′), 73.4 (C-4′), 73.3 (C-3′), 72.5 (C-5′), and six quaternary carbons at δC 154.2 (C-5), 153.8 (C-7), 132.5 (C-3a), 121.8 (C-7a), 122.6 (C-4), 118.2 (C-6). According to the 1H, 13C NMR, and DEPT spectra, 1 has a fully substituted benzene ring. The COSY spectrum (Fig. 1a ) showed the contacts between the fragments of n-hexyl and the rhamnosyl moeity of 1. The HMBC spectrum (Fig. 1a ) showed key correlations from CH2-3 to C-2, C-4, C-9, C-3a, C-7a, and CH2-8 to C-2, C-7, C-3a, C-7a, which establishes the skeleton of 1, from CH2-3 to C-9 and H-2 to C-10, which confirms the n-hexyl moiety attached to C-2, and from H-1′ to C-7, which shows that the rhamnosyl moiety is connected to C-7 with the α-orientation of the O-linkage. The other key correlations of HMBC are illustrated in Fig. 1a .
The relative configuration of 1 was deduced from the NOESY spectrum (Fig. 1b ). The α-orientation of the n-hexyl unit at C-2 was deduced from the observation of the quasi-1,3-diaxial interactions between H β -2 and H β -8/H β -3. From the above data, compound 1 was unambiguously characterized as rel-(2S)-4,6-dimethyl-2-hexyl-7-O-α-L-rhamnosylisochroman-5-ol, named monascupurpurin, and its structure is shown in Fig. 1, which was further confirmed by COSY, HMBC (Fig. 1a ), and NOESY (Fig. 1b ) experiments.
Experimental
General. Optical rotations were measured on a Jasco P-1020 digital polarimeter, UV spectra were obtained on a Jasco UV-240 spectrophotometer in MeOH, and IR spectra (KBr or neat) were taken on a PerkinElmer System 2000 FT-IR spectrometer. 1D (1H, 13C, DEPT) and 2D (COSY, NOESY, HSQC, HMBC) NMR spectra using CDCl3 as solvent were recorded on a Varian VNMRS 600 (600 MHz for 1H NMR, 150 MHz for 13C NMR) spectrometers. Chemical shifts were internally referenced to the solvent signals in CDCl3 (1H, δ 7.26; 13C, δ 77.0) with TMS as the internal standard. Low-resolution ESI-MS spectra were obtained on an API 3000 spectrometer (Applied Biosystems) and high-resolution ESI-MS spectra on a Bruker Daltonics APEX II 30e spectrometer. Low-resolution EI-MS spectra were recorded on a Quattro GC/MS spectrometer having a direct inlet system. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography, and silica gel 60 F-254 (Merck) was used for TLC and preparative TLC.
Fungus Material. Monascus purpureus BCRC 31499 was used throughout this study, and specimens were deposited at the Bioresource Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (FIRDI). M. purpureus was maintained on potato dextrose agar (PDA, Difco). The strain was cultured on PDA slants at 25°C for 7 days and the spores harvested by sterile water. The spores (5 × 105) were seeded into 300 mL shake flasks containing 50 mL RGY medium (3% rice starch, 7% glycerol, 1.1% polypeptone, 3.2% soybean powder, 0.2% MgSO4, 0.2% NaNO3) and cultivated with shaking (150 rpm) at 25°C for 3 days. After the mycelium enrichment step, an inoculum mixing 100 mL mycelium broth and 100 mL RGY medium was inoculated into plastic boxes (25 cm × 30 cm) containing 3 kg sterile rice and cultivated at 25°C for producing red mold rice (RMR; also called beni-koji in Japan). At day 7, 150 mL RGY medium was added to maintain the growth of the cells. After 21 days of cultivation, the RMR was harvested and lyophilized for the extraction of metabolites.
Extraction and Separation of Compounds. The dried red mold rice of Monascus purpureus BCRC 31499 (3.0 kg) was extracted five times with 95% EtOH at room temperature. The ethanolic syrup extract was partitioned between n-BuOH–H2O (1:1) to afford n-BuOH (3.2 g) and H2O (5.5 g) soluble fractions. The n-BuOH-soluble fraction (Fr. A, 3.2 g) was chromatographed by CC (100 g SiO2, 70–230 mesh), eluting with EtOAc and enriched with MeOH to produce eight fractions: Fr. A1–A8. Fraction A6 (980 mg) was re-subjected to CC (35 g SiO2, 70–230 mesh; CH2Cl2–EtOAc 10:1→1:1) to yield 15 subfractions: Subfr. A6.1–A6.15. Subfraction A6.6 (16 mg) was purified by preparative TLC (SiO2; CH2Cl2–acetone, 4:1) to gain 1 (1.7 mg).
Monascupurpurin (1). Oil, [α] 25D – 125.4° (c 0.09, MeOH). IR spectrum (neat, νmax, cm–1): 3410 (OH), 1590, 1456 (benzene ring). UV (MeOH): 280 (3.85). 1H and 13C NMR, see Table 1. ESI-MS m/z 447 ([M + Na]+). HR-ESI-MS m/z 447.2359 ([M + Na]+ (calcd for C23H36NaO7, 447.2355).
References
R. E. Mudgett, Monascus. In Natural Food Colorants: Science and Technology, G. J. Lauro, F. J. Francis (eds.), Marcel Dekker, New York, 2000, p. 31.
J. Ma, Y. Li, Q. Ye, J. Li, Y. Hua, D. Ju, D. Zhang, R. Cooper, and M. Chang, J. Agric. Food Chem., 48, 5220 (2000).
Y. C. Su, J. J. Wang, T. T. Lin, and T. M. Pan, J. Ind. Microbiol. Biotechnol., 30, 40 (2003).
P. J. Blanc, M. O. Loret, A. L. Santerre, A. Pareilleux, D. Prome, J. C. Prome, J. P. Laussac, and G. Goma, J. Food Sci., 59, 862 (1994).
H. C. Wong and P. E. Koehler, J. Food Sci., 46, 589 (1981).
Y. Aniya, I. I. Ohtani, T. Higa, C. Miyagi, H. Gibo, M. Shimabukuro, H. Nakanish, and J. Taira, Free Radical Biol. Med., 286, 999 (1999).
G. F. Wu and X. C. Wu, Acta Microbiol. Sin., 40, 394 (2000).
P. J. Blanc, J. P. Laussac, J. Le Bars, P. Le Bars, M. O. Loret, A. Pareilleux, D. Prome, J. C. Prome, A. L. Santerre, and G. Goma, Int. J. Food Microbiol., 27, 201 (1995).
P. J. Blanc, M. O. Loret, and G. Goma, Biotechnol. Lett., 17, 291 (1995).
W. A. Ayer and S. Miao, Can. J. Chem., 71, 487 (1993).
Acknowledgment
This investigation was supported by a grant from the Ministry of Economic Affairs of the Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2016, pp. 547–549.
Rights and permissions
About this article
Cite this article
Cheng, MJ., Wu, MD., Cheng, YC. et al. One New Compound from the Extract of the Fungus Monascus purpureus BCRC 31499. Chem Nat Compd 52, 634–636 (2016). https://doi.org/10.1007/s10600-016-1727-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1727-z