An effective strategy has been developed for the preparation of isoxazole-containing thiadiazepine derivatives via the reaction of 1,3-diallyl sulfamide with the corresponding 3-aryl-5-bromomethylisoxazoles using ring-closing metathesis as the key synthetic step. This route grants access to such thiadiazepine derivatives which cannot be synthesized by other methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sulfamides, being a well-known class of synthetic bacteriostatic antibiotics, exhibit the most diverse biological activity.1,2,3 Some of them have been popular commercial drugs for many years that have not lost their relevance even today.4,5,6,7,8 Sulfamide functional group can be found in a number of drugs that are currently marketed or being developed for the treatment of a wide range of diseases. Cyclic sulfamides are also promising compounds with great potential for use in medicinal chemistry. HIV-1 proteinase inhibitors9,10 were found among N,N'-disubstituted thiadiazepine derivatives, some of them having already been patented as antiHIV agents.11 The growing interest in cyclic sulfamides stimulates the development of new synthetic approaches, including those employing ringclosing metathesis.12,13,14 Metathesis reactions are one of the promising modern methods of constructing molecules that are very difficult or impossible to obtain in other ways. Given the constant need to search for new potentially biologically active substances, as well as the diverse biological activity of isoxazole derivatives,15,16,17,18,19 the development of convenient and effective methods for the synthesis of compounds of this class is an important and urgent task.
We published a method for the preparation of novel isoxazole-containing thiadiazepines from the corresponding aryl-substituted isoxazolylamines.20 However, given the difficulty in synthesizing the starting amines, an alternative route was developed for the synthesis of compounds of this type from the much more accessible arylisoxazole bromo derivatives. This made it possible to significantly expand the synthesis capabilities of new compounds that could not be obtained by the previously described method.20
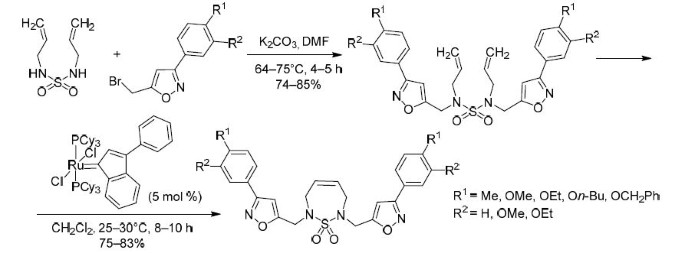
To this end, 1,3-diallyl sulfamide (2)21 was synthesized via the previously undescribed reaction of allylamine (1) with sulfuryl chloride in the presence of Et3N (Scheme 1). The reaction was carried out in dry CH2Cl2 solution in the presence of Et3N at a temperature of about 0°C for 1.5–2 h. The target 1,3-diallyl sulfamide (2) was obtained in 75% yield. Further, 1,3-diallyl sulfamide (2) was alkylated at both nitrogen atoms by the reaction with 2.2 equiv of the corresponding 3-aryl-5-bromomethylisoxazole 3a–e (10% excess) in a DMF solution at temperature of 64–75°С in the presence of 2.3 equiv of K2CO3 (15% excess). Diallyl derivatives 4a–e were isolated in 74–85% yields. Their structure was confirmed by chromato-mass spectrometry, 1H, 13C NMR spectroscopy, and elemental analysis.

Scheme 1
Metathesis reactions with the ring closing of derivatives 4a–e was carried out in dry degassed CH2Cl2 solution under an atmosphere of dry argon at temperature of 25–30°C for 8–10 h in the presence of bis(tricyclohexylphosphine) phenylindenylidene ruthenium complex, which was synthesized according to a literature method.22 The reaction products 5a–e were isolated after chromatographic purification in 75–83% yields. Their structures were confirmed by liquid chromato-mass spectrometry, 1H, 13C NMR spectroscopy data and elemental analysis. The formation of a thiadiazepine ring is indicated by the absence in the 1H NMR spectra of the signals of the four protons of the terminal =СН2 groups at 5.33 ppm and the signals of the two allyl groups –CH= in the 5.75–5.90 ppm range, which are observed in the spectra of the starting compounds, as well as the appearance of a specific signal in the 3.88–3.91 ppm range characteristic of protons of the CH2 group of the thiadiazepine ring. The 13C NMR spectra of compounds 5a–e contain a signal at 128.2 ppm characteristic of sp2-hybridized carbon atoms of the sevenmembered ring, and there are no signals of carbon atoms of the terminal =CH2 groups at 120.7 ppm that are present in the spectra of the starting diallyl derivatives 4a–e.
To conclude, we have developed a more convenient and efficient strategy for the preparation of isoxazolecontaining thiadiazepine derivatives using ring-closure metathesis allowing one to access derivatives that cannot be synthesized by the methods described previously.
Experimental
1H and 13C NMR spectra were acquired on a Bruker Avance DRX-500 spectrometer (500 and 125 MHz, respectively) in СDCl3, with TMS as internal standard. Liquid chromato-mass spectra were recorded on an Agilent 1100 Series LC-MS system equipped with an Agilent LC/MSD SL diode array mass selective detector, atmospheric pressure electrospray ionization. Zorbax SB-C18, 1.8 μm, 4.6 × 15 mm column; eluents: а) MeCN–H2O, 95:5, 0.1% CF3CO2H, b) 0.1% aqueous CF3CO2H; 3 ml/min flow; 1 μm infection volume; 215, 254, 285 nm detection wavelengths. The carbon and hydrogen contents were determined using the Pregl's gravimetric method, nitrogen content was determined using the Dumas method, and sulfur was determined using the Schöniger method.23 Merck Grade 9385, 60 Å, 230–400 mesh silica gel was used for column chromatography, eluent CH2Cl2.
Commercially available reagents and solvents were used, 3-aryl-5-bromomethylisoxazoles 3a–e were synthesized following a published method.24
N,N'-Diallyl sulfamide (2).21 A solution of sulfuryl chloride (0.01 mol) in CH2Cl2 (15 ml) was added to a solution of allylamine (1) (0.02 mol) and Et3N (0.02 mol) in CH2Cl2 slowly, dropwise at –5–0°С over 1.5–2 h. After adding all sulfuryl chloride, the mixture was stirred for 1.5–2 h at room temperature. After completion of the reaction, the reaction mixture was poured into cold H2O and extracted with CH2Cl2. The reaction product was purified by column chromatography on silica gel. The analytical data of the compounds were identical to those described in the literature.
Synthesis ofN,N'-diallyl-N,N'-bis[(3-arylisoxazol-5-yl)methyl]sulfamides 4a–е (General method). With vigorous stirring, K2CO3 (0.0115 mol) and the corresponding 3-aryl-5-bromomethylisoxazole 3а–е (0.0011 mol) were added to a solution of 1,3-diallyl sulfamide (2) (0.005 mol) in DMF (25–30 ml). The reaction mixture was stirred at 65–75°С for 4–5 h. The solvent was removed under reduced pressure, the residue was washed with H2O, extracted with CH2Cl2 (2×15 ml). The extract was dried over anhydrous Na2SO4, the product was isolated by column chromatography on silica gel, and recrystallized from aqueous EtOH.
N,N'-Diallyl-N,N'-bis{[3-(4-methylphenyl)isoxazol-5-yl]-methyl}sulfamide (4а). Yield 2.07 g (80%), white crystals, mp 97–98°С. 1H NMR spectrum, δ, ppm (J, Hz): 2.38 (6Н, s, 2CH3); 3.83 (4Н, d, 3J = 6.5, 2CH2); 4.50 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.24–5.34 (4H, m, 2СН2=); 5.75–5.88 (2Н, m, 2–СН=); 6.54 (2Н, s, Н isoxazole); 7.22 (4Н, d, 3J = 8.5, H Ar); 7.65 (4Н, d, 3J = 8.5, H Ar). 13C NMR spectrum, δ, ppm: 21.4; 41.9; 50.9; 101.8; 120.7; 125.8; 126.7; 129.6; 131.8; 140.3; 162.6; 168.0. Mass spectrum, m/z: 519 [M+Н]+. Found, %: С 64.80; H 5.85; N 10.77; S 6.23. C28H30N4O4S. Calculated, %: С 64.84; H 5.83; N 10.80; S 6.18 .
N,N'-Diallyl-N,N'-bis{[3-(4-butoxyphenyl)isoxazol-5-yl]methyl}sulfamide (4b). Yield 2.59 g (82%), white crystals, mp 90–91°С. 1H NMR spectrum, δ, ppm (J, Hz): 1.00 (6Н, t, 3J = 7.0, 2СН3); 1.48–1.56 (4H, m, 2СН2); 1.77–1.83 (4Н, m, 2СН2); 3.86 (4Н, d, 3J = 6.5, 2CH2); 4.01 (4H, t, 3J = 7.0, 2CH2); 4.52 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.28–5.37 (4H, m, 2СН2=); 5.81–5.89 (2Н, m, 2–СН=); 6.53 (2Н, s, Н isoxazole); 6.94 (4Н, d, 3J = 8.5, H Ar); 7.71 (4H, d, 3J = 8.5, H Ar). 13C NMR spectrum, δ, ppm: 13.8; 19.2; 31.2; 41.9; 50.9; 67.8; 101.6; 114.8; 120.6; 120.9; 128.2; 131.8; 160.7; 162.2; 167.9. Mass spectrum, m/z: 635 [M+Н]+. Found, %: С 64.32; H 6.65; N 8.84; S 5.07. C34H42N4O6S. Calculated, %: С 64.33; H 6.67; N 8.83; S 5.05.
N,N'-Diallyl-N,N'-bis{[3-(4-benzyloxyphenyl)isoxazol-5-yl]methyl}sulfamide (4c). Yield 2.98 g (85%), white crystals, mp 116–117°С. 1H NMR spectrum, δ, ppm (J, Hz): 3.85 (4Н, d, 3J = 6.5, 2CH2); 4.51 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.11 (4H, s, 2OCH2); 5.28–5.35 (4H, m, 2СН2=); 5.78–5.90 (2Н, m, 2–СН=); 6.53 (2Н, s, Н isoxazole); 7.03 (4Н, d, 3J = 8.5, H Ar); 7.33–7.45 (10Н, m, H Ar); 7.72 (4H, d, 3J = 8.5, H Ar). 13C NMR spectrum, δ, ppm: 41.9; 50.9; 70.1; 101.7; 115.3; 120.7; 121.4; 127.5; 128.2; 128.3; 128.7; 131.9; 136.5; 160.3; 162.2; 168.0. Mass spectrum, m/z: 703 [M+Н]+. Found, %: С 68.39; H 5.40; N 7.94; S 4.61. C40H38N4O6S. Calculated, %: С 68.36; H 5.45; N 7.97; S 4.56.
N,N'-Diallyl-N,N'-bis{[3-(3,4-dimethoxyphenyl)isoxazol-5-yl]methyl}sulfamide (4d). Yield 2.26 g (74%), white crystals, mp 108–109°С. 1H NMR spectrum, δ, ppm (J, Hz): 3.84 (4Н, d, 3J = 8.0, 2CH2); 3.89 (6Н, br. s, 2ОCH3); 3.90 (6Н, s, 2ОCH3); 4.49 (4H, s, 2CH2NSO2); 5.24–5.33 (4H, m, 2СН2=); 5.75–5.87 (2Н, m, 2–СН=); 6.54 (2Н, s, Н isoxazole); 6.86 (2Н, d, 3J = 10.5, H Ar); 7.23 (2Н, d, 3J = 10.5, H Ar); 7.36 (2Н, s, H Ar). 13C NMR spectrum, δ, ppm: 41.9; 50.9; 55.9; 56.0; 101.7; 109.3; 111.1; 120.0; 120.7; 121.3; 131.8; 149.3; 150.8; 162.3; 168.0. Mass spectrum, m/z: 611 [M+Н]+. Found, %: С 59.04; H 5.60; N 9.15; S 5.24. C30H34N4O8S. Calculated, %: С 59.00; H 5.61; N 9.17; S 5.25.
N,N'-Diallyl-N,N'-bis{[3-(3,4-diethoxyphenyl)isoxazol-5-yl]methyl}sulfamide (4e). Yield 2.53 g (76%), white crystals, mp 89–90°С. 1H NMR spectrum, δ, ppm (J, Hz): 1.39–1.49 (12H, m, 3,4-OCH2CH3); 3.83 (4Н, d, 3J = 8.0, 2CH2); 4.06–4.15 (8H, m, 3,4-OCH2CH3); 4.49 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.24–5.33 (4H, m, 2СН2=); 5.75–5.87 (2Н, m, 2–СН=); 6.50 (2Н, s, Н isoxazole); 6.86 (2Н, d, 3J = 10.0, H Ar); 7.21 (2Н, d, 3J = 10.0, H Ar); 7.36 (2Н, s, H Ar). 13C NMR spectrum, δ, ppm: 14.8; 41.9; 50.8; 64.5; 64.7; 101.7; 111.3; 112.9; 120.0; 120.6; 121.2; 131.8; 149.0; 150.5; 162.4; 167.9. Mass spectrum, m/z: 667 [M+Н]+. Found, %: С 61.20; H 6.33; N 8.42; S 4.82. C34H42N4O8S. Calculated, %: С 61.24; H 6.35; N 8.40; S 4.81.
Synthesis of 2,7-bis[(3-arylisoxazol-5-yl)methyl]-2,3,6,7-tetrahydro-1,2,7-thiadiazepine 1,1-dioxides 5a–е (General method). Ruthenium carbene catalyst (0.028–0.03 mmol) was added under an argon atmosphere to a solution of diallyl derivative 4а–е (0.6 mmol) in dry, degassed CH2Cl2 (15 ml). The mixture was kept at 25–30°С for 8–10 h. After completion of the reaction, the target products were isolated from the reaction mixture by column chromatography on silica gel. They were recrystallized from 70% aqueous EtOH.
2,7-Bis{[3-(4-methylphenyl)isoxazol-5-yl]methyl}-2,3,6,7-tetrahydro-1,2,7-thiadiazepine 1,1-dioxide (5a). Yield 0.24 g (82%), white crystals, mp 165–166°С. 1H NMR spectrum, δ, ppm (J, Hz): 2.40 (6Н, s, 2CH3); 3.91 (4Н, s, 2CH2); 4.61 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.85 (2Н, s, 2–СН=); 6.62 (2Н, s, Н isoxazole); 7.26 (4Н, d, 3J = 8.0, H Ar); 7.69 (4Н, d, 3J = 8.0, H Ar). 13C NMR spectrum, δ, ppm: 21.4; 44.7; 45.2; 101.4; 125.7; 126.7; 128.2; 129.6; 140.4; 162.6; 168.2. Mass spectrum, m/z: 491 [M+Н]+. Found, %: С 63.63; H 5.36; N 11.40; S 6.57. C26H26N4O4S. Calculated, %: С 63.66; H 5.34; N 11.42; S 6.54.
2,7-Bis{[3-(4-butoxyphenyl)isoxazol-5-yl]methyl}-2,3,6,7-tetrahydro-1,2,7-thiadiazepine 1,1-dioxide (5b). Yield 0.23 g (75%), white crystals, mp 117–118°С. 1H NMR spectrum, δ, ppm (J, Hz): 0.98 (6Н, t, 3J = 9.0, 2СН3); 1.45–1.55 (4H, m, 2СН2); 1.74–1.83 (4Н, m, 2СН2); 3.90 (4Н, s, 2CH2); 4.00 (4H, t, 3J = 9.0, 2CH2); 4.59 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.84 (2H, s, 2=СН–);6.58 (2Н, s, Н isoxazole); 6.95 (4Н, d, 3J = 11.0, H Ar); 7.71 (4H, d, 3J = 11.0, H Ar). 13C NMR spectrum, δ, ppm: 13.8; 19.2; 31.2; 44.7; 45.2; 67.8; 101.3; 114.9; 120.9; 128.2; 128.3; 160.8; 162.4; 168.0. Mass spectrum, m/z: 607 [M+Н]+. Found, %: С 63.32; H 6.32; N 9.24; S 5.29. C32H38N4O6S. Calculated, %: С 63.35; H 6.31; N 9.23; S 5.28.
2,7-Bis{[3-(4-benzyloxyphenyl)isoxazol-5-yl]methyl}-2,3,6,7-tetrahydro-1,2,7-thiadiazepine 1,1-dioxide (5с). Yield 0.31 g (78%), white crystals, mp 176–177°С. 1H NMR spectrum, δ, ppm (J, Hz): 3.88 (4Н, s, 2CH2); 4.58 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.10 (4H, s, 2OCH2); 5.82 (2Н, s, 2–СН=); 6.57 (2Н, s, Н isoxazole); 7.03 (4Н, d, 3J = 8.5, H Ar); 7.30–7.47 (10Н, m, H Ar); 7.72 (4H, d, 3J = 8.5, H Ar). 13C NMR spectrum, δ, ppm: 44.7; 45.2; 70.1; 101.3; 115.3; 121.4; 127.5; 128.1; 128.3; 128.7; 136.5; 160.3; 162.3; 168.1. Mass spectrum, m/z: 675 [M+Н]+. Found, %: С 67.68; H 5.06; N 8.32; S 4.71. C38H34N4O6S. Calculated, %: С 67.64; H 5.08; N 8.30; S 4.75.
2,7-Bis{[3-(3,4-dimethoxyphenyl)isoxazol-5-yl]methyl}-2,3,6,7-tetrahydro-1,2,7-thiadiazepine 1,1-dioxide (5d). Yield 0.26 g (76%), white crystals, mp 157–158°С. 1H NMR spectrum, δ, ppm (J, Hz): 3.89 (4Н, d, 3J = 2.0, 2CH2); 3.91 (6Н, br. s, 2ОCH3); 3.93 (6Н, s, 2ОCH3); 4.49 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.83 (2Н, s, 2–СН=); 6.59 (2Н, s, Н isoxazole); 6.90 (2Н, d, 3J = 10.0, H Ar); 7.27 (2Н, d, 3J = 10.0, H Ar); 7.34 (2Н, s, H Ar). 13C NMR spectrum, δ, ppm: 44.8; 45.2; 56.0; 56.1; 101.3; 109.3; 111.1; 120.0; 121.3; 128.3; 149.4; 150.8; 162.5; 168.2. Mass spectrum, m/z: 583 [M+Н]+. Found, %: С 57.76; H 5.22; N 9.60; S 5.45. C28H30N4O8S. Calculated, %: С 57.72; H 5.19; N 9.62; S 5.50.
2,7-Bis{[3-(3,4-diethoxyphenyl)isoxazol-5-yl]methyl}-2,3,6,7-tetrahydro-1,2,7-thiadiazepine 1,1-dioxide (5e). Yield 0.32 g (83%), white crystals, mp 120–121°С. 1H NMR spectrum, δ, ppm (J, Hz): 1.42–1.48 (12H, m, 3,4-OCH2CH3); 3.88 (4Н, s, 2CH2); 4.05–4.18 (8H, m, 3,4-OCH2CH3); 4.57 (4H, s, HetCH2N(Allyl)SO2N(Allyl)CH2Het); 5.82 (2Н, s, 2–СН=); 6.56 (2Н, s, Н isoxazole); 6.89 (2Н, d, 3J = 10.0, H Ar); 7.24 (2Н, d, 3J = 10.0, H Ar); 7.36 (2Н, s, H Ar). 13C NMR spectrum, δ, ppm: 14.7; 14.8; 44.7; 45.2; 64.5; 64.7; 101.3; 111.3; 113.0; 120.0; 120.2; 128.3; 149.0; 150.5; 162.5; 168.1. Mass spectrum, m/z: 639 [M+Н]+. Found, %: С 60.20; H 6.02; N 8.74; S 5.00. C32H38N4O8S. Calculated, %: С 60.17; H 6.00; N 8.77; S 5.02.
Elemental analysis was performed in the Analytical chemistry laboratory at the V. P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine.
References
Ahuja, P.; Singh, J.; Asthana, M. B.; Sardana, V.; Anand, N. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1989, 28, 1034.
Jadhav, P. K.; Woerner, F. J. Tetrahedron Lett. 1995, 36, 6383.
Jadhav, P. K.; Daneker, W. F.; Woerner, F. J. US Patent 5506355, CO7D, 1996; Chem. Abstr.1996, 125, 34038h.
Drews J. Science2000, 287, 1960.
Supuran, C. T.; Scozzafava, A. Curr. Med. Chem. – Immunol., Endocr. Metab. Agents2001, 1, 61.
Protease Inhibitors in AIDS Therapy; Ogden, R. C.; Flexner C. W., Eds.; Marcel Dekker: New York, Basel, 2001.
Supuran, C. T. Expert Opin. Invest. Drugs2003, 12, 283.
Supuran, C. T.; Scozzafava, A. Expert Opin. Ther. Pat.2000, 10, 575.
Hultén, J.; Bonham, N. M.; Nillroth, U.; Hansson, T.; Zuccarello, G.; Bouzide, A.; Åqvist, J.; Classon, B.; Danielson, H. U.; Karlén, A.; Kvarnström, I.; Samuelsson, B.; Hallberg, A. J. Med. Chem. 1997, 40, 885.
Hultén, J; Andersson, H. O.; Schaal, W.; Danielson, H. U.; Classon, B.; Kvarnström, I.; Karlén, A.; Unge, T.; Samuelsson, B; Hallberg, A. Med. Chem. 1999, 42, 4054.
Ax, A.; Schaal, W.; Vrang, L.; Samuelsson, B.; Hallberg, A.; Karlén, A. Bioorg. Med. Chem.2005, 13, 755.
Hanson, P. R.; Probst, D. A.; Robinson, R. E.; Yau, M. Tetrahedron Lett. 1999, 40, 4761.
Brown, R. C. D.; Castro, J. L.; Moriggi, J.-D. Tetrahedron Lett. 2000, 41, 3681.
Long, D. D.; Termin, A. P. Tetrahedron Lett. 2000, 41, 6743.
Kenji, T; Yoshiyuki, T; Tsutomu, I; Takashi, K; Toshiyuki, A; Shuichi, S; T. Yoshinori, T; Masafumi, I. EP Patent 2 952 503 A1. 2015.
Kanō, H; Adachi, I; Kido, R; Hirose, K. J. Med. Chem. 1967, 10, 411.
Selvam, C; Jachak, S. M; Tilagavathi, R; Chakraborti, A. K. Bioorg. Med. Chem. Lett.2005, 15, 1793
Talley, J. J.; Brown, D. L.; Carter, J. S., Graneto, M. J.; Koboldt, C. M.; Masferrer, J. L.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S.; Seibert, K. J. Med. Chem.2000, 43, 775.
Ruthu, M.; Pradeepkumar, Y., Madhusudhana chetty, C.; Prasanthi, G.; Reddy, V. J. S. J. Global Trends Pharm. Sci.2011, 2, 55.
Pavliuk, O.; Bezugly, Y.; Kashkovsky, V. Fr.–Ukr. J. Chem.2019, 7, 104.
Fornwald, R. M.; Fritz, J. A.; Wolfe, J. P. Chem.–Eur. J.2014, 20, 8782.
Schanz, H.-J.; Jafarpour, L.; Stevens, E. D.; Nolan, S. P. Organometallics1999, 18, 5187.
Klimova, V. А. Osnovnye mikrometody analiza organicheskikh soedinenii [in Russian]; Moscow: Khimiya, 1975.
Pavliuk, A. V.; Bezugly, Yu. V.; Kashkovsky, V. I. Fundamental'nye i prikladnye issledovaniya v sovremennoi khimii [in Russian]; Sukhoveev, V. V., Ed.; Nizhyn, 2016, p. 98.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(12), 1274–1277
Rights and permissions
About this article
Cite this article
Pavliuk, A.V., Bezugly, Y.V., Sukhoveev, V.V. et al. A convenient method for efficient synthesis of isoxazole-containing thiadiazepine derivatives. Chem Heterocycl Comp 55, 1274–1277 (2019). https://doi.org/10.1007/s10593-019-02612-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02612-4