Two new triterpenoids, 28-β-(E)-2′,3′-dimethyltridec-2′-enyl-3-oxofriedelan-11-en-28-oate and 3β-hydroxyolean-12-ene, were isolated from the stems of Drypetes cumingii by silica gel column chromatography and purified by recrystallization. Their structures were elucidated on the basis of spectroscopic analysis including 1D and 2D NMR techniques and HR-ESI-MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Drypetes cumingii Pax et K. Hoffm. is one of the many species of the genus Drypetes (Euphorbiaceae) encountered in China. There are about 200 species on earth, and more than 10 species have been identified in Hainan tropical monsoon forests [1]. These plants are well known in the folk medicine of Africa and Hainan, and many are used to treat various diseases, including tumors, gonorrhea, toothache, dysentery, coryza, sinusitis, boils, and swellings in West and Central Africa [2–4]. Up to now, the chemical constituents of Drypetes cumingii have been rarely reported. In our previous results, we isolated two new triterpenoids from the leaves and stems of Drypetes cumingii.
Their structures were established as 28-β-(E)-2′,3′-dimethyltridec-2′-enyl-3-oxofriedelan-11-en-28-oate (1) and 3β-hydroxyolean-12-ene (2) on the basis of spectroscopic analysis and chemical evidence, including 1D and 2D NMR data.
The novel compound 1 was obtained as white needle crystals with mp 277–279°C. Its molecular formula C45H74O3 was deduced on the basis of HR-ESI-MS (m/z 685.3321 [M + Na]+. The 1H NMR spectrum (Table 1) revealed the presence of seven singlet resonances for methyl groups at δ 0.72–1.17 (each 3H, s). The 13C NMR spectrum showed 45 carbon signals, including ten methyls, 19 methylenes, six methines, and ten quaternary carbons (Table 1). The 13C NMR spectra of compound 1 showed two carbonyl groups at δ 213.4 and 170.4. The carbon signal at δ 7.0 (C-23) was characteristic of a friedelane-type triterpene skeleton with one of the carbonyl groups located at the C-3 position [5, 6].
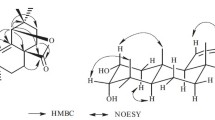
All the proton signals were assigned to the corresponding carbons through direct 1H and 13C correlations in the HSQC spectrum, and the basic skeleton was also further supported by the 2D 1H–1H COSY spectrum. In the HMBC spectrum, the key correlations between the proton H-18 (δ 1.74) and carbons C-28 (δ 170.4), C-20 (δ 28.4) and between the proton H-1′ (δ 4.22) and carbon C-28 (δ 170.4), together with the above molecular formula, confirm the position of connectivity of the (E)-2′,3′-dimethyltridec-2′-enyl ester to be to the 28-carboxyl group [7]. Accordingly, compound 1 was elucidated as 28-β-(E)-2′,3′-dimethyltridec-2′-enyl-3-oxofriedelan-11-en-28-oate.
Compound 2 was assigned the molecular formula C30H50O from the positive HR-ESI-MS at m/z 449.3417 [M + Na]+. The 1H NMR spectrum (Table 1) revealed the presence of seven singlet resonances for methyl groups at δ 0.78–1.13 (each 3H, s) and a triplet at δ 5.17 (1H, J = 3.6 Hz, H-12). These signals and the 13C NMR signals (Table1) at δ 121.9 and 145.4 were in agreement with reported data for olean-12-ene type triterpenes [7]. The 13C NMR spectra of 2 showed an oxymethine carbon signal at δ 79.1, indicating that it was present as a carbohydroxyl group. The HMBC spectrum showed correlations between the proton signal (δ 3.22) and carbons C-3 (δ 79.1) and C-4 (δ 38.9), confirming the position of the hydroxyl group at C-3. Therefore, the structure of compound 2 was established as 3β-hydroxyolean-12-ene.
Experimental
General. HR-ESI-MS: Bruker Apex, FT-ICR. NMR: Varian Unity 600.
Plant. The stems of Drypetes cumingii were collected in Hainan Province, China and were taxonomically indentified by Prof. Pinghuai Liu of Hainan University. The stems were dried, then pulverized into powdered and stored in a dryer.
Extraction and Isolation. Air-dried powdered stems of Drypetes cumingii (19 kg) were extracted consecutively with EtOH (95%) at room temperature three times. The extracts were concentrated and the residue suspended in water and partitionend with EtOAc to yield 177.0 g. Then the EtOAc extract was subjected to repeated silica gel column chromatography to yield two new triterpenoids. Their structures were elucidated on the basis of spectroscopic analysis including 1D and 2D NMR techniques and HR-ESI-MS.
References
B. T. Li, Flora of China, Vol. 44, Science Press, Beijing, 1994.
R. F. Irvine, Woody Plants of Ghana, Oxford University Press, London, 1961, 223 p.
Deli Chen, Xu Cheng, Yuwan Sun, and Pinghuai Liu, Chem. Nat. Compd., 50, 93 (2014).
J. M. Dalziel, The Useful Plants of West Tropical Africa, The Crown Agents for the Colonies, London, 1937, 140 p.
Y. L. Li, Y. X. Gao, X. W. Yang, H.-Z. Jin, J. Ye, L. Simmons, N. Wang, A. Steinmetz, and W.-D.Zhang, Phytochemistry, 81, 159 (2012).
D. W. Ou-Yang, L. Wu, Y. L. Li, D. Y. Kong, X. W. Yang, and W. D. Zhang, Phytochemistry, 72, 2197 (2011).
S. S. Awanchiri, H. Trinh-Van-Dufat, J. C. Shirri, M. D. J. Dongfack, G. M. Nguenang, S. Boutefnouchet, Z. T. Fomum, E. Seguin, P. Verite, F. Tillequin, and J. Wandji, Phytochemistry, 70, 419 (2009).
Acknowledgment
The authors acknowledge the technical assistance of the Institute of Medicinal Plant Development. This work is supported by the National Science and Technology Support Program of China (2011BAD14B01), the Provincial Science and Technology Program on Modernization of Traditional Chinese Medicine of Hainan (ZY201327), and the Innovation Fund Project for Technology Based Firms (13C2624464892).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2016, pp. 372–373.
Rights and permissions
About this article
Cite this article
Zhang, L., Liu, P., Sun, Y. et al. Two New Triterpenoids from the Leaves and Stems of Drypetes cumingii . Chem Nat Compd 52, 424–426 (2016). https://doi.org/10.1007/s10600-016-1664-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1664-x