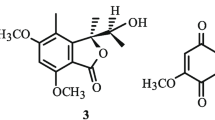
A new phthalide was isolated from the roots of Pittosporum illicioides Makino. Its structure was established as (S)-3-ethyl-4,7-dimethoxyphthalide based on physicochemical properties and extensive spectroscopic analysis including 1D, 2D NMR, and HR-MS spectra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pittosporum illicioides Makino (Pittosporaceae) is an evergreen shrub found in medium to high altitude in forests of China. It has been recorded as a folk remedy for the treatment of neurasthenia, insomnia, dreaminess, and hypertension in Guizhou Province, China [1]. Previous phytochemical investigation revealed that phthalides are the main active constituents in P. illicioides, which display inhibitory activity on superoxide generation and elastase release by neutrophils [2]. Our studies showed that an ethanol root extract of P. illicioides had remarkable antidepressant activity [3]. Further fractionation of the extract and experiments on the duration of immobility using either the tail suspension test or the forced swimming test in mice led to the discovery of an active fraction that contains the EtOAc-soluble fraction [4]. This paper reports the isolation and structure identification of a new natural product 1 from the EtOAc-soluble active fraction.
Compound 1, colorless needles, has the molecular formula C12H14O4, as established by HR-ESI-MS at m/z 245.07837 [M + Na]+ (calcd for M + Na, 245.07843), which was further confirmed by the 1H, 13C, and DEPT NMR data (Table 1). The presence of a carbonyl group was revealed by a band at 1761 cm–1 in the IR spectrum and was confirmed by the resonance at δ 168.7 in the 13C NMR spectrum. The 1H NMR spectrum of 1 showed the presence of two methoxyl groups, two ortho-coupled aromatic protons, an oxymethine proton, and an ethyl group. The NOESY spectrum revealed cross-peaks between a proton signal at δ 7.05 and a methoxyl signal at δ 3.85, and between a proton signal at δ 6.86 and a methoxyl signal at δ 3.94; the two methoxyl groups were assigned to C-4 and C-7. HMBC correlations were observed between a proton signal at δ 6.86 and a carbonyl carbon signal at δ 168.7 (C-1), and between a proton signal at δ 7.05 and a carbon signal at δ 80.5 (C-3); the aromatic protons were attached to C-6 and C-5. The ethyl group was placed at C-3 by HMBC correlations between H-9 and H-5 signals and a carbon signal of C-3. Compound 1 was levorotatory ([α] 25D –101.2°) as in the case of (S)-3-ethylphthalide ([α] 25D –73.5°), and the absolute configuration at C-3 in 1 has to be S [5, 6]. The structure of 1 was thus elucidated as (S)-3-ethyl-4,7-dimethoxyphthalide. This structure was supported by 1H–1H COSY and NOESY experiments, and the 13C NMR assignments were confirmed by DEPT, HMQC, and HMBC techniques.

Experimental
General Experimental Procedures. 1H NMR and 13C NMR were recorded on a JNM-ECA-400 model operating at 400 MHz and 100 MHz (DMSO-d6), using TMS as the internal standard. EI mass spectra were recorded on a Zabspec mass spectrometer. HR-ESI mass spectra were recorded on a Bruker APEX II mass spectrometer. Optical rotations were measured using a PolAAr 3005 polarimeter in CHCl3. UV spectra were obtained on a Shimadzu UV-2501PC spectrometer. IR spectra were recorded on a VERTEX 70 FT-IR spectrometer as pressed KBr discs. Melting points were measured on a Fisher-Johns apparatus and are uncorrected. Silica gel (200–300 mesh) for column chromatography was a product of the Qingdao Marine Chemical Company. Sephadex LH-20 for chromatography was purchased from Amersham Biosciences.
Plant Material. The roots of P. illicioides Makino were collected from Zunyi District, Guizhou Province, P. R. China, in August, 2009 and identified by Assoc. Prof. Bin Li, Beijing Institute of Radiation Medicine. Voucher specimens are deposited at the Herbarium of the Beijing Institute of Radiation Medicine.
Extraction and Isolation. The dried roots of P. illicioides Makino (35.8 kg) were pulverized and extracted with EtOH under reflux (150 L × 3, each 3 h). After the solvent was evaporated under vacuum, the EtOH extract (980 g) was partitioned between EtOAc and H2O (1:1). The EtOAc layer was concentrated to give a residue (165 g). The EtOAc-soluble fraction was subjected to column chromatography on silica gel and eluted with CH2Cl2–CH3OH (100:0→8:2) to give five fractions. Fraction 1 was purified on Sephadex LH-20 with CH3OH–CHCl3 (1:1) and crystallized in EtOAc to obtain compound 1 (25 mg).
( S )-3-Ethyl-4,7-dimethoxyphthalide (1). Colorless needles, mp 133–135°C, [α] 25D –101.2° (c 0.084, CHCl3). UV spectrum (CH3OH, λmax, nm) (log ε): 322 (4.35), 237 (3.78), 216 (3.63). IR (KBr, ν, cm–1): 3031, 2976, 2936, 2875, 1761 (C=O), 1607, 1509 (Ar), 1466, 1440, 1273, 1072, 1032, 975, 815. EI-MS (m/z, I rel, %): 222 (M+, 20), 194 (9), 193 (100), 165 (11). HR-ESI-MS m/z 245.07837 [M + Na]+ (calcd for C12H14O4Na, 245.07843).
References
Jiangsu New MEDICINE College, Dictionary of Chinese Traditional Medicine [in Chinese], Shanghai Science and Technology Press, Shanghai, 2001, 184 pp.
T. H. Chou, I. S. Chen, T. L. Hwing, T. C. Wang, T. H. Lee, L. Y. Cheng, Y. C. Chang, J. Y. Cho, and J. J. Chen, J. Nat. Prod., 71, 1692 (2008).
X. N. Zuo, B. K. Xiao, Y. R. Liu, J. Y. Yang, B. Zhen, and R. Q. Huang, Lishizhen Med. Mater. Med. Res., 24, 530 (2013).
X. N. Zuo, B. K. Xiao, Y. R. Liu, J. Y. Yang, B. Zhen, and R. Q. Huang, Milit. Med. Sci., 37, 283 (2013).
H. Takahashi, T. Tsubuki, and K. Higashiyama, Chem. Pharm. Bull., 39, 3136 (1991).
Sh. Sh. Afiyatullov, E. V. Leshchenko, M. P. Sobolevskaya, A. V. Gerasimenko, Yu. V. Khudyakova, N. N. Kirichuk, and V. V. Mikhailov, Chem. Nat. Compd., 51, 98 (2015).
Acknowledgment
This work was supported by Beijing Natural Science Foundation (No. 7142125), National Natural Science Foundation of China (No. 21375147), and the Integrated Drug R&D Platform (No. 2012ZX09301003-001-010) for "the significant creation of new drugs" under major projects of National Science and Technology, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2015, pp. 548–549.
Rights and permissions
About this article
Cite this article
Xiao, BK., Yang, JY., Liu, YR. et al. A New Phthalide from Pittosporum illicioides . Chem Nat Compd 51, 634–636 (2015). https://doi.org/10.1007/s10600-015-1372-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1372-y