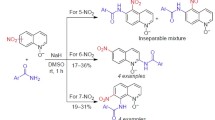
S N H Arylamination of 5-, 6-, 7-, and 8-nitroquinolines in anhydrous DMSO gave not only the arylamino derivatives of the respective nitroquinolines, but also the arylamino derivatives of nitrosoquinolines. In the case of 6-nitroquinoline, the first representatives of polycyclic structures on the basis of pyrido[3,2-a]phenazine 7-oxide were isolated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The quinoline system is often found in nature as a structural motif in a large alkaloid family, many of which, similarly to their synthetic analogs, show a broad spectrum of biological and pharmaceutical effects, including antimalarial, anticancer, antimycobacterial, antimicrobial, anticonvulsant, anti-inflammatory, cardiovascular, and antioxidant activity.1 As it has been pointed out in a recent review article,2 there is a continued interest in searching for new synthetic routes to quinoline derivatives. Current developments of organic chemistry have enabled direct functionalization of C–H bonds in aromatic compounds, in particular the formation of C–N bonds,3 using procedures that are in agreement with the principles of green chemistry and atom economy.4
Reactions involving nucleophilic substitution of hydrogen (SNH) are excellent examples of such procedures, which are generally divided into two types: oxidative and vicarious substitution reactions.5 Both types of reactions proceed through an addition step that results in the formation of σH-adduct, followed by its aromatization in the presence of external oxidant or elimination of simple HX molecules. Such SNH reactions have already been implemented in chemical industry6 and in many cases offer suitable alternatives for transition metal-catalyzed crosscoupling reactions.7
The goal of the current work was to study the possibilities for oxidative SNH arylamination of nitroquinolines containing a nitro group in the benzene ring of the molecule. It is known that quinoline itself can be used in oxidative SNH amination reaction with potassium amide in liquid ammonia – KMnO4 system, forming predominantly 2- or 4-aminoquinolines, depending on the reaction conditions.8 However, the relatively π-electron poor 5-, 6-, and 7-nitroquinolines already do not require KNH2 for the introduction of an NH2 group, because ammonia itself is sufficient as a nucleophile, but the regioselectivity of oxidative amination is determined only by the location of nitro group, regardless of its position in the pyridine or benzene ring of the quinoline molecule.8,9 Vicarious amination using 1,1,1-trimethylhydrazinium iodide as reagent proceeded analogously,10 forming both o- and p-aminonitroquinolines in both cases, with a significant predominance of the former.
However, the amination reaction conditions were not suitable for oxidative SNH arylamination due to the lower nucleophilicity of arylamide anions typically used in these reactions and their high sensitivity toward traditional oxidants. For these reasons, similar reactions are still quite rare. They have been reported for such azines as 5-azacinnoline,11 1,2,4-triazine,12 3-nitropyridine,7,13a 1,3,7-tri-azapyrene,14 as well as some nitroarenes.6,13 Suitable mild oxidants for the oxidation of σH-adducts in these reactions include air oxygen11,14 and nitrobenzene,7 while stable intermediates can be subjected to anodic oxidation.15 In the absence of external oxidant, the NO2 group13 or C=N bond in the ring of substrate molecule12 can also act as hydride ion acceptors.
During arylamination of nitroarenes, the oxidative mechanism (Scheme 1, path a) is accompanied by another route involving the aromatization of σH-adduct through its disproportionation, leading to the respective nitroso compounds (Scheme 1, path b).2,5d Thus, anions of primary arylamines react with nitrobenzenes, forming ortho-σH adducts that under alkaline reaction conditions are converted to N-aryl-2-nitrosoanilines.16a–d It is interesting to note that para-σH-adducts were also formed, but they were oxidized under the reaction conditions to the respective 4-nitrodiarylamines.16b We recently used the example of 3-nitropyridine to demonstrate that the SNH reactions of carbamoylamination17a and arylamination17b proceeded in anhydrous DMSO exclusively at the para position relative to the NO2 group and this did not prevent the formation of mixtures containing nitro and nitroso derivatives. Furthermore, the major arylamination products were specifically p-nitroso compounds.

Scheme 1
Taking into account the aforementioned observations, the interest in oxidative SNH-arylamination of 5-, 6-, 7-, and 8-nitroquinolines was motivated by the expected formation of arylamino derivatives not only on the basis of the respective nitroquinolines, but also nitrosoquinolines.
In the case of reaction between 5-nitroquinoline (1) and aniline, it was found that it was optimal to use 2 equiv of anilide anion for 1 equiv of substrate. The anion was generated by treating aniline solution in anhydrous DMSO with NaH at room temperature. The reaction was complete within 1 h after the addition of 5-nitroquinoline (1), giving a mixture of three products that were separated by chromatography on silica gel. The products were identified as 5-nitro-N-phenylquinolin-8-amine (2a), 5-nitro-N-phenylquinolin-6-amine (3a), and 5-nitroso-N-phenylquinolin-6-amine (4a) with the overall yield of 58% (Scheme 2, Table 1, entry 1). Thus, the nucleophile at the first stage of the reaction added at the ortho and para positions relative to the NO2 group, with the para-σH-adduct further undergoing oxidative aromatization with the formation of nitroamine 2a, while its ortho analog was aromatized in two directions, forming nitroamine 3a and nitrosoamine 4a. Practically the same results were obtained by performing this reaction under argon atmosphere (Table 1, entry 2). This indicates that, similarly to the case of 3-nitropyridine, 17b nitroquinoline 1 exhibited two types of reactivity, serving not only as substrate but also as the oxidant of σH-adducts during the formation of nitroamines 2a and 3a. As a result, their yields were lower and products from the reduction of substrate were observed, which had oligomeric structures according to 1H NMR spectra.

Scheme 2
The anions of other para-substituted anilines, such as p-toluidine, p-anisidine, p-bromoaniline, p-fluoroaniline, and p-trifluoromethylaniline reacted analogously with the formation of the respective compounds 2b–f, 3b–f, and 4b–f (Table 1, entries 3–7). An exception was found in the case of p-nitroaniline, which, similarly to m-nitropyridine,17b formed only nitro products 2g and 3g (entry 8). It is probable that it itself acted as an oxidant toward the respective intermediates. It was shown that o-chloroaniline also did not form nitrosoamine, while the yield of nitroamine 3h reached only 3% (entry 9). In our opinion, this indicated that the ortho substituent in aniline and the nitro group at position 5 of quinoline ring created substantial steric obstacles in the transition state during the formation of the ortho-σH-adduct. Indeed, the presence of two ortho substituents, as in the case of 2,4,6-tribromoaniline, led to the formation of only p-nitroamine 2i (entry 10).
A characteristic feature in 1H NMR spectra of nitrosoamines 4a–f that were acquired in CDCl3 solutions was the pronounced downfield shift of NH proton signals (15.9–16.0 ppm), pointing to a strong intramolecular NH···O=N hydrogen bond. The structure of nitrosoamine 4b was confirmed by X-ray structural analysis (Fig. 1).
8-Nitroquinoline (5) under the same conditions formed a mixture of 5-arylamino-8-nitroquinolines 6a–f and 7-arylamino-8-nitrosoquinolines 7a–f, with the latter as the major products (Scheme 3, Table 2, entries 1–6). The use of p-nitroaniline resulted in a mixture of two nitro compounds 6g and 8, while o-chloroaniline and 2,4,6-tribromoaniline gave only the para-substitution products 6h,i (Scheme 3, Table 2, entries 7–9). Similarly to the case of compounds 4a–f, 1H NMR spectra of nitrosoamines 7a–f in CDCl3 solutions indicated the presence of a strong intramolecular hydrogen bond (the chemical shifts of NHAr protons were in the range of 15.7–15.8 ppm).

Scheme 3
It is known that SNH-arylamination reaction of substrates lacking a free para position relative to the NO2 group, as in para-substituted nitrobenzenes16a or 5-nitroindole,16e produces only the respective o-nitrosoamines or products of their subsequent transformations. Similar disproportionation products were also expected from the arylamination of 6- and 7-nitroquinolines. However, in none of these two cases the respective nitroso compounds could be detected or isolated. 7-Nitroquinoline (9) reacted regio- and chemoselectively at position 8, forming 8-arylamino-7-nitroquinolines 10a–g as the only reaction products in 29–63% yields (Scheme 4).

Scheme 4
6-Nitroquinoline (11) also reacted regioselectively at position 5, forming oxidative arylamination products – 5-arylamino-6-nitroquinolines 12a–g in 23–61% yields (Scheme 5). In several cases we were also able to isolate and identify the minor products of these transformations. Thus, in the case of p-anisidine, 9-methoxypyrido[3,2-a]-phenazine 7-oxide (13c) was obtained in 6% yield, while the use of p-bromo- and p-trifluoromethylanilines led to the formation of 9-bromo-5-[(4-bromophenyl)amino]pyrido-[3,2-a]phenazine 7-oxide (14d) and 9-(trifluoromethyl)-5-{[4-(trifluoromethyl)phenyl]amino}pyrido[3,2-a]phenazine 7-oxide (14f) in 15 and 7% yields, respectively.

Scheme 5
Dedicated experiments showed that nitroamines 12c,d,f under the same reaction conditions were not converted to compounds 13 and 14. However, it is known that N-aryl-2-nitrosoanilines are readily cyclized with the formation of phenazine derivatives.16c,18 Taking this into account, we proposed that the starting materials for the synthesis of products 13 and 14 are the respective 5-arylamino-6-nitrosoquinolines 15, which are formed in small amounts during the arylamination of 6-nitroquinoline (11) and further undergo cyclization, for example, according to Scheme 6.

Scheme 6
It could be expected that the cyclization of nitrosoamines 15 should lead to N-oxides 13 as the final products. However, in two cases out of three they were further arylaminated while leaving the N-oxide functional group unchanged, with the reaction proceeding regioselectively at the benzene ring of phenazine oxide that is fused with pyridine ring. The structure of N-oxide 14f was confirmed by X-ray structural analysis (Fig. 2).
Taking into account the reversibility of the first step involving the addition of nucleophile, such differences in the outcome of SNH arylamination reactions of isomeric nitroquinolines can be explained by the different thermodynamic stability of σH-adducts, as well as by the competition between aromatization reaction rates in the two directions (the kinetic factor). Compared to nitroarenes, the π-electron poor pyridine ring certainly facilitated the addition step, but probably did not substantially improve the stability of the σH-intermediate, since the delocalization of negative charge involving the pyridine nitrogen atom that is possible in the case of 6- and 8-nitroquinolines would simultaneously disrupt the aromaticity of two rings. As a polar aprotic solvent, DMSO does not significantly solvate anionic species, thus enhancing the nucleophilicity of arylamide ions, but without stabilization of anionic intermediates. The strongly polar nature of DMSO promotes the formation of more polar anionic σH-adducts, which probably affects the regioselectivity of the reaction. In our experiments it was impossible to exclude the presence of oxidant, in order to direct the reaction toward the formation of nitroso compounds, since substrate itself acted as an oxidant (Table 1, entry 2).
Thus, the use of arylamine anions as nucleophilic agents in the reaction with 5-, 6-, and 8-nitroquinolines in anhydrous DMSO produced mixtures of arylamino derivatives of nitroquinolines and their nitroso analogs. This result was explained by the aromatization of the respective σH-adducts simultaneously in two directions, which has not been previously observed in nitroquinoline series. The nitroso products in the case of 6-nitroquinoline participated in cyclization process with the formation of pyrido[3,2-a]phenazine 7-oxide derivatives. The use of 7-nitroquinoline led only to 8-arylamino-7-nitroquinolines.
Experimental
1H and 13C NMR spectra (400 and 100 MHz, respectively) were acquired, as well as two-dimensional NMR experiments performed on a Bruker Avance HD 400 instrument for samples in DMSO-d6 or CDCl3 solutions. In the case of DMSO-d6 solutions, the residual solvent signals were used as internal standard (2.50 ppm for 1H nuclei, 40.45 ppm for 13С nuclei).19 TMS was used as internal standard for NMR spectra acquired in CDCl3 solutions. The signals of 13С NMR spectra for key compounds were assigned on the basis of two-dimensional 1Н –13С HMBC and 1H–13C HSQC experiments. High-resolution mass spectra were recorded on a Bruker maXis Impact UHR-TOF mass spectrometer (electrospray ionization). Melting points were determined on a REACH Devices RD-MP apparatus. The reaction progress and purity of the obtained compounds were controlled by TLC using Silufol UV-254 plates.
Nitroquinolines and sodium hydride (60% suspension in paraffin oil) were commercially available from abcr GmbH and were used without additional purification.
Preparation of compounds 2a–i, 3a–h, 4a–f, 6a–i, 7a–f, 8, 10a–g, 12a–g, 13c, 14d,f by arylamination of nitroquinolines 1, 5, 9, 11 (General method). Sodium hydride (60% suspension in paraffin oil, 40 mg, 1.0 mmol) and the appropriate nitroquinoline 1, 5, 9, or 11 (87 mg, 0.5 mmol) were added to a solution of arylamine (1.0 mmol) in anhydrous DMSO (2.0 ml) at room temperature. The mixture was vigorously stirred at room temperature for 1 h and then poured into saturated NaCl solution that was cooled to 5°С. The obtained precipitate was filtered off, washed with water, and dried. The obtained mixture was separated into individual compounds by dry silica gel flash chromatography.20
5-Nitro- N -phenylquinolin-8-amine (2a). The first fraction of yellow color, eluent PhMe. Yield 21 mg (16%), yellow crystals, mp 108–109°С (PhH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 7.20 (1H, d, J = 9.1, H-7 Quin); 7.24 (1H, t, J = 7.4, H-4' Ph); 7.47 (2H, dd, J = 7.9, J = 7.4, H-3',5' Ph); 7.51 (2H, d, J = 7.9, H-2',6' Ph); 7.90 (1H, dd, J = 8.8, H-3 Quin); 8.51 (1H, J = 9.1, H-6 Quin); 8.98 (1H, br. d, J = 4.1, H-2 Quin); 9.29 (1H, br. d, J = 8.8, H-4 Quin); 9.95 (1H, br. s, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 104.1 (C-7); 123.0 (C-4a); 123.5 (C-2',6' Ph); 125.1 (C-4' Ph); 125.6 (C-3); 129.5 (C-3',5' Ph); 130.3 (C-6); 132.1 (C-5); 132.7 (C-4); 136.1 (C-8a); 139.1 (C-1' Ph); 148.3 (C-2); 148.4 (C-8). Found, m/z: 288.0745 [М+Na]+. C15H11N3NaO2. Calculated, m/z: 288.0743.
5-Nitro- N -phenylquinolin-6-amine (3a). The second fraction of orange color, eluent PhMe. Yield 33 mg (25%), light-orange crystals, mp 69–70°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.29–7.35 (3H, m, H-2',4',6' Ph); 7.45–7.50 (2H, m, H-3',5' Ph); 7.52–7.57 (2H, m, H-3,7 Quin); 8.03 (1H, d, J = 9.6, H-8 Quin); 8.75 (1H, dd, J = 4.3, J = 1.4, H-2 Quin); 9.05 (1H, br. d, J = 8.7, H-4 Quin); 10.05 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 120.2; 124.3; 124.5; 124.9; 126.8 (2C); 130.2; 131.8; 137.5; 138.1; 142.6; 143.7; 147.8. Found, m/z: 266.0931 [М+H]+. C15H12N3O2. Calculated, m/z: 266.0924.
5-Nitroso- N -phenylquinolin-6-amine (4a). The third fraction of brown color, eluent PhMe. Yield 21 mg (17%), light-brown crystals, mp 106–107°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.31 (2H, d, J = 8.6, H-2',6' Ph); 7.35–7.41 (2H, m, H-7 Qu, H-4' Ph); 7.46–7.50 (2H, m, H-3',5' Ph); 7.60 (1H, dd, J = 8.3, J = 4.4, H-3 Quin); 7.95 (1H, d, J = 9.8, H-8 Quin); 8.83 (1H, dd, J = 4.4, J = 1.2, H-2 Quin); 9.43 (1H, dd, J = 8.3, J = 1.2, H-4 Quin); 15.93 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 119.7; 124.8; 125.3; 127.9; 129.6; 130.0; 130.3; 136.5; 136.6; 142.8; 143.3; 147.1; 149.6. Found, m/z: 272.0796 [М+Na]+. C15H11N3NaO. Calculated, m/z: 272.0794.
5-Nitro- N -( p -tolyl)quinolin-8-amine (2b). The first fraction of yellow color, eluent PhMe. Yield 38 mg (27%), yellowish-orange crystals, mp 94–95°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.40 (3H, s, CH3); 7.13 (1H, d, J = 9.1, H-7 Quin); 7.27 (2H, d, J = 8.5, H-2',6' Ar); 7.31 (2H, d, J = 8.5, H-3',5' Ar); 7.69 (1H, dd, J = 8.8, J = 4.1, H-3 Quin); 8.53 (1H, d, J = 9.1, H-6 Quin); 8.82 (1H, dd, J = 4.1, J = 1.5, H-2 Quin); 9.00 (1H, br. s, NH); 9.45 (1H, dd, J = 8.8, J = 1.5, H-4 Quin). 13C NMR spectrum (DMSO-d6), δ, ppm: 21.2; 103.5; 123.3; 123.7; 125.1; 130.4; 130.5; 132.9; 133.8; 135.5; 136.2; 136.5; 145.4; 147.8. Found, m/z: 302.0914 [М+Na]+. C16H13N3NaO2. Calculated, m/z: 302.0900.
5-Nitro- N -( p -tolyl)quinolin-6-amine (3b). The second fraction of orange color, eluent PhMe. Yield 8 mg (6%), dark-orange crystals, mp 91–92°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.41 (3H, s, CH3); 7.19 (2H, d, J = 8.2, H-2',6' Ar); 7.27 (2H, d, J = 8.2, H-3',5' Ar); 7.47 (1H, d, J = 9.6, H-7 Quin); 7.52 (1H, dd, J = 8.8, J = 4.2, H-3 Quin); 7.97 (1H, d, J = 9.6, H-8 Quin); 8.72 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 9.06 (1H, br. d, J = 8.8, H-4 Quin); 10.11 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 21.2; 120.0; 124.2; 124.6; 125.3; 126.2; 130.7; 131.6; 135.3; 137.0; 137.8; 142.8; 144.4; 147.9. Found, m/z: 280.1082 [М+H]+. C16H14N3O2. Calculated, m/z: 280.1081.
5-Nitroso- N -( p -tolyl)quinolin-6-amine (4b). The third fraction of brown color, eluent PhMe. Yield 34 mg (26%), light-brown crystals, mp 133–134°С (decomp., PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.42 (3H, s, CH3); 7.19 (2H, d, J = 8.1, H-2',6' Ar); 7.29 (2H, d, J = 8.1, H-3',5' Ar); 7.38 (1H, d, J = 9.8, H-7 Quin); 7.60 (1H, dd, J = 8.4, J = 4.4, H-3 Quin); 7.94 (1H, d, J = 9.8, H-8 Quin); 8.83 (1H, dd, J = 4.4, J = 1.4, H-2 Quin); 9.43 (1H, dd, J = 8.4, J = 4.4, H-4 Quin); 15.91 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 21.3; 119.9; 124.7; 125.2; 129.6; 130.4; 130.6; 133.6; 136.6; 138.1; 142.7; 143.2; 147.1; 149.5. Found, m/z: 286.0961 [М+Na]+. C16H13N3NaO. Calculated, m/z: 286.0951.
N -(4-Methoxyphenyl)-5-nitroquinolin-8-amine (2c). The first fraction of yellow color, eluent PhMe. Yield 19 mg (13%), yellow crystals, mp 148–149°С (PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.86 (3H, s, CH3O); 6.97 (1H, d, J = 9.1, H-7 Quin); 7.00 (2H, d, J = 8.8, H-2',6' Ar); 7.33 (2H, d, J = 8.8, H-3',5' Ar); 7.69 (1H, dd, J = 8.8, J = 4.1, H-3 Quin); 8.52 (1H, d, J = 9.1, H-6 Quin); 8.81 (1H, dd, J = 4.1, J = 1.5, H-2 Quin); 8.90 (1H, br. s, NH); 9.46 (1H, dd, J = 8.8, J = 1.5, H-4 Quin). 13C NMR spectrum (DMSO-d6), δ, ppm: 55.7; 103.2; 115.1; 123.7; 125.2; 125.6; 130.5; 131.5; 132.7; 133.8; 136.3; 147.7; 149.2; 157.8. Found, m/z: 318.0854 [М+Na]+. C16H13N3NaO3. Calculated, m/z: 318.0849.
N -(4-Methoxyphenyl)-5-nitroquinolin-6-amine (3c). The second fraction of orange color, eluent PhMe. Yield 16 mg (11%), orange-red crystals, mp 127–128°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.88 (3H, s, CH3O); 6.99 (2H, d, J = 8.8, H-2',6' Ar); 7.23 (2H, d, J = 8.8, H-3',5' Ar); 7.38 (1H, d, J = 9.6, H-7 Quin); 7.53 (1H, dd, J = 8.8, J = 4.2, H-3 Quin); 7.96 (1H, d, J = 9.6, H-8 Quin); 8.70 (1H, dd, J = 4.2, J = 1.0, H-2 Quin); 9.10 (1H, br. d, J = 8.8, H-4 Quin); 10.14 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 55.7; 115.3; 119.9; 124.3; 124.7; 125.6; 127.4; 130.6; 131.7; 137.9; 142.7; 145.2; 147.8; 158.7. Found, m/z: 318.0854 [М+Na]+. C16H13N3NaO3. Calculated, m/z: 318.0849.
N -(4-Methoxyphenyl)-5-nitrosoquinolin-6-amine (4c). The third fraction of brown color, eluent PhMe. Yield 17 mg (12%), dark-bordeaux crystals, mp 120–121°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.87 (3H, s, OCH3); 7.00 (2H, d, J = 8.8, H-2',6' Ar); 7.23 (2H, d, J = 8.8, H-3',5' Ar); 7.35 (1H, d, J = 9.8, H-7 Quin); 7.59 (1H, dd, J = 8.3, J = 4.3, H-3 Quin); 7.94 (1H, d, J = 9.8, H-8 Quin); 8.82 (1H, br. d, J = 3.2, H-2 Quin); 9.43 (1H, br. d, J = 8.3, H-4 Quin); 15.92 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 55.8; 115.2; 119.9; 124.7; 126.8; 128.8; 129.6; 130.4; 137.0; 142.7; 143.2; 147.1; 149.5; 159.2. Found, m/z: 302.0907 [М+Na]+. C16H13N3NaO2. Calculated, m/z: 302.0900.
N -(4-Bromophenyl)-5-nitroquinolin-8-amine (2d). The first fraction of yellow color, eluent PhMe. Yield 29 mg (17%), dark-yellow crystals, mp 183–184°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.20 (1H, d, J = 9.0, H-7 Quin); 7.31 (2H, d, J = 8.7, H-2',6' Ar); 7.57 (2H, d, J = 8.7, H-3',5' Ar); 7.71 (1H, dd, J = 8.8, J = 4.1, H-3 Quin); 8.54 (1H, d, J = 9.0, H-6 Quin); 8.85 (1H, dd, J = 4.1, J = 1.5, H-2 Quin); 9.03 (1H, br. s, NH); 9.44 (1H, dd, J = 8.8, J = 1.5, H-4 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 103.8; 118.0; 123.5; 124.3; 125.2; 129.9; 133.0; 133.8; 133.9; 136.7; 138.2; 147.2; 148.0. Found, m/z: 365.9847 [М(79Br)+Na]+. C15H10BrN3NaO2. Calculated, m/z: 365.9849.
N -(4-Bromophenyl)-5-nitroquinolin-6-amine (3d). The second fraction of orange color, eluent PhMe. Yield 17 mg (10%), light-red crystals, mp 166–167°С (PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.19 (2H, d, J = 8.6, H-2',6' Ar); 7.52 (1H, d, J = 9.6, H-7 Quin); 7.50–7.61 (3H, m, H-3 Qu, H-3',5' Ar); 8.05 (1H, d, J = 9.6, H-8 Quin); 8.77 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 8.98 (1H, br. d, J = 8.8, H-4 Quin); 9.80 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 119.7; 119.9; 124.3; 124.4; 126.2; 131.0; 131.6; 133.3; 137.4; 137.6; 142.6; 142.9; 148.3. Found, m/z: 365.9852 [М(79Br)+Na]+. C15H10BrN3NaO2. Calculated, m/z: 365.9849.
N -(4-Bromophenyl)-5-nitrosoquinolin-6-amine (4d). The third fraction of brown color, eluent PhMe. Yield 44 mg (27%), brown crystals, mp 159–160°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.18 (2H, d, J = 8.7, H-2',6' Ar); 7.32 (1H, d, J = 9.8, H-7 Quin); 7.58–7.63 (3H, m, H-3',5' Ar, H-3 Quin); 7.96 (1H, d, J = 9.8, H-8 Quin); 8.84 (1H, dd, J = 4.4, J = 1.6, H-2 Quin); 9.40 (1H, dd, J = 8.4, J = 1.6, H-4 Quin); 15.91 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 119.4; 121.3; 124.9; 126.7; 129.8; 130.2; 133.2; 136.1; 137.0; 143.1; 143.5; 146.9; 149.9. Found, m/z: 328.0082 [М(79Br)+H]+. C15H11BrN3O. Calculated, m/z: 328.0080.
N -(4-Fluorophenyl)-5-nitroquinolin-8-amine (2e). The first fraction of yellow color, eluent PhMe. Yield 16 mg (11%), yellow crystals, mp 231–232°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.05 (1H, d, J = 9.0, H-7 Quin); 7.15–7.20 (2H, m, H-2',6' Ar); 7.36–7.41 (2H, m, H-3',5' Ar); 7.70 (1H, dd, J = 8.8, J = 4.2, H-3 Quin); 8.53 (1H, d, J = 9.0, H-6 Quin); 8.83 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 8.94 (1H, br. s, NH); 9.45 (1H, dd, J = 8.8, J = 1.4, H-4 Quin). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 103.3; 116.8 (d, 2JCF = 22.6); 123.6; 125.2; 125.4 (d, 3JCF = 8.2); 130.1; 133.4; 133.8; 134.9 (4JCF = 3.0); 136.4; 147.9; 148.4; 160.4 (d, 1JCF = 244.1). Found, m/z: 306.0657 [М+Na]+. C15H10FN3NaO2. Calculated, m/z: 306.0649.
N -(4-Fluorophenyl)-5-nitroquinolin-6-amine (3e). The second fraction of orange color, eluent PhMe. Yield 10 mg (7%), light-orange crystals, mp 144–145°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.15–7.21 (2H, m, H-2',6' Ar); 7.25–7.31 (3H, m, H-3',5' Ar); 7.41 (1H, d, J = 9.6, H-7 Quin); 7.55 (1H, dd, J = 8.8, J = 4.2, H-3 Quin); 8.01 (1H, d, J = 9.6, H-8 Quin); 8.75 (1H, dd, J = 4.2, J = 1.2, H-2 Quin); 9.04 (1H, br. d, J = 8.8, H-4 Quin); 9.96 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 117.1 (2JCF = 22.7); 119.6; 124.4; 124.5; 126.5; 127.3 (3JCF = 8.2); 131.6; 134.1; 137.9; 142.8; 144.0; 148.1; 161.3 (d, 1JCF = 245.8). Found, m/z: 306.0653 [М+Na]+. C15H10FN3NaO2. Calculated, m/z: 306.0649.
N -(4-Fluorophenyl)-5-nitrosoquinolin-6-amine (4e). The third fraction of brown color, eluent PhMe. Yield 24 mg (18%), dark-brown crystals, mp 146–147°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.16–7.22 (2H, m, H-2',6' Ar); 7.31–7.25 (3H, m, H-3',5' Ar, H-7 Quin); 7.60 (1H, dd, J = 8.3, J = 4.4, H-3 Quin); 7.97 (1H, d, J = 9.8, H-8 Quin); 8.83 (1H, br. d, J = 4.4, H-2 Quin); 9.42 (1H, br. d, J = 8.3, H-4 Quin); 15.90 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 117.1 (d, 2JCF = 22.8); 119.4; 124.9; 127.2 (d, 3JCF = 8.5); 129.7; 130.3; 132.6 (d, 4JCF = 3.2); 137.0; 143.0; 143.3; 147.0; 149.7; 161.8 (d, 1JCF = 247.2). Found, m/z: 290.0708 [М+Na]+. C15H10FN3NaO. Calculated, m/z: 290.0700.
5-Nitro- N -[4-(trifluoromethyl)phenyl]quinolin-8-amine (2f). The first fraction of yellow color, eluent PhMe. Yield 22 mg (13%), pale-yellow crystals, mp 141–142°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.39 (1H, d, J = 9.0, H-7 Quin); 7.53 (2H, d, J = 8.3, H-2',6' Ar); 7.58–7.75 (3H, m, H-3 Qu, H-3',5' Ar); 8.57 (1H, d, J = 9.0, H-6 Quin); 8.87 (1H, br. d, J = 4.1, H-2 Quin); 9.24 (1H, br. s, NH); 9.43 (1H, br. d, J = 8.8, H-4 Quin). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 104.5; 121.3; 123.3; 124.2 (q, 1JCF = 269.9); 126.4 (q, 2JCF = 32.8); 127.2 (q, 3JCF = 3.7); 129.5; 133.8; 134.6; 136.9; 142.5; 146.1; 148.2. Found, m/z: 356.0629 [М+Na]+. C16H10F3N3NaO2. Calculated, m/z: 356.0617.
5-Nitro- N -[4-(trifluoromethyl)phenyl]quinolin-6-amine (3f). The second fraction of orange color, eluent PhMe. Yield 17 mg (10%), dark-orange crystals, mp 146–147°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.39 (2H, d, J = 8.2, H-2',6' Ar); 7.57 (1H, dd, J = 8.8, J = 4.2, H-3 Quin); 7.67 (1H, d, J = 9.5, H-7 Quin); 7.70 (2H, d, J = 8.2, H-3',5' Ar); 8.11 (1H, d, J = 9.5, H-8 Quin); 8.75 (1H, br. d, J = 4.2, H-2 Quin); 8.88 (1H, br. d, J = 8.8, H-4 Quin); 9.61 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 120.2; 123.0; 123.9; 124.0 (q, 1JCF-= 270.1); 124.4; 127.3 (q, 3JCF = 3.6); 127.6 (q, 2JCF = 32.8); 129.4; 131.3; 137.4; 140.7; 142.0; 143.3; 148.8. Found, m/z: 356.0619 [М+Na]+. C16H10F3N3NaO2. Calculated, m/z: 356.0617
5-Nitroso- N -[4-(trifluoromethyl)phenyl]quinolin-6-amine (4f). The third fraction of brown color, eluent PhMe. Yield 40 mg (25%), light-brown crystals, mp 144–145°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.34 (1H, d, J = 9.8, H-7 Quin); 7.42 (2H, d, J = 8.2, H-2',6' Ar); 7.61 (1H, dd, J = 8.4, J = 4.4, H-3 Quin); 7.76 (2H, d, J = 8.2, H-3',5' Ar); 7.98 (1H, d, J = 9.8, H-8 Quin); 8.86 (1H, br. d, J = 4.4, H-2 Quin); 9.39 (1H, br. d, J = 8.4, H-4 Quin); 16.00 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 119.3; 122.1; 123.0; 123.8 (q, 1JCF = 270.3); 125.0; 127.2 (q, 3JCF = 3.7); 129.5 (q, 2JCF = 32.9); 129.9; 137.5; 141.0; 143.3; 143.8; 146.7; 150.1. Found, m/z: 340.0679 [М+Na]+. C16H10F3N3NaO. Calculated, m/z: 340.0689.
4-Nitro- N -(4-nitrophenyl)quinolin-8-amine (2g). The first fraction of yellow color, eluent PhMe. Yield 45 mg (29%), dark-yellow crystals, mp 299–300°С (PhMe). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 7.70 (1H, d, J = 9.0, H-7 Quin); 7.78 (2H, d, J = 8.7, H-2',6' Ar); 7.95 (1H, dd, J = 8.8, J = 4.1, H-3 Quin); 8.28 (2H, d, J = 8.7, H-3',5' Ar); 8.56 (1H, d, J = 9.0, H-6 Quin); 9.07 (1H, br. d, J = 4.1, H-2 Quin); 9.24 (1H, br. d, J = 8.8, H-4 Quin); 10.40 (1H, br. s, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 108.1; 120.3; 122.5; 125.5; 125.6; 128.9; 132.7; 135.2; 137.4; 141.9; 145.2; 146.7; 149.2. Found, m/z: 333.0603 [М+Na]+. C15H10N4NaO4. Calculated, m/z: 333.0594.
4-Nitro- N -(4-nitrophenyl)quinolin-6-amine (3g). The second fraction of yellowish-orange color, eluent PhMe. Yield 20 mg (13%), light-orange crystals, mp 297–298°С (PhH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 7.70 (1H, d, J = 9.0, H-7 Quin); 7.78 (2H, d, J = 9.1, H-2',6' Ar); 7.94 (1H, dd, J = 8.8, J = 4.0, H-3 Quin); 8.28 (2H, d, J = 9.1, H-3',5' Ar); 8.56 (1H, d, J = 9.0, H-8 Quin); 9.05 (1H, br. d, J = 4.0, H-2 Quin); 9.25 (1H, br. d, J = 8.8, H-4 Quin); 10.37 (1H, br. s, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 108.1; 120.3; 122.5; 125.5; 125.6; 128.9; 132.8; 135.3; 137.4; 142.0; 145.2; 146.7; 149.2. Found, m/z: 333.0598 [М+Na]+. C15H10N4NaO4. Calculated, m/z: 333.0594.
N -(2-Chlorophenyl)-5-nitroquinolin-8-amine (2h). The first fraction of yellow color, eluent PhMe. Yield 87 mg (58%), dark-yellow crystals, mp 184–185°С (PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.18 (1H, dd, J = 8.0, J = 7.8, H-5' Ar); 7.22 (1H, d, J = 9.0, H-7 Quin); 7.38 (1H, dd, J = 8.0, J = 7.8, H-4' Ar); 7.55 (1H, dd, J = 8.0, J = 1.3, H-6' Ar); 7.68 (1H, dd, J = 8.0, J = 1.1, H-3' Ar); 7.72 (1H, dd, J = 8.8, J = 4.2, H-3 Quin); 8.55 (1H, d, J = 9.0, H-6 Quin); 8.90 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 9.27 (1H, br. s, NH); 9.43 (1H, dd, J = 8.8, J = 1.4, H-4 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 104.2; 122.8; 123.5; 125.2; 125.9; 127.8; 127.9; 129.7; 130.8; 133.7; 134.2; 136.2; 137.0; 146.7; 148.3. Found, m/z: 322.0343 [М(35Cl)+Na]+. C15H10ClN3NaO2. Calculated, m/z: 322.0354.
N -(2-Chlorophenyl)-5-nitroquinolin-6-amine (3h). The second fraction of yellow-orange color, eluent PhMe. Yield 5 mg (3%), yellow crystals, mp 154–155°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.23 (1H, dd, J = 8.2, J = 7.8, H-5' Ar); 7.35 (1H, dd, J = 8.2, J = 7.8, H-4' Ar); 7.43 (1H, dd, J = 8.2, J = 1.1, H-6' Ar); 7.49 (1H, d, J = 9.5, H-7 Quin); 7.52–7.57 (2H, m, H-3 Qu, H-3' Ar); 8.06 (1H, d, J = 9.5, H-8 Quin); 8.78 (1H, dd, J = 4.2, J = 1.3, H-2 Quin); 8.92 (1H, d, J = 8.8, H-4 Quin); 9.68 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 120.1; 124.1; 124.3; 124.7; 127.1; 128.0; 128.6; 129.2; 130.9; 131.4; 135.8; 137.4; 141.5; 143.1; 148.5. Found, m/z: 322.0369 [М(35Cl)+Na]+. C15H10ClN3NaO2. Calculated, m/z: 322.0354.
5-Nitro- N -(2,4,6-tribromophenyl)quinolin-8-amine (2i). The first fraction of yellow color, eluent PhMe. Yield 110 mg (44%), light-brown crystals, mp 196–197°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.36 (1H, d, J = 8.8, H-7 Quin); 7.73 (1H, dd, J = 8.9, J = 4.1, H-3 Quin); 7.88 (2H, s, H-3',5' Ar); 8.49 (1H, d, J = 8.8, H-6 Quin); 8.66 (1H, br. s, NH); 8.90 (1H, dd, J = 4.1, J = 1.5, H-2 Quin); 9.42 (1H, dd, J = 8.9, J = 1.5, H-4 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 104.9; 121.8; 123.3; 125.0; 125.1; 129.2; 133.7; 134.8; 135.6; 136.1; 136.3; 146.8; 148.5. Found, m/z: 521.8053 [М(79Br)+Na]+. C15H5Br3N3NaO2. Calculated, m/z: 521.8059.
8-Nitro- N -phenylquinolin-5-amine (6a). The first fraction of yellow color, eluent PhMe. Yield 16 mg (12%), light-yellow crystals, mp 201–202°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.58 (1H, s, NH); 7.15 (1H, d, J = 8.6, H-6 Quin); 7.21 (1H, t, J = 7.4, H-4' Ph); 7.25 (2H, d, J = 8.1, H-2',6' Ph); 7.40–7.46 (2H, m, H-3',5' Ph); 7.52 (1H, dd, J = 8.6, J = 4.1, H-3 Quin); 8.15 (1H, d, J = 8.6, H-7 Quin); 8.40 (1H, dd, J = 8.6, J = 1.5, H-4 Quin); 9.13 (1H, dd, J = 4.1, J = 1.5, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 107.3; 119.6; 121.4; 122.1 2С); 125.0; 128.1; 129.7; 130.1; 140.2; 141.9; 145.4; 152.8. Found, m/z: 266.0943 [М+H]+. C15H12N3O2. Calculated, m/z: 266.0924.
8-Nitroso- N -phenylquinolin-7-amine (7a). The second fraction of brown color, eluent EtOAc. Yield 60 mg (48%), dark-green crystals, mp 137–138°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.23 (1H, d, J = 9.6, H-6 Quin); 7.30 (2H, d, J = 7.5, H-2',6' Ph); 7.37 (1H, t, J = 7.5, H-4' Ph); 7.45–7.51 (3H, m, H-3 Qu, H-3',5' Ph); 7.71 (1H, d, J = 9.6, H-5 Quin); 8.00 (1H, dd, J = 8.0, J = 1.6, H-4 Quin); 9.09 (1H, dd, J = 4.5, J = 1.6, H-2 Quin); 15.78 (1H, br. s, NH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 7.23 (1H, d, J = 9.6, H-6 Quin); 7.41 (1H, t, J = 7.3, H-4' Ph); 7.45 (2H, d, J = 7.6, H-2',6' Ph); 7.49–7.53 (2H, m, H-3',5' Ph); 7.61 (1H, dd, J =8.0, J = 4.4, H-3 Quin); 8.03 (1H, d, J = 9.6, H-5 Quin); 8.29 (1H, dd, J = 8.0, J = 1.5, H-4 Quin); 8.98 (1H, dd, J = 4.4, J = 1.5, H-2 Quin); 15.43 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 117.6 (C-6); 121.5 (C-4a); 121.8 (C-3); 125.5 (C-2',6'); 127.8 (C-4'); 130.0 (C-3',5'); 136.4 (C-1'); 136.6 (C-4); 137.0 (C-7); 140.2 (C-5); 149.0 (C-8,8а); 152.7 (C-2). Found, m/z: 250.0973 [М+H]+. C15H12N3O. Calculated, m/z: 250.0975.
8-Nitro- N -( p- tolyl)quinolin-5-amine (6b). The first fraction of yellow color, eluent PhMe. Yield 20 mg (14%), yellow crystals, mp 191–192°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.39 (3H, s, CH3); 6.55 (1H, s, NH); 7.02 (1H, d, J = 8.7, H-6 Quin); 7.16 (2H, d, J = 8.3, H-2',6' Ar); 7.25 (2H, d, J = 8.3, H-3',5' Ar); 7.52 (1H, dd, J = 8.7, J = 4.1, H-3 Quin); 8.16 (1H, d, J = 8.7, H-7 Quin); 8.38 (1H, dd, J = 8.7, J = 1.5, H-4 Quin); 9.13 (1H, dd, J = 4.1, J = 1.5, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 21.1 (CH3); 106.2 (C-6); 118.9 (C-4a); 121.2 (C-3); 123.1 (C-2',6'); 128.5 (C-7); 129.5 (C-4); 130.7 (C-3',5'); 135.3 (C-4'); 137.2 (C-1'); 139.2 (C-8); 142.0 (C-8a); 146.3 (C-5); 152.7 (C-2). Found, m/z: 280.1087 [М+H]+. C16H14N3O2. Calculated, m/z: 280.1081.
8-Nitroso- N -( p -tolyl)quinolin-7-amine (7b). The second fraction of brown color, eluent EtOAc. Yield 58 mg (44%), light-brown crystals, mp 241–242°С (decomp., EtOAc). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.41 (3H, s, CH3); 7.17 (2H, d, J = 8.2, H-2',6' Ar); 7.23 (1H, d, J = 9.6, H-5 Quin); 7.27 (2H, d, J = 8.2, H-3',5' Ar); 7.47 (1H, dd, J = 8.0, J = 4.4, H-3 Quin); 7.69 (1H, d, J = 9.6, H-6 Quin); 7.99 (1H, dd, J = 8.0, J = 1.6, H-4 Quin); 9.07 (1H, dd, J = 4.4, J = 1.6, H-2 Quin); 15.76 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 21.3; 117.7; 121.4; 121.7; 125.4; 130.6; 133.5; 136.5; 137.1; 137.9; 140.0; 149.0; 149.1; 152.7. Found, m/z: 264.1148 [М+H]+. C16H14N3O. Calculated, m/z: 264.1131.
N -(4-Methoxyphenyl)-8-nitroquinolin-5-amine (6c). The first fraction of yellow color, eluent PhMe. Yield 12 mg (8%), orange crystals, mp 194–195°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.86 (3H, s, CH3); 6.57 (1H, s, NH); 6.82 (1H, d, J = 8.7, H-6 Quin); 6.99 (2H, d, J = 8.8, H-2',6' Ar); 7.22 (2H, d, J = 8.8, H-3',5' Ar); 7.51 (1H, dd, J = 8.6, J = 4.1, H-3 Quin); 8.15 (1H, d, J = 8.7, H-7 Quin); 8.36 (1H, dd, J = 8.6, J = 1.4, H-4 Quin); 9.12 (1H, dd, J = 4.1, J = 1.4, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 55.7; 105.1; 115.4; 118.2; 121.1; 126.0; 128.9; 129.3; 132.2; 138.5; 142.0; 147.3; 152.7; 157.9. Found, m/z: 296.1032 [М+H]+. C16H14N3O3. Calculated, m/z: 296.1030.
N -(4-Methoxyphenyl)-8-nitrosoquinolin-7-amine (7c). The second fraction of brown color, eluent EtOAc. Yield 58.6 mg (42%), dark-brown crystals, mp 134–135°С (decomp., EtOAc). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.86 (3H, s, CH3); 6.99 (2H, d, J = 8.8, H-2',6' Ar); 7.18 (1H, d, J = 9.6, H-5 Quin); 7.22 (2H, d, J = 8.8, H-3',5' Ar); 7.46 (1H, dd, J = 8.0, J = 4.5, H-3 Quin); 7.69 (1H, d, J = 9.6, H-6 Quin); 7.99 (1H, dd, J = 8.0, J = 1.5, H-4 Quin); 9.06 (1H, dd, J = 4.5, J = 1.5, H-2 Quin); 15.74 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 55.7; 115.2; 117.7; 121.3; 121.7; 126.9; 128.7; 136.5; 137.5; 140.0; 149.0; 149.1; 152.6; 159.1. Found, m/z: 280.1081 [М+H]+. C16H14N3O2. Calculated, m/z: 280.1081.
N -(4-Bromophenyl)-8-nitroquinolin-5-amine (6d). The first fraction of yellow color, eluent PhMe. Yield 10 mg (6%), yellowish-orange crystals, mp 194–195°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.47 (1H, s, NH); 7.11 (2H, d, J = 8.7, H-2',6' Ar); 7.15 (1H, d, J = 8.6, H-6 Quin); 7.50–7.56 (3H, m, H-3 Qu, H-3',5' Ar); 8.14 (1H, d, J = 8.6, H-7 Quin); 8.38 (1H, dd, J = 8.6, J = 1.6, H-4 Quin); 9.13 (1H, dd, J = 4.2, J = 1.6, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 108.3; 117.2; 120.0; 121.6; 123.2; 127.6; 129.7; 133.1; 139.7; 140.7; 141.7; 144.6; 152.9. Found, m/z: 344.0035 [М(79Br)+H]+. C15H11BrN3O2. Calculated, m/z: 344.0029.
N -(4-Bromophenyl)-8-nitrosoquinolin-7-amine (7d). The second fraction of brown color, eluent PhMe–EtOAc, 7:3. Yield 59 mg (36%), brown crystals, mp 150–151°С (decomp., PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.15–7.20 (3H, m, H-2',6' Ar, H-5 Quin); 7.50 (1H, dd, J = 8.0, J = 4.5, H-3 Quin); 7.60 (2H, d, J = 8.6, H-3',5' Ar); 7.73 (1H, d, J = 9.6, H-6 Quin); 8.01 (1H, br. d, J = 7.9, H-4 Quin); 9.09 (1H, br. d, J = 3.3, H-2 Quin); 15.73 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 117.2; 121.2; 121.7; 122.0; 127.0; 133.2; 135.8; 136.6; 137.0; 140.4; 148.8; 148.9; 152.9. Found, m/z: 328.0078 [М(79Br)+H]+. C15H11BrN3O. Calculated, m/z: 328.0080. Found, m/z: 349.9921 [М(79Br)+Na]+. C15H10BrN3NaO. Calculated, m/z: 349.9899.
N -(4-Fluorophenyl)-8-nitroquinolin-5-amine (6e). The first fraction of yellow color, eluent PhMe. Yield 13 mg (9%), yellowish-green crystals, mp 215–216°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.53 (1H, s, NH); 6.94 (1H, d, J = 8.6, H-6 Quin); 7.11–7.16 (2H, m, H-2',6' Ar); 7.21–7.26 (2H, m, H-3',5' Ar); 7.53 (1H, dd, J = 8.6, J = 4.2, H-3 Quin); 8.14 (1H, d, J = 8.6, H-7 Quin); 8.38 (1H, dd, J = 8.6, J = 1.3, H-4 Quin); 9.13 (1H, dd, J = 4.2, J = 1.3, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 106.3; 117.0 (d, 2JCF = 22.7); 119.0; 121.4; 125.1 (d, 3JCF = 8.1); 128.2; 129.4; 135.9; 139.7; 141.8; 146.1; 152.8; 160.3 (d, 1JCF = 244.0). Found, m/z: 284.0839 [М+H]+. C15H11FN3O2. Calculated, m/z: 284.0830.
N -(4-Fluorophenyl)-8-nitrosoquinolin-7-amine (7e). The second fraction of brown color, eluent EtOAc. Yield 60 mg (45%), orange-brown crystals, mp 127–128°С (EtOAc). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.14 (1H, d, J = 9.6, H-5 Quin); 7.19 (2H, d, J = 8.2, H-2',6' Ar); 7.26–7.30 (2H, m, H-3',5' Ar); 7.49 (1H, dd, J = 8.0, J = 4.4, H-3 Quin); 7.73 (1H, d, J = 9.6, H-6 Quin); 8.01 (1H, dd, J = 8.0, J = 1.6, H-4 Quin); 9.09 (1H, dd, J = 4.4, J = 1.6, H-2 Quin); 15.68 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 117.1 (d, 2JCF = 22.7) 117.2; 121.5; 121.9; 127.4 (d, 3JCF = 8.4); 132.4; 136.6; 137.3; 140.4; 148.9; 149.0; 152.9; 161.7 (d, 1JCF = 247.0). Found, m/z: 268.0882 [М+H]+. C15H11FN3O. Calculated, m/z: 268.0881.
8-Nitro- N -[4-(trifluoromethyl)phenyl]quinolin-5-amine (6f). The first fraction of yellow color, eluent PhMe. Yield 17 mg (10%), bright-orange crystals, mp 199–200°С (PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.54 (1H, s, NH); 7.22 (2H, d, J = 8.5, H-2',6' Ar); 7.38 (1H, d, J = 8.5, H-6 Quin); 7.56 (1H, dd, J = 8.7, J = 4.1, H-3 Quin); 7.62 (2H, d, J = 8.5, H-3',5' Ar); 8.14 (1H, d, J = 8.5, H-7 Quin); 8.41 (1H, dd, J = 8.7, J = 1.5, H-4 Quin); 9.14 (1H, dd, J = 4.1, J = 1.5, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 111.3; 119.1; 121.5; 122.0; 124.2 (q, 1J = 269.7); 125.2 (q, 2J = 32.6); 126.8; 127.4; 130.4; 141.4; 142.2; 143.0; 144.7 (2С); 152.9. Found, m/z: 334.0798 [М+H]+. C16H11F3N3O2. Calculated, m/z: 334.0798.
8-Nitroso- N -[4-(trifluoromethyl)phenyl]quinolin-7-amine (7f). The second fraction of brown color, eluent EtOAc. Yield 25 mg (16%), dark-green crystals, mp 158–159°С (PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.21 (1H, d, J = 9.6, H-6 Quin); 7.41 (2H, d, J = 8.2, H-2',6' Ar); 7.52 (1H, dd, J = 8.0, J = 4.5, H-3 Quin); 7.72–7.77 (3H, m, H-5 Qu, H-3',5' Ar); 8.02 (1H, br. d, J = 7.7, H-4 Quin); 9.08–9.10 (1H, m, H-2 Quin); 15.80 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 117.0 (C-6); 122.0 (C-1'); 122.2 (C-3); 123.8 (q, 1J = 270.8, CF3); 125.2 (C-2',6'); 127.2 (q, 3J = 3.5, C-3',5'); 129.2 (q, 2J = 32.8, C-4'); 136.7 (C-7); 136.9 (C-4); 140.5 (C-4a); 140.7 (C-5); 148.6 (C-8,8a); 152.9 (C-2). Found, m/z: 318.0847 [М+H]+. C16H11F3N3O. Calculated, m/z: 318.0849.
8-Nitro- N -(4-nitrophenyl)quinolin-5-amine (6g). The first fraction of yellow color, eluent PhMe. Yield 28 mg (18%), light-yellow crystals, mp 261–262°С (PhMe). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 7.32 (2H, d, J = 9.2, H-2',6' Ar); 7.64 (1H, d, J = 8.4, H-6 Quin); 7.77 (1H, dd, J = 8.5, J = 4.2, H-3 Quin); 8.18 (2H, d, J = 9.2, H-3',5' Ar); 8.28 (1H, d, J = 8.4, H-7 Quin); 8.38 (1H, dd, J = 8.5, J = 0.8, H-4 Quin); 9.08 (1H, dd, J = 4.2, J = 0.8, H-2 Quin); 9.84 (1H, br. s, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 114.3; 116.4, 122.2; 122.4; 125.4; 126.0; 132.1; 140.0; 140.2; 141.3; 142.7; 149.8; 153.0. Found, m/z: 311.0774 [М+H]+. C15H11N4O4. Calculated, m/z: 311.0775.
8-Nitro- N -(4-nitrophenyl)quinolin-7-amine (8). The second fraction of orange color, eluent PhMe. Yield 12 mg (8%), dark-orange crystals, mp 271–272°С (PhMe). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 7.32 (2H, d, J = 9.1, H-2',6' Ar); 7.64 (1H, d, J = 8.4, H-5 Quin); 7.77 (1H, dd, J = 8.6, J = 4.2, H-3 Quin); 8.19 (2H, d, J = 9.1, H-3',5' Ar); 8.28 (1H, d, J = 8.4, H-6 Quin); 8.73 (1H, dd, J = 8.6, J = 1.1, H-4 Quin); 9.08 (1H, dd, J = 4.2, J = 1.1, H-2 Quin); 9.84 (1H, br. s, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 114.3; 116.4; 122.2; 122.4; 125.4; 126.0; 132.2; 140.0; 140.1; 141.3; 142.7; 149.8; 153.0. Found, m/z: 311.0768 [М+H]+. C15H11N4O4. Calculated, m/z: 311.0775.
N -(2-Chlorophenyl)-8-nitroquinolin-5-amine (6h). The fraction of yellow color, eluent PhMe. Yield 63 mg (42%), orange crystals, mp 178–179°С (PhH). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.75 (1H, s, NH); 7.09 (1H, m, H-4' Ar); 7.26–7.30 (2H, m, H-6 Qu, H-5' Ar); 7.34 (1H, br. d, J = 7.5, H-6' Ar); 7.51 (1H, br. d, J = 8.0, H-3' Ar); 7.58 (1H, dd, J = 8.6, J = 4.1, H-3 Quin); 8.15 (1H, d, J = 8.5, H-7 Quin); 8.47 (1H, dd, J = 8.6, J = 0.8, H-4 Quin); 9.15 (1H, dd, J = 4.1, J = 0.8, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 110.3; 120.4, 121.2; 121.9; 124.4; 125.1; 127.1; 128.0; 130.2; 130.5; 137.9; 141.6 (2С); 143.4; 152.9. Found, m/z: 300.0542 [М(35Cl)+H]+. C15H11ClN3O2. Calculated, m/z: 300.0534.
8-Nitro- N -(2,4,6-tribromophenyl)quinolin-5-amine (6i). The fraction of yellow color, eluent PhMe. Yield 45 mg (18%), brown crystals, mp 190–191°С (PhMe). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.38 (1H, d, J = 8.5, H-6 Quin); 6.44 (1H, br. s, NH); 7.61 (1H, dd, J = 8.6, J = 4.2, H-3 Quin); 7.85 (2H, s, H-3',5' Ar); 8.07 (1H, d, J = 8.5, H-7 Quin); 8.53 (1H, br. d, J = 8.4, H-4 Quin); 9.15 (1H, br. d, J = 3.2, H-2 Quin). 13C NMR spectrum (CDCl3), δ, ppm: 107.7; 119.5; 120.7; 121.8; 123.2; 127.2; 129.8; 135.7; 136.5; 141.1; 141.5; 143.6; 152.8. Found, m/z: 499.8222 [М(79Br)+H]+. C15H9Br3N3O2. Calculated, m/z: 499.8239.
7-Nitro- N -phenylquinolin-8-amine (10a). The fraction of yellow color, eluent PhMe. Yield 81 mg (61%), yellowishorange crystals, mp 162–163°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.03 (2H, d, J = 8.0, H-2',6' Ph); 7.11 (1H, t, J = 7.4, H-4' Ph); 7.25 (1H, d, J = 9.2, H-5 Quin); 7.32 (2H, m, H-3',5' Ph); 7.59 (1H, dd, J = 8.2, J = 4.2, H-3 Quin); 8.05 (1H, d, J = 9.2, H-6 Quin); 8.17 (1H, dd, J = 8.2, J = 1.4, H-4 Quin); 8.84 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 9.37 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 116.9; 119.4; 123.5; 124.4 (2С); 129.2; 130.6; 133.0; 136.5; 136.6; 140.9; 141.1; 148.8. Found, m/z: 288.0737 [М+Na]+. C15H11N3NaO2. Calculated, m/z: 288.0743.
7-Nitro- N -( p -tolyl)quinolin-8-amine (10b). The fraction of yellow color, eluent PhMe. Yield 80 mg (57%), orange crystals, mp 133–134°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.33 (3H, s, CH3); 6.93 (2H, d, J = 8.3, H-2',6' Ar); 7.12 (2H, d, J = 8.3, H-3',5' Ar); 7.21 (1H, d, J = 9.2, H-5 Quin); 7.58 (1H, dd, J = 8.2, J = 4.2, H-3 Quin); 8.03 (1H, d, J = 9.2, H-6 Quin); 8.15 (1H, dd, J = 8.2, J = 1.5, H-4 Quin); 8.82 (1H, dd, J = 4.2, J = 1.5, H-2 Quin); 9.33 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 21.1 (C-CH3); 116.4 (C-5); 119.5 (C-2',6'); 123.6 (C-6); 124.4 (C-3); 129.7 (C-3',5'); 130.6 (C-4a); 132.6 (C-7); 134.2 (C-4'); 136.4 (C-4); 137.0 (C-8); 138.3 (C-1'); 141.1 (C-8a); 148.6 (C-2). Found, m/z: 302.0900 [М+Na]+. C16H13N3NaO2. Calculated, m/z: 302.0898.
N -(4-Methoxyphenyl)-7-nitroquinolin-8-amine (10c). The fraction of yellow color, eluent PhMe. Yield 93 mg (63%), pale-yellow crystals, mp 118–119°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.80 (3H, s, OCH3); 6.86 (2H, d, J = 8.8, H-2',6' Ar); 7.00 (2H, d, J = 8.8, H-3',5' Ar); 7.18 (1H, d, J = 9.2, H-5 Quin); 7.57 (1H, dd, J = 8.2, J = 4.2, H-3 Quin); 8.01 (1H, d, J = 9.2, H-6 Quin); 8.15 (1H, br. d, J = 8.2, H-4 Quin); 8.81 (1H, br. d, J = 4.2, H-2 Quin); 9.32 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 55.6; 114.4; 116.0; 121.3; 123.7; 124.4; 130.6; 132.1; 134.1; 136.4; 137.6; 141.1; 148.5; 156.7. Found, m/z: 318.0853 [М+Na]+. C16H13N3NaO3. Calculated, m/z: 318.0849.
N -(4-Bromophenyl)-7-nitroquinolin-8-amine (10d). The fraction of yellow color, eluent PhMe. Yield 100 mg (58%), yellowish-orange crystals, mp 188–189°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.90 (2H, d, J = 8.7, H-2',6' Ar); 7.29 (1H, d, J = 9.2, H-5 Quin); 7.41 (2H, d, J = 8.7, H-3',5' Ar); 7.60 (1H, dd, J = 8.2, J = 4.2, H-3 Quin); 8.07 (1H, d, J = 9.2, H-6 Quin); 8.18 (1H, dd, J = 8.2, J = 1.4, H-4 Quin); 8.84 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 9.32 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 116.9; 117.5; 121.1; 123.3; 124.6; 130.7; 132.1; 133.3; 136.4; 136.6; 140.2; 141.0; 148.9. Found, m/z: 365.9853 [М(79Br)+Na]+. C15H10BrN3NaO2. Calculated, m/z: 365.9849.
N -(4-Fluorophenyl)-7-nitroquinolin-8-amine (10e). The fraction of yellow color, eluent PhMe. Yield 69 mg (49%), orange crystals, mp 156–157°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.00–7.02 (4H, m, H-2',3',5',6' Ar); 7.25 (1H, d, J = 9.2, H-5 Quin); 7.59 (1H, dd, J = 8.2, J = 4.1, H-3 Quin); 8.04 (1H, d, J = 9.2, H-6 Quin); 8.17 (1H, dd, J = 8.2, J = 1.1, H-4 Quin); 8.83 (1H, dd, J = 4.2, J = 1.1, H-2 Quin); 9.32 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 116.0 (d, 2JCF = 22.7); 116.9; 121.5 (d, 3JCF = 8.1); 123.5; 124.5; 130.7; 132.7; 136.5; 137.2 (2С, d, 4JCF = 2.8); 140.9; 148.7; 159.7 (d, 1JCF = 242.0). Found, m/z: 306.0642 [М+Na]+. C15H10FN3NaO2. Calculated, m/z: 306.0649.
7-Nitro- N -[4-(trifluoromethyl)phenyl)]quinolin-8-amine (10f). The fraction of yellow color, eluent PhMe. Yield 62 mg (37%), orange crystals, mp 142–143°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.05 (2H, d, J = 8.4, H-2',6' Ar); 7.38 (1H, d, J = 9.2, H-5 Quin); 7.55 (2H, d, J = 8.4, H-3',5' Ar); 7.62 (1H, dd, J = 8.2, J = 4.1, H-3 Quin); 8.12 (1H, d, J = 9.2, H-6 Quin); 8.21 (1H, br. d, J = 8.2, H-4 Quin); 8.87 (1H, dd, J = 4.2, J = 1.2, H-2 Quin); 9.42 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 118.6; 118.7; 123.2; 124.3 (q, 1J = 267.8); 124.7; 125.7 (q, 2J = 25.2); 126.4 (q, 3J = 3.7); 130.8; 134.3; 135.5; 136.6; 141.1; 144.2; 149.2. Found, m/z: 356.0629 [М+Na]+. C16H10F3N3NaO2. Calculated, m/z: 356.0617.
7-Nitro- N -(4-nitrophenyl)quinolin-8-amine (10g). The fraction of yellow color, eluent PhMe. Yield 45 mg (29%), bright-yellow crystals, mp 152–153°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.00 (2H, d, J = 8.9, H-2',6' Ar); 7.50 (1H, d, J = 9.2, H-5 Quin); 7.65 (1H, dd, J = 8.3, J = 4.2, H-3 Quin); 8.14–8.20 (3H, m, H-6 Quin and H-3',5' Ar); 8.25 (1H, br. d, J = 8.3, H-4 Quin); 8.90 (1H, br. d, J = 4.2, H-2 Quin); 9.46 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 118.0; 120.2; 122.9; 124.8; 125.3; 130.9; 134.4; 135.6; 136.7; 141.1; 143.0; 147.3; 149.7. Found, m/z: 333.0583 [М+Na]+. C15H10N4NaO4. Calculated, m/z: 333.0549.
6-Nitro- N -phenylquinolin-5-amine (12a). The fraction of yellow color, eluent PhMe. Yield 70 mg (53%), darkorange crystals, mp 129–130°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.94 (2H, d, J = 7.9, H-2',6' Ph); 7.09 (1H, t, J = 7.4, H-4' Ph); 7.18 (1H, dd, J = 8.7, J = 4.2, H-3 Quin); 7.26 (2H, m, H-3',5' Ph); 7.69 (1H, d, J = 9.5, H-8 Quin); 8.25 (1H, br. d, J = 8.7, H-4 Quin); 8.38 (1H, d, J = 9.5, H-7 Quin); 8.93 (1H, dd, J = 4.2, J = 1.4, H-2 Quin); 9.72 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 120.4 (C-3); 120.8 (C-2',6'); 121.9 (C-4a); 123.4 (C-8); 124.3 (C-4'); 125.1 (C-7); 129.8 (C-3'); 135.4 (C-6); 136.2 (C-4); 140.2 (C-5); 143.7 (C-1'); 151.6 (C-8a); 153.4 (C-2). Found, m/z: 266.0929 [М+H]+. C15H12N3O2. Calculated, m/z: 266.0924.
6-Nitro- N -( p -tolyl)quinolin-5-amine (12b). The fraction of yellow color, eluent PhMe. Yield 85 mg (61%), dark-red crystals, mp 110–111°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 2.32 (3H, s, CH3); 6.87 (2H, d, J = 8.3, H-2',6' Ar); 7.07 (2H, d, J = 8.3, H-3',5' Ar); 7.16 (1H, dd, J = 8.7, J = 4.2, H-3 Quin); 7.63 (1H, d, J = 9.5, H-8 Quin); 8.24 (1H, br. d, J = 8.7, H-4 Quin); 8.37 (1H, d, J = 9.5, H-7 Quin); 8.91 (1H, dd, J = 4.2, J = 1.5, H-2 Quin); 9.85 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 21.0; 120.1; 121.3; 121.7; 122.7; 125.2; 130.4; 134.3; 134.6; 136.3; 141.0; 141.1; 151.7; 153.3. Found, m/z: 280.1070 [М+H]+. C16H14N3O2. Calculated, m/z: 280.1081.
N -(4-Methoxyphenyl)-6-nitroquinolin-5-amine (12c). The fraction of yellow color, eluent PhMe. Yield 61 mg (41%), bright-red crystals, mp 141–142°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 3.80 (3H, s, OCH3); 6.83 (2H, d, J = 8.8, H-2',6' Ar); 6.96 (2H, d, J = 8.8, H-3',5' Ar); 7.14 (1H, dd, J = 8.6, J = 4.2, H-3 Quin); 7.58 (1H, d, J = 9.5, H-8 Quin); 8.22 (1H, br. d, J = 8.6, H-4 Quin); 8.38 (1H, d, J = 9.5, H-7 Quin); 8.89 (1H, br. d, J = 4.2, H-2 Quin); 10.02 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 55.7; 115.1; 120.0; 121.3; 122.1; 123.5; 125.3; 133.7; 136.3; 136.6; 142.0; 151.9; 153.2; 157.0. Found, m/z: 296.1027 [М+H]+. C16H14N3O3. Calculated, m/z: 296.1030.
N -(4-Bromophenyl)-6-nitroquinolin-5-amine (12d). The fraction of yellow color, eluent PhMe. Yield 43 mg (25%), bright-yellow crystals, mp 142–143°С (petroleum ether). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.79 (2H, d, J = 8.7, H-2',6' Ar); 7.25 (1H, dd, J = 8.6, J = 4.2, H-3 Quin); 7.36 (2H, d, J = 8.7, H-3',5' Ar); 7.75 (1H, d, J = 9.5, H-8 Quin); 8.24 (1H, br. d, J = 8.7, H-4 Quin); 8.37 (1H, d, J = 9.5, H-7 Quin); 8.91 (1H, dd, J = 4.2, J = 1.5, H-2 Quin); 9.85 (1H, s, NH). 13C NMR spectrum (CDCl3), δ, ppm: 116.6; 120.7; 121.9; 122.0; 124.2; 125.0; 132.8; 135.9; 136.1; 139.2; 142.9; 151.5; 153.6. Found, m/z: 344.0022 [М(79Br)+H]+. C15H11BrN3O2. Calculated, m/z: 344.0029.
N -(4-Fluorophenyl)-6-nitroquinolin-5-amine (12e). The fraction of yellow color, eluent PhMe. Yield 54 mg (38%), orange crystals, mp 147–148°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.91–6.99 (4H, m, H-2'3',5',6' Ar); 7.20 (1H, dd, J = 8.7, J = 4.2, H-3 Quin); 7.68 (1H, d, J = 9.5, H-8 Quin); 8.19 (1H, br. d, J = 8.7, H-4 Quin); 8.38 (1H, d, J = 9.5, H-7 Quin); 8.93 (1H, dd, J = 4.2, J = 1.0, H-2 Quin); 9.73 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 116.7 (d, 2J = 22.7); 120.4; 121.6; 122.8 (d, 3J = 8.0); 123.4; 125.1; 135.1; 136.0; 139.8 (d, 4J = 2.9); 140.6; 151.7; 153.5; 159.6 (d, 1J = 242.9). Found, m/z: 284.0837 [М+H]+. C15H11FN3O2. Calculated, m/z: 284.0830.
6-Nitro- N -[4-(trifluoromethyl)phenyl]quinolin-5-amine (12f). The fraction of yellow color, eluent PhMe. Yield 38 mg (23%), orange crystals, mp 113–114°С (petroleum ether). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.91 (2H, d, J = 8.5, H-2',6' Ar); 7.33 (1H, dd, J = 8.7, J = 4.2, H-3 Quin); 7.48 (2H, d, J = 8.5, H-3',5' Ar); 7.87 (1H, d, J = 9.4, H-8 Quin); 8.23 (1H, br. d, J = 8.7, H-4 Quin); 8.39 (1H, d, J = 9.4, H-7 Quin); 9.01 (1H, br. d, J = 4.2, H-2 Quin); 9.29 (1H, br. s, NH). 13C NMR spectrum (CDCl3), δ, ppm (J, Hz): 118.8; 121.3; 122.5; 124.2 (q, 1J = 269.7); 124.6; 124.9; 125.3 (q, 2J = 23.6); 127.0 (q, 3J = 3.7); 135.6; 137.4; 137.5; 146.9; 151.1; 153.8. Found, m/z: 334.0805 [М+H]+. C16H11F3N3O2. Calculated, m/z: 334.0798.
6-Nitro- N -(4-nitrophenyl)quinolin-5-amine (12g). The fraction of yellow color, eluent PhMe. Yield 76 mg (49%), bright-orange crystals, mp 199–200°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 6.83 (2H, d, J = 9.0, H-2',6' Ar); 7.40 (1H, dd, J = 8.6, J = 4.2, H-3 Quin); 8.00 (1H, d, J = 9.4, H-8 Quin); 8.11 (2H, d, J = 9.0, H-3',5' Ar); 8.22 (1H, br. d, J = 8.6, H-4 Quin); 8.39 (1H, d, J = 9.4, H-7 Quin); 9.04–9.08 (2H, m, H-2 Quin, NH). 13C NMR spectrum (CDCl3), δ, ppm: 117.4; 121.9; 123.0; 124.7; 126.0; 127.1; 135.1; 135.2; 139.0; 142.4; 149.6; 150.9; 154.1. Found, m/z: 311.0766 [М+H]+. C15H11N4O4. Calculated, m/z: 311.0775.
9-Methoxypyrido[3,2- a ]phenazine 7-oxide (13c). The third fraction of pale-yellow color, eluent PhMe–EtOAc, 7:3. Yield 8 mg (6%), dark-orange crystals, mp 232–233°С (CHCl3). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 4.08 (3H, s, OCH3); 7.55 (1H, dd, J = 9.3, J = 2.5, H-10); 7.70 (1H, dd, J = 8.2, J = 3.0, H-2); 8.01 (1H, d, J = 2.5, H-8); 8.21 (1H, d, J = 9.3, H-11); 8.22 (1H, d, J = 9.8, H-6); 8.82 (1H, d, J = 9.8, H-5); 9.09 (1H, d, J = 3.0, H-3); 9.57 (1H, d, J = 8.2, H-1). 13C NMR spectrum (CDCl3), δ, ppm: 56.6; 96.2; 120.1; 122.9; 126.1; 127.0; 131.9; 133.0; 133.2; 133.7; 136.4; 141.0; 141.6; 149.5; 152.3; 162.0. Found, m/z: 300.0729 [М+Na]+. С16Н11N3NaО2. Calculated, m/z: 300.0743.
9-Bromo-5-[(4-bromophenyl)amino]pyrido[3,2- a ]phenazine 7-oxide (14d). The second fraction of yellow color, eluent PhMe. Yield 37 mg (15%), orange-red crystals, mp 257–258°С (petroleum ether). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.45 (2Н, d, J = 8.8, H-2',6' Ar); 7.61 (2Н, d, J = 8.8, H-3',5' Ar); 7.76–7.87 (2H, m, H-2,10); 8.11 (1Н, d, J = 9.0, H-11); 8.21 (1Н, s, H-6); 8.90 (1Н, d, J-= 2.1, H-8); 8.97 (1Н, br. s, NH); 9.06 (1Н, dd, J = 4.5, J = 1.7, H-3); 9.61 (1Н, dd, J = 8.2, J = 1.7, H-1). 13C NMR spectrum (CDCl3), δ, ppm: 92.0; 117.5; 121.5; 123.6; 124.1; 124.9; 127.2; 131.7; 133.1; 133.1; 134.6; 136.1; 137.3; 138.8; 140.4; 140.9; 143.0; 143.2; 151.0. Found, m/z: 494.9447 [M(79Br)+H]+. С21Н13Br2N4О. Calculated, m/z: 494.9451.
9-(Trifluoromethyl)-5-[(4-(trifluoromethyl)phenyl]-amino)pyrido[3,2- a ]phenazine 7-oxide (14f). The second fraction of orange color, eluent PhMe. Yield 17 mg (7%), orange crystals, mp 231–232°С (petroleum ether). 1H NMR spectrum (CDCl3), δ, ppm (J, Hz): 7.65 (2Н, d, J = 8.6, H-2',6' Ar); 7.72 (2Н, d, J = 8.6, H-3',5' Ar); 7.82 (1Н, s, H-6); 7.86 (1H, dd, J = 8.1, J = 4.5, H-2); 7.90 (1H, dd, J = 8.8, J = 1.7, Н-10); 8.38 (1Н, d, J = 8.8, H-11); 8.47 (1Н, br. s, H-8); 9.10 (1Н, dd, J = 4.5, J = 1.6, H-3); 9.14 (1Н, br. s, NH); 9.67 (1Н, dd, J = 8.1, J = 1.6, H-1). 13C NMR spectrum (CDCl3), δ, ppm: 102.7; 116.8; 120.7; 120.9; 122.6; 123.0; 123.7 (2C); 123.8; 124.1 (2C); 125.3 (2C); 125.7 (2C); 126.7 (2C); 126.8; 127.0; 127.1 (3C); 128.4; 129.2; 129.3; 130.9; 131.8; 132.1; 132.2; 134.5; 140.6; 141.2; 142.0; 142.5; 143.2; 143.7; 146.5; 150.9. Found, m/z: 475.0982 [M+H]+. С23Н13F6N4О. Calculated, m/z: 475.0988.
X-ray structural studies of compounds 4b and 14f were performed on an Agilent SuperNova diffractometer using microfocus X-ray source with copper anode and Atlas S2 CCD detector. Crystals suitable for X-ray structural analysis were obtained by slow evaporation of chloroform solution at room temperature. The collection of reflexes, determination and refinement of unit cell parameters were performed by using the specialized CrysAlisPro 1.171.38.41 software suite (Rigaku Oxford Diffraction, 2015).21 The structures were solved by using ShelXT program (Sheldrick, 2015),22 structure refinement was also performed with ShelXL program (Sheldrick, 2015).23 Molecular graphics were rendered and prepared for publication with the Olex2 version 1.2.10 software suite.24 The complete X-ray diffraction datasets were deposited at the Cambridge Crystallographic Data Center (deposits CCDC 1854534 (compound 4b) and CCDC 1854919 (compound 14f)).
References
(a) Kumar, S.; Bawa, S.; Gupta, H. Mini-Rev. Med. Chem. 2009, 9, 1648. (b) Jain, S.; Chandra, V.; Jain, P. K.; Pathak , K.; Pathak, D.; Vaidya, A. Arab. J. Chem. 2016, DOI:https://doi.org/10.1016/j.arabjc.2016.10.009. (c) Gopaul, K.; Shintre, S. A.; Koorbanally, N. A. Anticancer Agents Med. Chem. 2015, 15(5), 631. (d) Sharma, V.; Mehta, D. K.; Das, R. Mini-Rev. Med. Chem. 2017, 17(16), 1557. (e) Musiol, R. Expert Opin. Drug Discovery 2017, 12(6), 583. (f) Hussaini, S. M. Expert Opin. Ther. Pat. 2016, 26(10), 1201. (g) Puskullu, M. O.; Tekiner, B.; Suzen, S. Mini-Rev. Med. Chem. 2013, 13, 365. (h) Michael, J. P. Nat. Prod. Rep. 1997, 14, 605.
Makosza, M.; Wojciechowski, K. Top. Heterocycl. Chem. 2014, 37, 51.
Baeten, M.; Maes, B. U.W. Adv. Organomet. Chem. 2017, 67, 401.
(a) Arends, I.; Sheldon, R.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, 2007. (b) Constable, D. J. C.; Dunn, P. J.; Hayler, J. D.; Humphrey, G. R.; Leazer, J. L. Linderman, R. J.; Lorenz, K.; Manley, J.; Pearlman, B. A.; Wells, A.; Zaks, A.; Zhang, T. Y. Green Chem. 2007, 9, 411. (c) Utepova, I. A.; Trestsova, M. A.; Chupakhin, O. N.; Charushin, V. N.; Rempel, A. A. Green Chem. 2015, 17, 4401.
(a) Chupakhin, O. N.; Charushin, V. N. Tetrahedron Lett. 2016, 57, 2665. (b) Charushin, V. N.; Chupakhin, O. N. Top. Heterocycl. Chem. 37, 2014, 1. (c) Gulevskaya, A. V.; Pozharskii, A. F. Top. Heterocycl. Chem. 2014, 37, 179. (d) Makosza, M. Synthesis 2017, 3247. (e) Chupakhin, O. N.; Charushin, V. N.; van der Plas, H. C. Nucleophilic Aromatic Substitution of Hydrogen; Academic Press: San Diego, 1994.
(a) Bashkin, J. K.; Rains, R.; Stern, M. Green Chem. 1999, 1, G41. (b) Triplett, R. D.; Rains, R. K. US Patent 7504539 B2, 2006.
Patriciu, O.-I.; Finaru, A.-L.; Sandulescu, I.; Guillaumet, G. Synthesis 2007, 3868.
Tondys, H.; van der Plas, H. C.; Wozniak, M. J. Heterocycl. Chem. 1985, 22, 353.
Wozniak, M.; Baranski, A.; Nowak, K.; van der Plas, H. C. J. Org. Chem. 1987, 52, 5643.
(a) Grzegozek, M. J. Heterocycl. Chem. 2008, 45, 1879. (b) Grzegozek, M.; Szpakiewicz, B.; Kowalski, P. ARKIVOC 2009, (vi), 84.
Budyka, M. F.; Terent'ev, P. B.; Kost, A. N. Chem. Heterocycl. Compd. 1978, 14, 663. [Khim. Geterotsikl. Soedin. 1978, 809.]
Garnier, E.; Audoux, J.; Pasquinet, E.; Suzenet, F.; Poullain, D.; Lebret, B.; Guillaumet, G. J. Org. Chem. 2004, 69, 7809.
Gulevskaya, A. V.; Tyaglivaya, I. N.; Verbeeck, S.; Maes, B. U. W.; Tkachuk, A. V. ARKIVOC 2011, (ix), 238.
Borovlev, I. V.; Demidov, O. P.; Saigakova, N. A.; Amangasieva G. A. Eur. J. Org. Chem. 2014, 7675.
(a) Shchepochkin, A. V.; Chupakhin, O. N.; Charushin, V. N.; Steglenko, D. V.; Minkin, V. I.; Rusinov, G. L.; Matern, A. I. RSC Adv. 2016, 6, 77834. (b) Makhaeva, G. F.; Lushchekina, S. V.; Boltneva, N. P.; Serebryakova, O. G.; Rudakova, E. V.; Ustyugov, A. A.; Bachurin, S. O.; Shchepochkin, A. V.; Chupakhin, O. N.; Charushin, V. N.; Richardson, R. J. Bioorg. Med. Chem. 2017, 25, 5981. (c) Shchepochkin, A. V.; Chupakhin, O. N.; Charushin, V. N.; Rusinov, G. L.; Subbotina, Yu. O.; Slepukhin, P. A.; Budnikova, Yu. G. Russ. Chem. Bull., Int. Ed. 2014, 62, 773. [Izv. Akad. Nauk, Ser. Khim. 2013, 772.]
(a) Wróbel, Z.; Kwast, A. Synlett 2007, 1525. (b) Wróbel, Z.; Kwast, A. Synthesis 2010, 3865. (c) Kwast, A.; Stachowska, K.; Trawczyński, A.; Wróbel, Z. Tetrahedron Lett. 2011, 52, 6484. (d) Wróbel, Z.; Stachowska, K.; Grudzień, K.; Kwast, A. Synlett 2011, 1439. (e) Wróbel, Z.; Wiecław, M.; Bujok, R.; Wojciechowski, K. Monatsh. Chem. 2013, 144, 1847.
(a) Avakyan, E. K.; Borovlev, I. V.; Demidov, O. P.; Amangasieva, G. A.; Pobedinskaya, D. Yu. Chem. Heterocycl. Compd. 2017, 53, 1207. [Khim. Geterotsikl. Soedin. 2017, 53, 1207.] (b) Borovlev, I. V.; Demidov, O. P.; Amangasieva, G. A.; Avakyan, E. K.; Borovleva, A. A.; Pobedinskaya, D. Yu. Synthesis 2018, DOI: 10.1055/s-0037-1610173.
Titova, S. P.; Arinich, A. K.; Gorelik, M. V. Russ. J. Org. Chem. 1986, 22, 1407. [Zh. Org. Khim. 1986, 1562.]
Gottlieb, H. E.; Kotlyar, V.; Nudelman, A. J. Org. Chem. 1997, 62, 7512.
Sharpe, D.; Gospy, I.; Rawley A. Organic Chemistry Laboratory [Russian translation]; Mir: Moscow, 1993, p. 240.
CrysAlisPro, version 1.171.38.41; Rigaku Oxford Diffraction, 2015. https://www.rigaku.com/en/products/smc/crysalis.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, А64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
This work was performed with financial support from the Ministry of Education and Science of the Russian Federation within the framework of State Assignment (project No. 4.6306.2017/8.9).
Author information
Authors and Affiliations
Corresponding author
Additional information
A Supplementary information file containing 1Н and 13С NMR spectra of all synthesized compounds is available at the journal website at http://springerlink.bibliotecabuap.elogim.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(9), 875–886
Electronic supplementary material
ESM 1
(PDF 9547 kb)
Rights and permissions
About this article
Cite this article
Demidov, O.P., Pobedinskaya, D.Y., Avakyan, E.K. et al. S N H Arylamination of Nitroquinolines: Access to Nitro and Nitroso Derivatives of Arylaminoquinolines. Chem Heterocycl Comp 54, 875–886 (2018). https://doi.org/10.1007/s10593-018-2368-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2368-x