Abstract
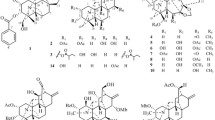
Study of the aerial parts of the two species of Ageratina: A. dictyoneura and A. illita afforded four new ent-labdane diterpenoids (1–4). Two known labdanes: 2β,3α,15-trihydroxy-ent-labd-7-ene (5), and 2β,3α-trihydroxy-ent-labd-7-en-15-oic acid (6); two sesquiterpene lactones: 8β-hydroxy-β-cyclocostunolide (7) and eupatoriopicrin (8), one benzofuran, and six flavonoids were also isolated. Their chemical structures were determined based on extensive spectroscopic study, comparison with reported data and chemical transformations. The cytotoxicity of the new ent-labdane diterpenoids 1–3, sesquiterpene lactone 7, and the flavonoid: quercetin 3,7-dimethylether were assessed against the human myeloid leukemia U-937 cell line and found that compound 7 and quercetin 3,7-dimethylether were cytotoxic against this cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Eupatorieae tribe (Asteraceae) comprises ~2000–2500 species, 19 subtribes, and about 182 genera. It is considered the richest tribe in terms of the number of species. The tribe seems to be limited to the west hemisphere, proposing a Neotropical origin with a small number of native species in the old world (Chen et al. 2011; Rivera et al. 2016). King and Robinson reclassified Eupatorieae tribe into more natural groups relying on morphological comparisons, geographic distribution, and chromosome numbers (King and Robinson 1987), and also removing many species from Eupatorium to other genera: for example; Ageratina and Austroeupatorium (King and Robinson 1970).
Sesquiterpenes, diterpenes, and thymol derivatives are the major chemically constituents in Ageratina genus, and play a critical role in the biological effects of several Ageratina species (Herz 2001; Liu et al. 2015; Ma et al. 2015).
As a part of our continuing search for novel, plant-derived anticancer chemotherapeutic agents, and our systematic investigation of the composition of Asteraceae plants (Castillo et al. 2016; Triana et al. 2016), we have investigated the chemical constituents of the aerial parts of two Ageratina species, endemic to Hispaniola Island: Ageratina dictyoneura (Urban) R.M. King & H. Rob [syn. Eupatorium dictyoneurum (Urban)] and Ageratina illita (Urban) R.M. King & H. Rob [syn. Eupatorium illitium (Urban)] (Pruski and Clase 2012). The isolation, structural elucidation, and the cytotoxic effects of the isolated compounds against U-937 myeloid leukemia cells are described herein.
Materials and methods
General experimental techniques
Optical rotations were recorded in a Perkin-Elmer model 343 polarimeter. IR spectra was recorded using a Bruker model IFS-55 spectrophotometer. 1H and 13C NMR spectra were obtained on a Bruker model AMX-500 NMR spectrometer with standard pulse sequences operating at 500 for 1H and 125 MHz for 13C NMR, respectively. CDCl3 was used as solvent. EIMS was taken on a Micromass model Autospec (70 eV) spectrometer. HRESIMS was performed with a LCT Premier XE Micromass Waters spectrometer in the positive-ionization mode (Waters Corporation). Column chromatography (CC) was carried out on silica gel 60 (Merck 230-400 mesh), and preparative TLC on silica gel 60 PF254+366 plates (20 × 20 cm, 1 mm thickness). The developed TLC plates were visualized by ultraviolet light (254; 366 nm) and then by spraying with staining system of H2SO4-H2O-CH3COOH (1:4:20), followed by heating of silica gel plates.
Plant material
A. dictyoneura (Urban) R.M. King & H. Rob [syn. E. dictyoneurum (Urban)] was collected at Cordillera Central, Province La Vega, (Constanza-Valle Nuevo), Dominican Republic, in August 2010, and was identified by botanist Mr. Teodoro Clase, at Dr. Rafael Ma. Moscoso National Botanic Garden, Santo Domingo, Dominican Republic. A voucher specimen (JBSD 121460) was deposited in the National Botanic Garden, Dominican Republic.
A. illita (Urban) R.M. King & H. Rob [syn. E. illitium (Urban)] was collected at Sierra de Bahoruco, Province Pedernales, Dominican Republic, in June 2010, and was identified by botanist Mr. Teodoro Clase, at Dr. Rafael Ma. Moscoso National Botanic Garden, Santo Domingo, Dominican Republic. A voucher specimen (JBSD 121457) was deposited in the National Botanic Garden, Dominican Republic.
Extraction and isolation
The aerial parts of the two Ageratina species were exhaustively extracted with 95% EtOH in a Soxhlet apparatus for 72 h. The solvents were concentrated under reduced pressure, and the extracts were subsequently fractionated by silica gel CC using hexane and ethyl acetate (EtOAc) mixtures of increasing polarity.
The extraction of 4.57 kg of A. dictyoneura gave 420 g of a viscous residue, which was subjected to CC on silica gel affording five fractions. Fraction 1 [hexane-EtOAc (4:1)] was purified using preparative TLC using hexane-EtOAc (9:1) to yield dictyolabadan B (2) (85 mg), and dictyolabdan C (3) (92 mg). Fraction 2 [hexane-EtOAc (3:2)] was submitted to a CC on silica gel [hexane-EtOAc (4:1)] and preparative TLC [hexane-EtOAc (9:1)] to yield rhamnocitrin (135 mg), quercetin 3,7-dimethylether (92 mg), pinocembrin (103 mg), penduletin (63 mg), 4-hydroxybenzoic acid (14 mg), and dictyolabdan D (4) (5.3 mg). Fraction 3 [hexane-EtOAc (1:1)] was subjected to a preparative TLC and eluted with hexane-EtOAc (2:3) to afford dictyolabdan A (1) (87 mg), betuletol 3-methylether (23 mg), and 2β,3α,15-trihydroxy-ent-labd-7-ene (5) (10 mg). Fraction 4 [hexane-EtOAc (2:3)] purified using preparative TLC [hexane-EtOAc (1:1)] gave quercetin 3-methylether (125 mg) and a mixture which, after acetylation with acetic anhydride-pyridine and preparative TLC [hexane-EtOAc (9:1)] yielded 2β,3α-diacetyloxy-ent-labd-7-en-15-oic acid, the diacetyl compound 6 (4 mg).
Dictyolabdan A (1)
White amorphous solid, [α]20D = –9.05 (c 0.760, CHCl3); IR (film, NaCl) νmax 3401, 2969, 2917, 1682, 1647, 1448, 1259, 1243, 1190, 1094, 1052 cm−1; HRESIMS m/z; 359.2187 [M + Na]+ (calcd. for C20H32O4Na, 359.2198); 1H NMR and 13C NMR data, see Table 1.
Acetylation of compound 1
A solution of 1 (9.3 mg) in a mixture of acetic anhydride (2 ml) and pyridine (1 ml) was allowed to stand at room temperature overnight. The reaction mixture was treated in the usual way, and the product was purified by silica gel CC with hexane-EtOAc (8:2) to afford the acetate 1a (10.6 mg). Colorless oil, IR (film, NaCl) νmax 2969, 2917, 1682, 1647, 1448, 1259, 1243, 1190, 1166, 1052 cm−1; HRESIMS m/z; 443.2420 [M + Na]+ (calcd. for C24H36O6Na, 443.2410); 1H NMR and 13C NMR data, see Table 1.
Dictyolabdan B (2)
Yellow oil, [α]20D = −21.12. (c 0.113, CHCl3); IR (film, NaCl) νmax 3442, 2972, 2.925, 1713, 1695, 1643, 1454, 1384, 1240, 1167, 1046 cm−1; HRESIMS m/z; 441.2606 [M + Na]+ (calcd. for C25H38O5Na, 441.2617). 1H NMR and 13C NMR data, see Table 1.
Acetylacion of compound 2
A solution of 2 (7.2 mg) in a mixture of acetic anhydride (2 ml) and pyridine (1 ml) was allowed to stand at room temperature overnight. The reaction mixture was treated in the usual way, and the product was purified by silica gel CC with hexane-EtOAc (8:2) to afford the acetate 2a (8.3 mg) whose spectroscopic data were totally coincident with those of compound 3.
Dictyolabdan C (3)
Yellow oil, [α]20D = −35.92 (c 1.35, CHCl3); IR (film, NaCl) νmax 2971, 2921, 1729, 1714, 1643, 1454, 1371, 1243, 1157, 1086, 1042 cm−1; HRESIMS m/z; 483.2741 [M + Na]+ (calcd. for C27H40O6Na, 483.2723); 1H NMR and 13C NMR data, see Table 1.
Dictyolabdan D (4)
Colorles oil, [α]20D = −15.05 (c 0.21, CHCl3); IR (film, NaCl) νmax IR νmax 3445, 2972, 2925, 1710, 1695, 1643, 1454, 1384, 1240, 1240, 1167, 1046 cm−1; HRESIMS m/z; 433.2574 [M − H]− (calcd. for C25H37O6, 433.2590); 1H NMR and 13C NMR data, see Table 1.
The extraction of 617 g of A. illita gave 91 g of a viscous residue, which was subjected to CC on silica gel affording five fractions. Fraction 1 [hexane-EtOAc (3:2)] was purified by preparative TLC [hexane-EtOAc (4:1)] to yield (8R)-8-hydroxy-β-cyclocostunolide (7) (7.6 mg). Fraction 2 [hexane-EtOAc (2:3)] was subjected to a preparative [TLC hexane-EtOAc (1:1)] to furnish 7-hydroxytoxol (5 mg) and eupatoriopicrin (33) (8.3 mg).
8β-Hydroxy-β-cyclocostunolide (7)
Colorless oil, [α]25D =+48.57 (c 0.35, CHCl3); IR νmax 3469, 2929, 1756, 1650, 1611, 1441, 1411, 1252, 1228, 1176, 1124, 1055, 989 cm−1; HRESIMS m/z 271.1315 [M + Na]+ (calcd. for C15H20O3Na, 271.1310); 1H NMR (CDCl3, 500 MHz): δ 1.09 (s, CH3-14), 1.35 (td, J = 5.0, 12.9 Hz, H-1α), 1.50 (m, overlapped, H-1β), 1.53 (dd, J = 2.9, 14.7 Hz, H-9α), 1.60-1.67 (m, overlapped, H-2α and 2β), 1.89 (dd, J = 2.4, 14.7 Hz, H-9β), 1.99 (m, overlapped, H-3a), 2.30 (d br, J = 11.0 Hz, H-5α), 2.35 (m, overlapped, H-3b), 2.74 (ddd, J = 2.9, 5.6, 8.6 Hz, H-7α), 4.54 (dd, J = 11.0 Hz, H-6β), 4.60 (dd, J = 2.7, 5.4 Hz, H-8α), 4.86 (d, J = 1.5 Hz, H-15a), 4.95 (d, J = 1.5 Hz, H-15b); 5.50 (d, J = 3.1 Hz, H-13a), 6.22 (d, J = 3.1 Hz, H-13b); 13C NMR (CDCl3, 125 MHz): 20.3 (C-14), 22.2 (C-2), 35.8 (C-3), 38.8 (C-10), 41.8 (C-1), 46.5 (C-9), 53.9 (C-7), 56.0 (C-5), 65.8 (C-8), 75.4 (C-6), 109.2 (C-15), 118.2 (C-13), 135.9 (C-11), 144.1 (C-4), 170.3 (C-12).
Cell culture and cytotoxicity assays
The human leukemia U-937 cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and grown in RPMI 1640 containing 2 mM l-glutamine supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 µg/ml streptomycin, and 100 U/ml penicillin. The cytotoxicity of the tested compounds was analyzed using colorimetric 3-(4,5-dimethyl-2-thiazolyl-)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay as previously described (Estévez-Sarmiento et al. 2017). Briefly, cells were cultured in the presence of increasing concentrations of compounds for 72 h and the IC50 values were determined graphically for each experiment by a nonlinear regression using the curve-fitting routine of the computer software Prism™ 4.0 (GraphPad) and the equation derived by DeLean et al. (1978). Values are means ± SE from at least three independent experiments, each performed in triplicate. Compounds were dissolved in DMSO and kept under dark conditions at 25 °C. Before each experiment, compounds were dissolved in culture medium at 37 °C and the final concentration of DMSO did not exceed 0.2% (v/v).
Results and discussion
Phytochemical study of the ethanol extract of the aerial part of A. dictyoneura led to the isolation of four new ent-labdane diterpenes: dictyolabdans A–D (1–4) (Fig. 1). Along with two known labdane derivatives: 2β,3α,15-trihydroxy-ent-labd-7-ene (5) and 2β,3α-trihydroxy-ent-labd-7-en-15-oic acid (6) (Zdero et al. 1990), and six known flavonoids: rhamnocitrin (Scio et al. 2003), quercetin 3,7-dimethylether (Yang et al. 1990), pinocembrin (Aboushoer et al. 2010), quercetin 3-methylether (Wang et al. 2010), penduletin (Sy and Brown 1998), and betuletol 3-methylether (Horie et al. 1998). The structures of the known compounds were identified by comparison of their spectroscopic data with those reported in the literature.
Compound 1 was obtained as a white amorphous solid. The IR spectrum revealed the presence of a hydroxyl group (3401 cm−1), a carboxylic group (2969 cm−1), and olefinic functions (1647 cm−1). Its molecular formula was assigned as C20H32O4, based on its HRESIMS (m/z 359.2175 [M + Na]+, calcd. 359.2198). The 1H NMR spectrum of 1 (Table 1) showed the presence of five methyls at quaternary carbons at δH 0.83, 0.87, 0.99, 1.76, 1.93 p.p.m; two oxygenated methines at δH 2.93 (d, J = 9.7 Hz, H-3) and 3.62 (ddd, J = 4.0, 9.5, 12.0 Hz, H-2), and two olefinic methines at δH 5.40 (br s, H-7) and 5.64 (s, H-14). The 13C NMR (Table 2) and DEPT spectra of 1 indicated the presence of five methyl groups, four methylene carbons, six methine carbons, two quaternary carbons, two disubstituted olefinic carbons, and a carbonyl carbon. Four olefinic signals were detected at δC 117.4 (C-14), 123.1 (C-7), and 136.1 (C-8), 161.5 (C-13), thus suggesting two trisubstituted double bonds. Both 1H and 13C NMR spectral data of compound 1 were close to the labdane-type diterpene: 2,18-dihydroxylabda-7,13(E)-dien-15-oic acid (Wabo et al. 2012). NMR assignments were carried out based on two-dimensional (2D) NMR data. Thus, COSY experiment disclosed three partial structures: CH2CH(O)CH(O), CHCH2CH, and CHCH2CH2, corresponding to the C-1, C-2, C-3; C-5, C-6, C-7; and C-9, C-11, C-12 fragments, respectively. HMBC experiment (Fig. 2) showed a cross peak between proton at δH 2.93 (d, J = 9.7 Hz, H-3) and the gem dimethyl carbons at δC 16.8 (C-18) and 22.3 (C-19), as well as, signals at δC 46.9 (C-1) and 51.6 (C-5), confirmed the location of hydroxyl groups on C-2 and C-3 carbons. The coupling constant between the protons H-3 (d, J = 9.7 Hz) and H-2 (ddd, J = 4.0, 9.5, 12.0 Hz) suggested the trans disposition of the hydroxyl groups, as reported in ent-labdanes by Zdero et al. (1990). The presence of two secondary OH groups was confirmed by acetylation of 1 with acetic anhydride and pyridine, yielding the diacetyl derivative 1a (Tables 1 and 2). The relative stereochemistry of the vicinal hydroxyls was determined based on the NOESY experiment of 1a; correlation between the signals of the protons at δH 1.35 (dd, J = 6.5, 9.6 Hz, H-5) and δH 4.68 (d, J = 10.3 Hz, H-3), clearly indicated that these protons are on the same face, having a β configuration. This implies an α configuration for the proton at H-2, supporting the correlation of δH 5.04 (ddd, J = 4.4, 10.3, 12.4 Hz, H-2) and the protons for CH3-18 (δH 0.90, s) and CH3-20 (δH 0.82, s). Pertinent cross peak correlations observed between CH3-20 (δH 0.82, s) and CH2-11 (δH 1.49 m; 1.30 m), suggested the β-orientation of the proton at C-9. The double bond at C-13 was determined as Z based on the observed correlation between the CH3-16 (δH 1.85, d, J = 1.4 Hz) and the proton H-14 (δH 5.59, d, J = 1.4 Hz) in the NOESY experiment. The absolute configuration of the ent labdane series was deduced from comparison between the negative sign of specific rotation of 1 [α]20D = −9.0 (CHCl3) with the value for 2β,3α,15-trihydroxy-ent-labd-7-ene [α]24D = −29.0 (CHCl3), isolated from Baccharis pingraea (Zdero et al. 1990). The above results confirmed the structure of 1 as (2 S,3 S,13Z)-2,3-dihydroxy-ent-labd-7,13-dien-15-oic acid, which we first named this series as dictyolabdan A.
Dictyolabdan B (2) was isolated as a yellow oil, with [α]20D = −35.9 (CHCl3). The molecular formula was determined as C25H35O5 by HRESIMS analysis (m/z 441.2606 [M + Na]+, calcd. 441.2617). The 13C NMR (Table 2) and DEPT spectra indicated the presence of seven methyl groups, four methylene groups, seven methine groups, two quaternary carbons, three di-substituted olefinic carbons, and two carbonyl carbons. The 1H NMR (Table 1) showed the presence of two additional methyls at δH 1.91 (dd, J = 1.6, 7.1 Hz, CH3-4′) and 1.83 (t, J = 1.6 Hz, CH3-5′), together with the signal at δH 5.99 (ddd, J = 1.6, 7.2, 8.8 Hz, CH-3′) suggested the presence of angeloyloxy group. Both the 1H and 13C NMR (Tables 1 and 2) spectral data of compound 2 were very similar to those of 1 and revealed that the two compounds are closely related in structure. The major difference is that 2 contains an additional angeloyl ester. The position of the angeloyl group was defined according to the low-field shift of the proton at δH 4.97 (ddd, J = 4.3, 10.0, 11.6 Hz, H-2) and the HMBC correlation between the signal at δH 4.97 and the signal at δC 168.8, corresponding to the carbonyl C-1′. Correlations in the NOESY experiment of 2 suggested the same relative stereochemistry as compound 1, leading to the conclusion that compound 2 is (2 S,3 S,13Z)-2-angeloyloxy-3-hydroxy-ent-labd-7(8),13(14)-dien-15-oic acid.
Compound 3 was isolated as a yellow oil whose IR absorption bands at 1730 and 1714 cm-1 indicated the presence of carbonyl groups. The molecular formula of 3 was determined as C27H40O6, with an additional acetyl group compared to compound 2, on the basis of its HRESIMS positive data (m/z 483.2741 [M + Na]+). Both the 1H and 13C NMR (Tables 1 and 2) spectral data of compound 3 were close to those of 2, the differences were evident between these compounds since the the proton at C-3 was low-field shift to δH 4.80 (dd, J = 3.3, 10.4 Hz, H-3), suggesting the presence of an acetyloxy group attached to C-3. Thus, treatment of 2 with Ac2O-pyridine gave a monoacetyl derivative whose physical and spectroscopic constants were identical with those of 3 and was determined as (2 S,3 S,13Z)-2-angeloyloxy-3-acetyloxy-ent-labd-7(8),13(14)-dien-15-oic acid, named as dictyolabdan C.
Dictyolabdan D (4) was obtained as a yellow oil and showed a molecular formula C25H38O6 assigned by HRESIMS (m/z 433.2574 [M-H]−, calcd. 433.2590). The IR spectrum of 4 showed absoption bands at 3434 cm−1 (hydroxyl group), and 1721 cm−1 (carbonyl group). The 1H NMR spectrum of 4 (Table 1) showed the presence of six methyl groups at δH 1.99 (dd, J = 1.5, 7.2 Hz), 1.90 (t, J = 1.5 Hz), 1.89 (d, J = 1.1 Hz), 1.05 (s), 0.86 (s), and 0.80 (s). The 13C NMR (Table 2) and DEPT experiments showed the presence of six methyls, five methylene carbons (including four aliphatic and one exomethylene), seven methine carbons (three oxygenated and one olefinic), and seven singlet carbons. The 1H and 13C NMR data were similar to those of compound 2. Significant differences between both compounds were the presence of a hydroxymethine at δC 73.8 (C-7) and exocyclic methylene at δC 111.2 (C-17) in 4, instead of the trisubstituted double bond for 2. HMBC correlations (Fig. 3) between the carbon at δC 111.2 (C-17) and the proton at δH 4.44 (t, J = 2.9 Hz, H-7), and between the carbon at δC 147.6 (C-8) and the protons at δH 2.16 (H-9), 1.86 (H-6β), and 1.58 (H-6α), confirmed the presence of a –CH2CH(OH)C(CH2)CH– partial structure. The resonance assigned to H-7 (t, J7-6 = 2.9 Hz) indicated approximately equal coupling values for the protons at C-6. Their values are in particular agreement with the average conformation of 55° dihedral angle, calculated from a molecular model for those three nuclei. Comparison of the coupling constants and the chemical shift of 4, with those reported for ent-labdanes isolated from Andrographis paniculata (Chen et al. 2008). Along with, the lack of correlation in the NOESY experiment (Fig. 3) between the proton at H-7 and the methyls at CH3-18, CH3-20, as well as the protons at H-5 and H-9 suggested a β-orientation for the hydroxyl group. The relative configuration of compound 4 was assigned with reference to the NOESY spectrum (Fig. 3) by analogy with the compounds 1 and 2. From this data, the new compound 4 was deduced to be (2 S,3 S,7 S,13Z)-2-angeloyloxy-3,7-dihydroxy-ent-labd-13(14)-en-15-oic acid.
Reinvestigation (Castillo et al. 2015) of non-polar fractions of the ethanolic extract of A. illita led us to the isolation of two sesquiterpene lactones compound 7, and eupatoriopicrin 8 (Drożdż et al. 1972), as well as 7-hydroxytoxol (Zhou et al. 2013).
Compound 7, a colorless oil, showed a molecular formula C15H20O3, assigned by HRESIMS (m/z 271.1310] [M + Na]+, calcd. 248.1412). The IR spectrum showed absorption bands at 3469 cm−1 (OH group), 1756 cm−1 (γ-lactone), and 1650, 1612 cm−1 (olefinic functions). The 1H NMR of 7 showed the presence of four olefinic methylene protons at δH 4.86 (d, J = 1.5 Hz, H-15a), 4.95 (d, J = 1.5 Hz, H-15b), 5.50 (d, J = 3.1 Hz, H-13a), 6.22 (d, J = 3.1 Hz, H-13b), and two oxygenated methines at δH 5.54 (dd, J = 11.0 Hz, H-6), and 4.60 (dd, J = 2.7, 5.4 Hz, H-8). The 13C NMR and DEPT data indicated the presence of one methyl carbon, six methylene groups (including two exomethylene double bonds), four methine carbons, one quaternary carbon, two di-substituted olefinic carbons, and one carbonyl group corresponding to a γ-lactone. The connectivities were established by analysis of its COSY experiment. Careful study of the 2D NMR and comparison with the partially reported 1H NMR data for 8β-hydroxy-β-cyclocostunolide (Jakupovic et al. 1988) led us to the conclusion that compound 7 could be identified as (8R)-8-hydroxy-β-cyclocostunolide. Complete 1H and 13C spectroscopic data of 7 is reported for the first time.
We were also interested in determining whether some of the isolated compounds displayed cytotoxic properties against U-937 cells. This cell line is a useful model to study the cell growth inhibition of leukemia cells by chemical, physical, and physiological agents. Antiproliferative studies in the U-937 cells of the new labdane diterpenoids 1–3 as well as, the known sesquiterpene lactone 7 and the flavonoid quercetin 3,7-dimethylether were assessed and it was observed that the latter compounds were the most cytotoxic compounds. Treatment with both compounds resulted in a dose-dependent inhibition of cellular proliferation. The IC50 values (the concentration that induces a 50% inhibition of cell growth) against U-937 cells were 6.0 ± 0.7 µM and 7.0 ± 0.5 µM for 8β-hydroxy-β-cyclocostunolide 7 and quercetin 3,7-dimethylether, respectively. However, the naturally occurring labdane diterpenoids 1–3 did not display any potent cytotoxic properties (IC50 >10 µM). The antitumor agent etoposide was used as a positive control (IC50 = 1.5 ± 0.2 µM).
References
Aboushoer MI, Fathy HM, Abdel-Kader MS, Goetz G, Omar AA (2010) Terpenes and flavonoids from Egyptian collection of Cleome droserifolia. Nat Prod Res 24:687–696
Castillo QA, Triana J, Eiroa JL, Calcul L, Rivera E, Wojtas L, Padrón JM, Boberieth L, Keramane M, Abel-Santos E, Báez LA, Germosén EA (2016) Ent-labdane diterpenoids from aerial parts of Eupatorium obtusissmum. J Nat Prod 79:907–913
Castillo QA, Triana J, Eiroa JL, Padrón JM, Plata GB, Abel-Santos EV, Báez LA, Rodríguez DC, Jiménez MA, Pérez-Pujols MG (2015) Flavonoids from Eupatorium illitum and their antiproliferative activities. Pharmacogn J 7:178–181
Chen L, Zhu H, Wang R, Zhou K, Jing Y, Qiu F (2008) Ent-labdane diterpenoids lactone stereoisomers from Andrographis paniculata. J Nat Prod 71:852–855
Chen YL, Kawahara T, Hind DNJ (2011) Eupatorieae. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China, vol 20–21. Science press-Missouri Botanical Garden Press, Beijing-St Louis, pp 879–891
DeLean A, Munson PJ, Rodbard D (1978) Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol 235:E97–E102
Drożdż B, Grabarczyk H, Samek Z, Holub M, Herout V, Šorm F (1972) On terpenes. CCXVI. Sesquiterpenic lactones from Eupatorium cannabinum L., revision of the structure of eupatoriopicrin. Collect Czech Chem Commun 37:1546–1554
Estévez-Sarmiento F, Said M, Brouard I, León F, García C, Quintana J, Estévez F (2017) 3′-Hydroxy-3,4′-dimethoxyflavone blocks tubulin polymerization and is a potent apoptotic inducer in human SK-MEL-1 melanoma cells. Bioorg Med Chem 25:6060–6070
Herz W (2001) Chemistry of eupatoriinae. Biochem Syst Ecol 20:1115–1137
Horie T, Ohtsuru Y, Shibata K, Yamashita K, Tsukayama M, Kawamura Y (1998) 13C NMR spectral assignment of the A-ring of polyoxygenated flavones. Phytochemistry 47:865–874
Jakupovic L, Lehmann L, Bohlmann F, King RM, Robinson H (1988) Sesquiterpene lactones and other constituents from Cassinia, Actinobole and Anaxeton species. Phytochemistry 27:3831–3839
King R, Robinson H (1970) Studies in the Eupatoriae (Compositae). XIX. New combinations in Ageratina. Phytologia 29:208–229
King RM, Robinson H (1987) The genera of the Eupatorieae (Asteraceae), Monogr Syst Bot Missouri Bot Gard, vol 22. Allen Press Inc., Lawrence, Kansas, USA
Liu PY, Liu D, Li WH, Zhao T, Sauriol F, Gu YC, Shi QW, Zhang ML (2015) Chemical constituents of plants from genus Eupatorium (1904-2014). Chem Biodivers 12:1841–1515
Ma QP, Cheng CR, Li XF, Liang XY, Ding J (2015) Chemistry, pharmacological activities and analysis of Ageratina adenophora. Asian J Chem 27:4311–4316
Pruski JF, Clase TG (2012) Studies of Neotropical compositae-VI. New species of Eupatorieae from Belize. Hispaniola Peru Phytoneuron 32:1–15
Rivera VL, Panero JL, Schilling EE, Crozier BS, Moraes MD (2016) Origins and recent radiation of Brazilian eupatorieae (Asteraceae) in the eastern cerrado and atlantic forest. Mol Phylogenet Evol 97:90–100
Scio E, Ribeiro A, Alves TMA, Romanha AJ, Filho JDdS, Cordell GA, Zani CL (2003) Diterpenes from Alomia myriadenia (Asteraceae) with cytotoxic and trypanocidal activity. Phytochemistry 64:1125–1131
Sy L, Brown GD (1998) Three sesquiterpenes from Artemisia annua. Phytochemistry 48:1207–1211
Triana J, Eiroa JL, Morales M, Perez FJ, Brouard I, Quintana J, Ruiz-Estévez M, Estévez F, León F (2016) Sesquiterpenoids isolated from two species of the Asteriscus alliance. J Nat Prod 79:1292–1297
Wabo HK, Chabert P, Tane P, Noté O, Tala MF, Peluso J, Muller C, Kikuchi H, Oshima Y, Lobstein A (2012) Labdane-type diterpenes and flavones from Dodonaea viscosa. Fitoterapia 83:859–863
Wang J, Gao H, Zhao J, Wang Q, Zhou L, Han J, Yu Z, Yang F (2010) Preparative separation of phenolic compounds from Halimodendron halodendron by high-speed counter-current chromatography. Molecules 15:5998–6007
Yang SL, King RA, Roberts MF (1990) The flavonoids of Ageratina deltoidea. Biochem Syst Ecol 18:485–486
Zdero C, Bohlmann F, Niemeyer HM (1990) Ent-labdane glycosides from Baccharis pingraea. Phytochemistry 29:2611–2616
Zhou ZY, Liu WX, Pei G, Ren H, Wang J, Xu QL, Xie HH, Wan FH, Tan JW (2013) Phenolics from Ageratina adenophora roots and their phytotoxic effects on Arabidopsis thaliana seed germination and seedling growth. J Agric Food Chem 61:11792–11799
Acknowledgements
This research was partially supported by the Ministerio de Educación Superior, Ciencia y Tecnología (MESCYT), Dominican Republic, FONDOCYT Program, under grants 2009-2C3-016 and 2013-1D4-003.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
In memoriam: Professor Dr. Francisco J. Pérez.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Eiroa, J.L., Triana, J., Pérez, F.J. et al. Secondary metabolites from two Hispaniola Ageratina species and their cytotoxic activity. Med Chem Res 27, 1792–1799 (2018). https://doi.org/10.1007/s00044-018-2192-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2192-y