Abstract
Cheetahs (Acinonyx jubatus) are a textbook example of how habitat loss, human-wildlife conflicts and historic bottlenecks depleted genetic variability, both genome-wide and at the major histocompatibility complex (MHC), which plays an integral role in the adaptive immune response. However, free-ranging Namibian cheetahs show no signs of impaired immunocompetence or health. This contradicts theoretical expectations and poses the question whether the manner by which MHC diversity is judged needs to be revised. Here, we show that free-ranging Namibian cheetahs still harbour MHC alleles that are divergent enough to cover several functionally distinct MHC supertypes and thus are probably capable of binding and presenting a relatively broad range of antigens to T-cells. We detected a similar pattern in three other free-ranging, strongly bottlenecked cat species, supporting the hypothesis that species with a low MHC allelic diversity might be able to retain functional diversity not within but across loci. Moreover, the allelic composition influences the level of MHC class I and class II expression which also might play a significant role in pathogen defence. Thus, our study indicates that the evolutionary role of MHC diversity goes beyond counting the remaining number of MHC alleles and offers an explanation as to how cat species might have avoided impaired immuno-competence, despite showing low MHC allelic diversity. Although the low MHC diversity currently seems to be sufficient to ensure the health of free-ranging cheetahs, it is currently unknown whether it can provide sufficient protection from future threats through emerging new pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conservation of genetic variability is considered a key aspect in ensuring evolutionary and adaptive processes enhancing the persistence of populations and species (Frankham et al. 2002). Diversity (i.e. the number of different alleles) in the immune genes of the major histocompatibility complex (MHC) coding for cell surface glycoproteins that bind and present antigens to T-cells is thought to have a strong impact on the functional plasticity of immune responses (Murphy and Weaver 2017). The best-known, mutually non-exclusive selection mechanisms driving high MHC diversity are ‘heterozygote advantage’ and ‘frequency-dependent selection’ (for reviews see Edwards and Hedrick 1998; Bernatchez and Landry 2003; Sommer 2005; Radwan et al. 2010). The ‘heterozygote advantage’ hypothesis (i.e. the overdominance model) states that MHC heterozygotes have a selective advantage over homozygotes because they might be able to mount a protective immune reaction against a wider range of different pathogens (Doherty and Zinkernagel 1975; Takahata and Nei 1990; Hughes and Nei 1992). The ‘frequency-dependent selection’ hypothesis assumes that the arms race between parasite and host gives an advantage to individuals carrying specific, less frequent alleles that will be particularly protective against a certain pathogen. Over time, different alleles will be protective such that a high MHC diversity is maintained in a host population (Clarke and Kirby 1966; Takahata and Nei 1990). Indeed, in most outbred vertebrate species, MHC genes are highly diverse in terms of their sequence variation among alleles, heterozygosity and population-wide number of alleles (Sommer 2005). Whereas pathogen-driven and sexual selection might retain resistance alleles in the population, strong population bottlenecks and inbreeding may lead to genetic drift and the loss of MHC alleles, which is often associated with high parasite loads and disease susceptibility (Meyer-Lucht and Sommer 2009; Siddle et al. 2010; Cheng et al. 2012). Moreover, the loss of MHC diversity poses the risk that deleterious mutations, which constitute a ‘sheltered load’ that remains unexpressed in most outbred and highly diverse populations, are expressed in bottlenecked species with usually high proportions of homozygous individuals (van Oosterhout 2009).
In recent years, however, examples of depleted MHC diversity but low susceptibility to parasites and diseases have been accumulating (reviewed by Radwan et al. 2010). This contradicts theoretical expectations and poses the question whether the manner by which MHC diversity is judged needs to be revised (Heinrich et al. 2017).
Cheetahs (Acinonyx jubatus) are a well-known textbook example of how habitat loss, human-wildlife conflicts and historic bottlenecks might have reduced genetic diversity, both genome-wide and at the MHC (Dobrynin et al. 2015). The free-ranging global cheetah population, with a large proportion of it occurring in Namibia, is estimated to comprise only ~ 7100 individuals confined to 9% of their historical range (Durant et al. 2017). The low genetic diversity has been proposed to explain disease vulnerability (O’Brien and Evermann 1988), absent ablation after skin grafts experiments between unrelated individuals (O’Brien et al. 1985), and inferior seminal quality compared with the domestic cat (Wildt et al. 1983). More recent studies revealed that free-ranging Namibian cheetahs have a slightly higher MHC class I diversity than initially reported but this is still relatively low when compared with outbreeding species (Castro-Prieto et al. 2011b). In contrast, the MHC class II diversity was as low as found in earlier studies in zoo animals (Castro-Prieto et al. 2011b). In contrast to earlier studies in zoo animals (O’Brien et al. 1985, 1986), however, impairment of the immunocompetence and high disease susceptibility has not been observed in wild cheetahs (Castro-Prieto et al. 2012; Heinrich et al. 2017). Studies of virus antibody seroprevalence and necropsies in free-ranging Namibian cheetahs indicated no clinical symptoms of acute viral diseases in cheetahs and generally a good health status (Munson et al. 2005; Thalwitzer et al. 2010).
One possible explanation for the puzzling finding that free-ranging Namibian cheetahs have a low MHC diversity and yet show no signs of apparent impaired health or immunocompetence is that the present MHC alleles might be divergent enough to cover several so-called MHC supertypes ensuring the defence against pathogens. This idea is based on the observation that the molecules encoded by different MHC alleles have different antigen-binding specificities, but that certain MHC molecules may recognise the same peptide motifs and that MHC alleles may be grouped accordingly into so-called MHC supertypes (Sidney et al. 1996). MHC alleles of the same supertype encode biochemically similar amino acids at antigen-binding sites and thus the molecules bind similar antigens, whereas molecules encoded by alleles from different supertypes recognise repertoires of different antigens. This concept has been confirmed by peptide prediction algorithms that have indicated that the overlap in bound antigens is smaller in molecules encoded by genetically divergent MHC allele pairs (Lenz 2011). The grouping of MHC alleles into supertypes is a widely used approach in human vaccine design (Sidney et al. 1996; Wang et al. 2016). Following the concept of the ‘divergent allele advantage’ hypothesis (Wakeland et al. 1990), the presence of MHC alleles from the different supertypes (and thus with different antigen-binding specificities) may be under pathogen-driven selection. Lighten et al. (2017) proposed that the supertypes cover different areas of the epitope space. Balancing selection is thought to act across the supertype variation when different parasite taxa challenge the host’s immune response. In other words, supertype frequencies rise and fall, depending on the presence of the parasite taxon that they recognise and bind (cf. negative frequency-dependent selection and Red Queen dynamics, Lighten et al. 2017).
Associations of certain MHC supertypes with susceptibility or resistance to parasites and diseases have been found in several free-ranging host species, for example, in associations of birds and avian malaria (Sepil et al. 2013), primates and intestinal parasites (Schwensow et al. 2007), rabbits and rabbit haemorrhagic disease virus (Schwensow et al. 2017) or amphibians and viral pathogens (Wang et al. 2017). Furthermore, in an experiment with a self-fertilising fish species (Kryptolebias marmoratus), Ellison and colleagues (2012) showed that the loss of alleles after bottlenecks was not random. After several generations of selfing, the most divergent alleles (i.e. alleles from different supertypes) were retained in the population; this was attributed to overdominant selection (Doherty and Zinkernagel 1975; Takahata and Nei 1990; Hughes and Nei 1992). Van Oosterhout (2013) proposed an intriguing alternative mechanism to overdominant (pathogen-driven) selection, namely that alleles from different MHC supertypes segregate at different loci. Even if alleles within loci become fixed, this means that supertypes can be preserved across different loci. This might explain the observation that the average MHC diversity of K. marmoratus is similar between the free-ranging population and laboratory lines after more than 10 generations of selfing (Ellison et al. 2012; van Oosterhout 2013).
Another possible explanation for the good health of the free-ranging Namibian cheetah is that the complexity of MHC diversity goes beyond the antigen-binding specificity of the specific alleles/supertypes. This may include the strength of immune responses that may be directed by the MHC expression level. As is becoming evident, genetic variation might exceed to the level at which the genes are expressed; this might modulate the efficacy of immune responses. For example, in humans, the expression of alleles at one MHC class I locus (HLA-C) has been associated with HIV control and non-progression to AIDS (Apps et al. 2013). Chicken MHC class I alleles differ from each other in their expression level on red blood cells, a feature that is associated with resistance against Marek’s disease (Kaufman et al. 1995; Kaufman and Salomonsen 1997). Moreover, MHC class II haplotype-specific mutations in regulatory gene regions influencing the transcription level might modulate immune reactions, and the frequently reported disease associations of human MHC alleles might be related to the level of gene expression, as well as to allelic differences in response to the antigen (Handunnetthi et al. 2010). In a natural population of yellow-necked mice (Apodemus flavicollis), cestode susceptibility is associated with high allele-specific MHC class II expression (Axtner and Sommer 2012). Studies investigating the role of MHC expression in wildlife, however, are still very limited. Whether the allelic composition at MHC genes influences their expression is often difficult to test in free-ranging species under natural conditions because of the aforementioned usually high MHC allelic variety and restrictions in sample size. Thus, free-ranging cheetahs represent a valuable study system, since only ten MHC class I sequences from four putative loci and only four MHC class II sequences from at least three loci have been found in > 140 free-ranging Namibian cheetahs (Castro-Prieto et al. 2011b).
In the present study, we used samples from this long-term study of free-ranging Namibian cheetahs (Thalwitzer et al. 2010; Castro-Prieto et al. 2011b, 2012; Heinrich et al. 2017) to test (1) whether MHC alleles have not been lost randomly in past bottlenecks and a certain degree of functional diversity is still retained in MHC supertypes and (2) whether the cheetah’s MHC allelic repertoire determines the expression level of both MHC class I and class II genes. According to the ‘heterozygote advantage’ hypothesis, we expected that heterozygotes (or individuals with a higher number of alleles) should have a higher MHC expression than homozygotes (or individuals with a lower number of alleles). We expect this only, if the level of MHC expression is selected for and simply determined by additive effects. This should not be the case if MHC alleles compete for surface expression. In this case, and also according to the ‘frequency-dependent selection’ hypothesis, the presence of specific alleles should influence the MHC expression level. This study is, to our knowledge, the first to address these basic questions about the role of MHC supertype diversity and expression in a free-ranging animal population. It also contributes to the gaining of a better understanding of the role of conservation genetics in a free-ranging charismatic cat species that is increasingly threatened through habitat loss and human-wildlife conflicts.
Materials and methods
Sampling of Namibian cheetahs
Between 2003 and 2012, 94 free-ranging cheetahs inhabiting commercial livestock or game farmlands in Namibia were trapped, immobilised, examined for their overall health status, sampled and released as described in Thalwitzer et al. (2010). For MHC sequence analyses, blood samples were centrifuged and the buffy coat fraction was stored in liquid nitrogen. For MHC expression analyses, PAX-gene blood RNA tubes (Qiagen, Hilden, Germany) and RNA protect animal blood tubes (Qiagen, Hilden, Germany) were filled with blood, incubated at room temperature for 24 h and stored at − 20 °C until transported to Germany. Paxgene samples were transported on dry ice, whereas all other samples were transported in a dry shipper in full compliance with the Convention on International Trade in Endangered Species (CITES) and stored at − 80 °C until further processing at the laboratory. Our study on free-ranging cheetahs was approved by the Ministry of Environment and Tourism of Namibia (permit numbers: 700/2003, 764/2004, 939/2005, 1089/2006, 1194/2007, 1300/2008, 1392/2009, 1514/2010, 1514/2011, 1689/2012) and the Internal Ethics Committee of the Leibniz Institute for Zoo and Wildlife Research (IZW, Berlin, Germany, permit number: 2002-04-01).
MHC class I and class II genotyping
Total genomic DNA was isolated from blood buffy coat by using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. We genotyped genes from both MHC class I and II genes. MHC class I molecules bind fragments from endogenous proteins such as those derived from viruses or cancer-infected cells and present them to CD8 + T-cells. MHC class II molecules present extracellularly derived antigens, for example, those from bacteria or helminths, to CD4 + T-cells (Doherty and Zinkernagel 1975). We focused our analyses on the second exons of MHC class I and II because these regions are highly polymorphic and include functionally important antigen-binding sites (ABS), i.e. amino acid positions postulated to interact directly with foreign antigens (Bjorkman et al. 1987; Brown et al. 1993). Moreover, previous investigations using Sanger-sequencing provided stronger evidence for positive selection on the ABS of MHC I exon 2 than exon 3 (Castro-Prieto et al. 2012). The study further suggested that the identified sequences correspond to MHC I alleles from different putative loci (Castro-Prieto et al. 2012). In the present study, we determined MHC class I allelic diversity by using a high throughput sequencing approach as described recently by Santos et al. (2017). The MHC loci were amplified with primers adjusted to the requirements of Illumina sequencing technology, and all individuals genotyped at a 229-bp fragment by using four primer pairs and two technical replicates per individual. Primer sequences, PCR protocols and fragment length are detailed in Supplementary Table 1. Indexing was performed with a Fluidigm Access Array Barcode Library for Illumina Sequencers − 384. Isomolarity for the downstream steps was achieved by measuring the DNA concentration with a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) and by adjusting volumes accordingly with H2O. Purification was performed with the MinElute 96 UF PCR Purification Kit (Qiagen). Sequencing was carried out on a MiSeq platform (Illumina, Inc.). We followed our previously published bioinformatics pipeline (Sommer et al. 2013). Results were checked for artefacts by first removing low-quality reads (mean Phred quality score < 20) and reads with any sequencing errors on primers or barcodes. All reads were then submitted to local BLAST against MHC class I sequences and clustering was performed in QIIME (Caporaso et al. 2010) with default settings. Putative chimeras were detected and removed by using UCHIME (Edgar 2004). The MHC allele calling of each sample was based only on variants that passed our conservative approach and had been identified in both replicates, i.e. originated in independent PCRs (Sommer et al. 2013; Clozato et al. 2015; Schuster et al. 2016; Santos et al. 2017). Since the MHC class II diversity in cheetahs is considerably lower than the one of MHC class I, no next-generation sequencing approach was required. Genotyping of the second exon (246 bp) of MHC II-DRB was conducted through single-stranded conformation polymorphism (SSCP) analysis followed by Sanger sequencing of the distinctive single-strand bands as described previously (Castro-Prieto et al. 2011b, 2012). To ensure that the sequences represented true alleles, the PCR-SSCP analysis was conducted twice per individual sample.
MHC class I and class II expression analyses
RNA was isolated from 2.5 ml whole blood by using the PAXgene blood RNA kit (Qiagen) or 500 µl whole blood by using the RNeasy Protect Animal Blood System (Qiagen) following the manufacturer’s instructions. To ensure the removal of genomic DNA from the isolated RNA, a second DNA digestion was performed by using the DNase I RNase-free Set (Fermentas, St. Leon-Rot, Germany). cDNA synthesis was obtained by using 200 U RevertAid™ H Minus M-MuLV Reverse Transcriptase (Fermentas) in a 20 µl reaction tube containing 2 µg total RNA as a template and 1 µl Oligo(dT)12−18 primer (0.5 µg/µl, Invitrogen, Karlsruhe, Germany), 0.5 µl ribonuclease inhibitor (20 U/µl), 2 µl dNTP mix (10 mM), 4 µl buffer (5×) and RNase-free water. The reaction was incubated at 42 °C for 60 min.
We performed quantitative PCRs on a Rotor Gene 3000 (Corbett Research) by using three different primer pairs each to amplify the different MHC class I loci/alleles and MHC class II loci/alleles, respectively (Supplementary Table 1). Four reference genes, glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), β2-microglobulin (B2M), 40 s ribosomal protein S7 (RPS7) and 14-3-3 protein zeta/delta (YWHAZ) were also included for each sample. Reference gene validation was performed using the RefFinder tool (http://150.216.56.64/referencegene.php?type=reference, for results see Supplementary File 1). All reactions were run in triplicates with a no-template control to check for contamination. The output (i.e. non-baseline corrected data) obtained from the real-time PCR was analysed by using the program LinRegPCR v. 2016.1, which performs baseline correction for each sample separately, determines a window-of-linearity per amplicon and calculates the mean PCR efficiency E per sample (results in Supplementary File 2). LinRegPCR further determined the individual PCR fluorescence threshold Nq (set at one cycle below the upper limit of the window of linearity) and Cq, the fractional cycle at which Nq was reached (Ruijter et al. 2009, 2014; Tuomi et al. 2010). The data were inspected visually for Cq or expression outliers. We then calculated the individual expression value Q = E− Cq for each gene. The arithmetic mean of Q for each MHC amplicon was calculated from the triplicate repeats and normalised to the geometric mean of the four reference genes GAPDH, B2M, RPS7 and YWHAZ (Vandesompele et al. 2002). As the Illumina sequencing results indicated a more complex genomic architecture of the cheetah class I MHC than Sanger sequencing (Castro-Prieto et al. 2011b) on which qPCR primer design was based, locus assignment of the qPCR MHC class I primer pairs could not be done with certainty in all cases. Therefore, we chose a conservative approach and measured the overall expression level at MHC class I as the sum of the expression of each of the three MHC class I primer pairs.
Statistical analyses
Phylogenetic relationships between MHC class I alleles were analysed by using Bayesian inference as implemented in Mr. Bayes 3.2.6 (Huelsenbeck and Ronquist 2001). The substitution model was GTR + Γ + I as previously established (Castro-Prieto et al. 2011b). The length of the MCMC chain was 5 × 10^6 generations, with a burn-in of 25% and a sampling every 5 × 103 generations.
To test whether a certain degree of functional diversity (i.e. different antigen-binding amino acid motifs) is present in MHC supertypes, we translated all known cheetah MHC class I nucleotide sequences (this study, Castro-Prieto et al. 2012) and all available Genbank sequences of the Asian lion (Panthera leo persica), two leopard subspecies (African leopard, Panthera pardus pardus and Amur leopard, Panthera pardus orientalis) and the domestic cat (Felis catus) into amino acid sequences and aligned them (for accession numbers see Supplementary File 3). Thereby, some nucleotide sequences translated into the same amino acid sequence. The amino acids at the putative antigen-binding sites (Bjorkman et al. 1987; Bjorkman and Parham 1990) were transformed into a matrix by using five physiochemical descriptor variables z1 (hydrophobicity), z2 (steric bulk), z3 (polarity), z4 and z5 (electronic effects) (Sandberg et al. 1998). We used discriminant Analyses of Principle Components (DAPC) implemented in the adegenet R package (Jombart 2008) to cluster the amino acid sequences into groups with putatively similar physiochemical characteristics and binding spectra, hereafter referred to as ‘MHC supertypes’ (Sepil et al. 2013; Lighten et al. 2017; Schwensow et al. 2017; Wang et al. 2017).
In order to test whether the MHC allelic repertoire of the cheetah determined the expression level of both MHC class I and class II genes, we first investigated whether the overall number of MHC alleles (as a surrogate for heterozygosity), the presence/absence of certain MHC alleles or the sex was associated with the overall individual MHC class I expression. Secondly, we tested whether the genotype, heterozygosity, the presence of a certain allele or the sex affected the individual MHC expression at specific MHC class I (MHC1 locus D, Castro-Prieto et al. 2011b) and II loci (MHC2 locus C, Castro-Prieto et al. 2011b). We constructed generalised linear models with a gamma error distribution by using the R software environment for statistical and graphical computing (R Development Core Team 2013) and the packages MuMIn (Barton 2015) and AICcmodavg (Mazerolle 2016). Full model selection and multimodel inference (Burnham and Anderson 2002) was conducted in order to determine a minimum adequate model set and variable influence. To avoid overparameterisation, we limited the number of explanatory variables per model to three and used Akaike’s Information Criterion (AICc) corrected for finite sample size to compare models. Finally, we evaluated whether the overall expression differed between the MHC class I and II genes and whether the individual MHC expression was correlated between the MHC classes. Therefore, we used a Kruskal–Wallis test (Kruskal and Wallis 1952) and compared the expression measured by the six different primer pairs (see Supplementary Table 1 for details). Posthoc pairwise multiple comparisons were calculated according to Dunn (1964) with a Bonferroni correction for multiple comparisons by using the R package PMCMR (Pohlert 2014). Deviation from Hardy–Weinberg equilibrium was tested by using the R package HardyWeinberg (Graffelman 2015).
Results
MHC class I and class II allelic diversity
High throughput sequencing of 94 individuals on an Illumina Miseq platform yielded 1,815,749 reads after quality filtering. The coverage per individual ranged from 8,081 to 29,051 reads (average: 19,316 ± 4,579 SD). The reads assembled into 11 sequence clusters that had a length of 229 bp and for simplicity are hereafter referred to as MHC class I alleles ignoring that sequences may come from different co-amplified loci. No stop codons and no shifted or disrupted reading frames were observed.
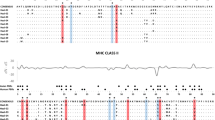
As in our previous study (Castro-Prieto et al. 2011b), we detected between three and eight MHC class I alleles per individual (average = 5.46 ± 1.18 SD). Seven out of ten previously known alleles were detected again in the present study (Fig. 1), which was carried out in the same study region in Namibia as before. These alleles also included the previously described not-expressed allele (Acju-MHC*12) belonging to a pseudogene (formerly MHC class I locus C, Castro-Prieto et al. 2011b). By using a NGS-Illumina approach, we detected four new MHC class I alleles (Acju-MHC*13–16, Genbank accession numbers MK411535-MK411538) that have not been found before when using a combined single-strand conformation polymorphism and Sanger sequencing approach in 149 individuals (Castro-Prieto et al. 2011b). The accordingly updated phylogenetic analysis (Fig. 1) showed that locus assignment of most alleles is more complex than suggested by Sanger sequencing (Castro-Prieto et al. 2011b). Only the monophyly of the previously reported MHC class I locus D could be confirmed (Castro-Prieto et al. 2011b). This locus encompassed the most prevalent allele Acju-MHCI*05 which was detected in 96% of all samples and the allele Acju-MHCI*07. The newly identified alleles Acju-MHCI*14, Acju-MHCI*15 and Acju-MHCI*16 formed a previously not detected monophyletic cluster (Fig. 1). Acju-MHCI*14 was the second most prevalent allele in the population and found in 86% of the cheetahs. The relative abundance of all other alleles ranged from 9 to 79%.
Comparison of inferred phylogenetic relationships of all known MHC class I and II alleles in cheetahs. a Phylogenetic relationships of MHC class I alleles and locus assignment (A–D) as previously inferred from SSCP and Sanger sequencing [adopted from Castro-Prieto et al. (2011a, b)]. b Phylogenetic tree of all reported MHC class I alleles (Castro-Prieto et al. 2011a, b, this study by Illumina sequencing) as estimated by using MrBayes. The alleles newly identified by the Illumina sequencing approach are coloured in blue. Cheetah MHC class I alleles that were detected previously (Castro-Prieto et al. 2011a, b) but not found in the individuals of the present study are indicated in grey. Values printed on branches show the posterior probability, values in brackets show the relative abundance of the respective allele in the sample. c Phylogenetic relationships of MHC class II alleles and locus assignment (A–C) as previously inferred from SSCP and Sanger sequencing [adopted from Castro-Prieto et al. (2011a, b)]
The DAPC analysis suggested that the available amino acid MHC class I sequences from the five different cat species group into 12 MHC supertypes (Fig. 2, Supplementary Fig. 1). Most MHC alleles were assigned to a supertype with high confidence (posterior probability > > 0.95, Supplementary file 3). Cheetah alleles probably represent six different supertypes, as did the alleles of the domestic cat. The alleles of the Amur leopard and the African leopard each fell into five different supertypes and the Asian lion alleles fell into seven supertypes. All cat species had a similar number of STs in spite of differences in the number of alleles (Supplementary Fig. 2). All cheetah individuals carried alleles belonging to ST11. Nine percent of the cheetahs had alleles from two supertypes but most cheetahs had alleles belonging to three (40%) or four (46%) different supertypes. Only 4% had alleles from 5 supertypes and 1% of the cheetahs had alleles from all six supertypes.
Probability of MHC allele assignment to MHC supertypes (based on the results of DAPC). MHC class I alleles are numbered as follows: Cheetah: 1–10, Asian lion: 11–51, African leopard: 52–57, Amur leopard: 58–69, domestic cat: 70–97. For membership probabilities, allele names and accession numbers of the sequences see Supplementary file 2. Note that cluster 7 was established by the principle component analysis but no allele was grouped into it with a high posterior probability by the DAPC
No further MHC class II alleles were detected and thus no changes to the previously proposed phylogenetic relationships were made (Fig. 1).
MHC class I and class II expression
Overall MHC class I expression
We first tested whether the total number of alleles (i.e. a surrogate of heterozygosity) or the presence of a specific MHC allele influenced the overall MHC class I expression. Whereas the total number of alleles had no effect, the overall MHC class I expression was lower when the allele AJUMHCAJUI1 was present (Fig. 3; Table 1). AJUMHCAJUI1 was retained in all top models (with a ΔAICc < 2, Supplementary Table 2). The best model according to AICc was the one including only the presence/absence of AJIMHCAJUI1 and this parameter was found to significantly contribute to explaining the variation of overall MHC class I expression (Table 1).
Effects of a the total number of alleles or b the presence of AJUMHCAJUI1 on the overall MHC class I expression of cheetahs. The mean (blue line) and the variance (grey band) are indicated (N3 alleles = 11, N4 alleles = 20, N5 alleles = 32, N6 alleles = 14, N7 alleles = 4, N8 alleles = 1). The small blue lines in b indicate the number of samples per group. For model estimates and information on the model selection see Table 1 and Suppl. Table 2, respectively
MHC class I locus D expression
In the next step, we investigated whether the genotype, heterozygosity, the presence of a specific allele and sex influenced the expression level at the monophyletic MHC class I D-locus (Fig. 4). The best model indicated that individuals carrying at least one copy of the MHC class I allele Acju-MHC1*07 had a higher expression at the D-locus than individuals not having a copy of this allele (AICc = 259.59, p = 0.05, Table 2; Fig. 4). The second and third best models both yielded highly similar AICc values and indicated that heterozygosity increased expression (Table 2; Fig. 4). The allele frequencies at this locus did not deviate significantly from Hardy–Weinberg equilibrium (HWE, χ2=0.19, p = 0.67). However, note that out of the 88 individuals included in this model, only four individuals (5%) were homozygous for Acju-MHC1*07, whereas 57% were homozygous for the lower expressed Acju-MHC1*05 and 39% were heterozygous. Furthermore, all three models indicated that males tended to have a higher expression at this locus than females (p = 0.06, Table 2; Fig. 4).
Effects of a genotype, b heterozygosity, or c the presence of a specific allele influence the expression level at the monophyletic MHC class I D-locus. d Sex was implemented in all models as an explanatory variable. The p values of the potentially influencing parameters are given and the means and variances are indicated. The small blue lines at the bottom and top indicate the number of samples per group. For model estimates and information on the model selection see Table 2
MHC class II locus C expression
We further investigated whether the genotype, heterozygosity, the presence of a specific allele and/or sex influenced the expression level at the monophyletic MHC class II C-locus. The best model revealed that heterozygotes were lower expressed than homozygous individuals (p = 0.05, Table 3; Fig. 5), probably because of the competition between the alleles *14 and *15. No differences in the expression of the respective genotypes, the presence of either allele and males and females were observed (Table 3; Fig. 5). The allele frequencies at this locus deviated from HWE (χ2=4.90, p = 0.03) with higher numbers of heterozygotes than expected.
Effects of a genotype, b heterozygosity, the presence of c allele Acju-MHCI*14 or d allele Acju-MHCI*15 on the expression level at the monophyletic MHC class II C-locus. Sex was implemented in all models but had no effect. The p-value of the potentially influencing parameter is given and the means and variances are indicated. The small blue lines at the bottom and top indicate the number of samples per group. For model estimates and information on the model selection see Table 3. Allele 14 = Acju-MHCI*14, Allele 15 = Acju-MHCI*15
Comparison of MHC class I and II expression
Finally, we compared the expression levels of MHC class I and II (Fig. 6). The expression level measured with the three MHC class I primer pairs was both significantly higher and more variable than the expression level measured with the three MHC class II primer pairs (Kruskal–Wallis χ 2 = 99.68, p < 2.2e−16). Posthoc pairwise comparisons using Dunn’s tests showed significant differences between MHC class I and class II (all Bonferroni-corrected p values ≤ 0.001) but no differences within classes (all Bonferroni-corrected p values > 0.49) (Fig. 6a). Furthermore, the individual expression of MHC class I and class II was strongly correlated (Pearson correlation, p = 1.68e−13, r = 0.72, Fig. 6b).
Comparison of the cheetah MHC class I and class II expression. a Expression measured with each of the three MHC class I and class II primer pairs. Posthoc pairwise comparisons showed no differences within MHC classes (Dunn’s test, all Bonferroni-corrected p values > 0.49) but the expression level differed between classes (Dunn’s test, all Bonferroni-corrected p values ≤ 0.001). b Correlation between the overall MHC class I and class II expression
Discussion
By using the more sensitive next generation Illumina sequencing technology, we identified four new MHC class I alleles in 94 free-ranging Namibian cheetahs. Three of these alleles grouped into a new cluster, potentially a new locus, in our phylogenetic analysis. Although the MHC class I variability in cheetahs is higher than previously thought, this species still shows dramatically low levels of MHC class I diversity and a high degree of homozygosity when compared with other felids (Castro-Prieto et al. 2011b; Dobrynin et al. 2015). One possible explanation for the finding that free-ranging Namibian cheetahs have a low MHC diversity and yet show no signs of apparent impaired health or immunocompetence is that the present MHC alleles may still be divergent enough to cover several functionally distinct MHC supertypes, such that (currently) a sufficient degree of diversity is given. Another possible explanation is that the complexity of MHC diversity may go beyond the antigen-binding specificity of certain MHC alleles/supertypes. The allelic diversity might affect the strength of the immune responses, which may be directed by the MHC expression level. Our study of free-ranging cheetahs demonstrates that functional MHC diversity is retained in MHC supertypes and that MHC expression is triggered by allelic diversity. This adds a new perspective to the conservation of genetic diversity in threatened species which possibly lose immune genetic sequence diversity linked to anthropogenic impact and environmental changes.
The amino acids at the antigen-binding sites (ABS) determine those antigens that can be bound by the MHC molecule. Therefore, in humans and wildlife, the grouping of MHC alleles into MHC supertypes according to their functional properties at the ABS has long been recognised as a valid approach for classifying functional diversity. It has been used in vaccine research and to investigate associations with pathogen resistance or disease susceptibility (Trachtenberg et al. 2003; Schwensow et al. 2007, 2017; Sepil et al. 2013; Wang et al. 2017). The publicly available Felidae MHC class I sequences used in this study belong to 12 different MHC supertypes. All free-ranging cat species included in our supertype assignment have small populations and have experienced strong bottlenecks. The IUCN status of Asiatic lions is currently classified as ‘endangered’ (IUCN 2017) with only a few hundred individuals existing in a single isolated relict population in the Gir forest, India (summarized by Sachdev et al. 2005). The Amur leopard is critically endangered (IUCN 2017) and both African leopards and cheetahs are currently classified as vulnerable (IUCN 2017), although strong evidence supports the view that the IUCN status of the cheetah should be upgraded to ‘endangered’ (Durant et al. 2017). Nevertheless, all species have retained functionally diverse alleles and all of them still harbour alleles from at least four MHC class I supertypes. Namibian leopards, for example, have a low MHC diversity, and only six MHC class I alleles were found in 25 sampled individuals (Castro-Prieto et al. 2011a). However, our analyses here indicate that these alleles are functionally highly divergent and cluster into five different supertypes. The MHC class I alleles detected in 20 Asiatic lions group into seven different supertypes. In our Namibian cheetah study population, only 9% of the individuals carried alleles clustering into two supertypes, whereas 86% carried alleles from three or four supertypes, 4% had alleles belonging to five supertypes and only 1% had alleles from six supertypes. This pattern of retained supertype variability in the four free-ranging cat species that went through past bottlenecks and strong population declines suggests that alleles are not lost randomly. Species with a low MHC diversity may still retain alleles that are divergent enough to cover several functional important MHC supertypes. Our data also support the idea that alleles assigned to different supertypes may derive from different loci (van Oosterhout 2013). Cheetahs carried up to four single-allele supertypes, meaning that at least two loci must encode the respective four supertypes. On the other hand, the same supertype can also be encoded by more than one locus. The alleles Acju-MHC*04, Acju-MHC*05 and Acju-MHC*07 are located at two different loci but all belong to ST11 (Fig. 1, Supplementary File 3), and we found that 13% of the sampled cheetahs carry simultaneously these three alleles and therefore had at least three copies of the same supertype. Moreover, several supertypes are shared in the investigated cat species; for example, Asian lions, Amur leopards and African leopards all have alleles belonging to ST2 (Supplementary File 3). The studied free-ranging cheetahs live sympatrically with African leopards (Castro-Prieto et al. 2011a) and we found that both species have alleles belonging to the same supertypes (ST1, ST9 and ST11, Supplementary File 3). This indicates that even MHC supertypes consisting of ancient and divergent allelic lineages that predate speciation in natural populations (known as trans-species polymorphism) are maintained over time, probably because of balancing selection by similar parasite and pathogen species (Wakeland et al. 1990; Richman 2000). Identical MHC supertypes were recently also found in three fish species (Poecilia reticulata, P. obscura and Micropoecilia picta) despite within-species population allelic differentiation and a simulation has indicated that these MHC supertypes have been maintained, despite changes in allele frequencies and the loss of alleles in individual populations (Lighten et al. 2017). This was attributed to Red Queen dynamics at the MHC supertype level, and evidence was presented that MHC alleles underlie positive selection in Red Queen arms races. Our results suggest that the evolutionary model developed by Lighten et al. (2017) is also applicable for MHC evolution in Felidae or, more generally, in other vertebrate species.
We further demonstrated that the MHC allelic repertoire of the cheetah influences the expression level of both MHC class I and class II genes indicating that the individual genetic make-up determines expression differences measured in the wild. Therefore, the level of expression may itself be a feature under selection in nature. Individual differences in immune gene expression in free-ranging species are likely to be consistent over time, as recently shown in individuals of free-ranging field voles (Microtus agrestis) investigated at various points in time (Arriero et al. 2017). Pathogen infections may select for an optimal MHC expression level in the host, for example, hosts with low expression of the B21 haplotype have a selective advantage over highly expressing haplotypes as higher MHC expression is associated with susceptibility to Marek’s disease in chicken (Kaufman and Salomonsen 1997). Pathogens such as viruses or bacteria can also directly influence the level of MHC expression to avoid immune recognition (Sengupta et al. 2017; Ende et al. 2018), and in this case, individuals that are more resistant to changes in their expression should have an advantage. For example, MHC class I and class II genes of some susceptible amphibian species are up-regulated following host infection by the fungal pathogen Batrachochytrium dendrobatidis, but resistant species did not show comparable changes in transcriptional expression (Fu and Waldman 2017).
Our results revealed that the presence of certain MHC alleles influenced the level at which MHC genes are expressed. Individuals carrying the MHC class I allele AJUMHCAJUI1 show lower overall MHC class I expression than individuals not carrying this allele. In contrast, the total number of alleles has no effect on the overall expression level. This indicates that the overall MHC expression is not determined by simple additive effects of alleles present in an individual carrier but by specific alleles differing in their individual expression. Different MHC class I alleles might compete for surface expression. This has been reported from an experimental study with mice in which certain MHC class I alleles were differentially expressed in the two homozygous parental strains compared to their heterozygous F1 hybrids (Tourdot and Gould 2002). The authors demonstrated a particularly strong effect of individuals being homozygous for the H-2b allele, which dominated the expression over H-2b/H-2k hybrids, and concluded that competition might occur between alleles of an individual determining its overall expression level. If cheetah alleles compete in a similar way, the expression of AJUMHCAJUI1 might be dominated by other alleles. However, AJUMHCAJUI1 could not be assigned to a specific locus with sufficient confidence, thus it was not possible to study locus-specific interaction effects in this case.
However, we conducted genotype-specific analyses for the MHCI-D and MHCII-C locus. All models suggested that males had a higher expression in MHCI-D than females. Sex differences in the expression level of some genes are widespread and may underlie various forces of selection (Ellegren and Parsch 2007). In the best model, the presence of the MHC I allele Acju-MHCI*07 increased the expression level at the MHC-I D locus (Fig. 4; Table 2). The second best model with a similar fit suggested that heterozygous individuals have a higher expression, with the third model that contained the genotypes showing a similar result. Most cheetahs (57%) were homozygous for the alternative Acju-MHCI*05 allele at this locus. If Acju-MHCI*07 is highly expressed, a higher expression level in heterozygotes than homozygotes carrying the alternative allele would explain this finding. Interestingly, only 5% of the sampled individuals carried Acju-MHCI*07 in a homozygous state. No significant deviation from HWE was observed at this locus but our sample size is too low for us to make inferences about the average level of homozygous Acju-MHCI*07 expression, or whether the rare observation of this genotype is of selective importance.
We did not find evidence that Acju-MHCI*07 influences the overall MHC class I expression. Nevertheless, the observation that distinct MHC class I alleles were differentially expressed agrees well with results from humans and rhesus macaques (Greene et al. 2011). The expression level of certain alleles was reported to possibly influence disease progression. For example, the allele-specific level of expression at the HLA-C locus influences disease progress and HIV viral loads in humans (Thomas et al. 2009), and MHC class I allele-specific resistance to Marek’s disease was demonstrated in chicken (Kaufman et al. 1995; Kaufman and Salomonsen 1997). For the cheetah MHC class II locus C, the best model revealed that heterozygotes had a lower expression than homozygous individuals (Table 3), presumably because of competition between alleles Acju-MHCI*14 and Acju-MHCI*15. At this locus, we observed deviation from HWE. More cheetahs (60%) were heterozygous and fewer cheetahs were homozygous than expected (18% homozygous for Acju-MHCI*14 and 22% for Acju-MHCI*15). This may suggest the presence of selection against high expression at this locus. No differences in the expression of the respective genotypes and males and females were observed (Fig. 5; Table 3). In general, cheetahs are extremely invariable at MHC class II loci. Three classical (= encoding antigen-presenting molecules) MHC class II loci were inferred (Castro-Prieto et al. 2011b), with two of them showing allelic fixation, i.e. all individuals are homozygotes with the same allele. Thus, only a single heterozygous MHC class II locus is currently known in cheetahs.
Our results suggest that the role of MHC diversity goes beyond sequence variability. Indeed, the idea that MHC sequence diversity and expression are connected may be even more complex. The expression level may be directly associated with the binding capacities of specific MHC molecules. A recent study demonstrated that some chicken and human MHC class I alleles have a wider antigen-binding spectrum (‘generalist’ alleles) than others (‘specialist’ alleles). The expression level of ‘generalist’ alleles was lower than the expression level of ‘specialist’ alleles, and the authors suggested that the negative selection of T-cells in the thymus explains the lower expression level of promiscuous alleles and that these differences ensure optimal T-cell responses against various pathogens (Chappell et al. 2015). They proposed that low expressing generalist alleles bind and present a wider range of different antigens that will activate a wider range of T-cell clones and that this strategy is beneficial for the immune response against slow evolving pathogens (e.g. large DNA viruses, bacteria or parasites). On the contrary, against fast evolving pathogens (e.g. RNA viruses) with limited scope for immune evasion, particular highly expressed fastidious specialist MHC alleles might be the optimal strategy (Chappell et al. 2015). Our comparison of the overall expression level between MHC class I and II genes revealed significantly lower MHC class II than class I expression. This may have been influenced by the fact that MHC class II is only expressed on specific, antigen-presenting cells while MHC class I is thought to be expressed on all nucleated blood cells which might lead to higher levels of MHC class I than class II RNA in the blood. Following Chappell et al. (2015), we suggest an alternative (or additional and non-exclusive) hypothesis, namely that the few molecules expressed at MHC class II in cheetahs might be lowly expressed generalist alleles that protect against a wider array of parasites and pathogens. This combination of low expressed MHC II alleles and high expressed MHC I alleles may be the reason why none of the free-ranging cheetahs of this study population showed any clinical or pathological evidence of disease (n = 62, Thalwitzer et al. 2010, Wachter et al., unpublished results). An interaction between two such generalist alleles might explain the reduced expression level of heterozygotes of the MHC class II locus C in a similar way as proposed by Chappell et al. (2015). However, future studies investigating MHC class II expression in different organs will be needed to test this hypothesis.
Conclusion
All four free-ranging, strongly bottlenecked cat species studied here carried alleles from several functionally divergent MHC supertypes, supporting the hypothesis that species with a low MHC allelic variability could maintain diversity across but not within loci. We further demonstrated that the MHC allelic repertoire of the cheetah determines the expression level of both MHC class I and class II genes indicating that the MHC expression is triggered by MHC diversity. This combined mechanism offers an explanation for the current lack of impairment of the immune system of the cheetah. It also adds a new perspective to the conservation of genetic diversity in threatened species losing MHC allelic diversity due to anthropogenic impact and environmental changes. On a cautionary note, one has to keep in mind that, although the low MHC allelic diversity currently seems to be sufficient to ensure the good health status of free-ranging cheetahs, it may not provide sufficient protection from emerging new pathogens. Cheetahs are globally declining and 77% of their current range lies outside of protected areas (Durant et al. 2017). This makes it likely that contact zones between cheetahs and pathogens of humans, pets or livestock will increase. Low MHC diversity may, thus, at some stage become insufficient for these potential future threats.
References
Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, Brumme CJ, John M, Mallal S, Nelson G, Bosch R, Heckerman D, Stein JL, Soderberg KA, Moody MA, Denny TN, Zeng X, Fang J, Moffett A, Lifson JD, Goedert JJ, Buchbinder S, Kirk GD, Fellay J, McLaren P, Deeks SG, Pereyra F, Walker B, Michael NL, Weintrob A, Wolinsky S, Liao W, Carrington M (2013) Influence of HLA-C expression level on HIV control. Science 340:87–91
Arriero E, Wanelik KM, Birtles RJ, Bradley JE, Jackson JA, Paterson S, Begon M (2017) From the animal house to the field: Are there consistent individual differences in immunological profile in wild populations of field voles (Microtus agrestis)? PLoS ONE 12:e0183450
Axtner J, Sommer S (2012) The functional importance of sequence versus expression variability of MHC alleles in parasite resistance. Genetica 140:407–420
Barton K (2015) MuMIn: multi-model inference. R package version 1.14.0., http://CRAN.R-project.org/package=MuMIn
Bernatchez L, Landry C (2003) MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years? J Evol Biol 16:363–377
Bjorkman P, Parham P (1990) Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem 59:253–288
Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC (1987) The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 329:512–518
Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC (1993) Three-dimensional structure of the human class II histocompatibility antigen HLA DR1. Nature 364:33–39
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. edn. Springer, New York
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335
Castro-Prieto A, Wachter B, Melzheimer J, Thalwitzer S, Sommer S (2011a) Diversity and evolutionary patterns of immune genes in free-ranging Namibian leopards (Panthera pardus pardus). J Hered 102:653–665
Castro-Prieto A, Wachter B, Sommer S (2011b) Cheetah paradigm revisited: MHC diversity in the world’s largest free-ranging population. Mol Biol Evol 28:1455–1468
Castro-Prieto A, Wachter B, Melzheimer J, Thalwitzer S, Hofer H, Sommer S (2012) Immunogenetic variation and differential pathogen exposure in free-ranging cheetahs across Namibian farmlands. PLoS ONE 7:e49129
Chappell PE, Meziane EK, Harrison M, Magiera Ł, Hermann C, Mears L, Wrobel AG, Durant C, Nielsen LL, Buus S, Ternette N, Mwangi W, Butter C, Nair V, Ahyee T, Duggleby R, Madrigal A, Roversi P, Lea SM, Kaufman J (2015) Expression levels of MHC class I molecules are inversely correlated with promiscuity of peptide binding. eLife 4:e05345
Cheng Y, Sanderson C, Jones M, Belov K (2012) Low MHC class II diversity in the Tasmanian devil (Sarcophilus harrisii). Immunogenetics 64:525–533
Clarke B, Kirby DR (1966) Maintenance of histocompatibility polymorphisms. Nature 211:999–1000
Clozato CL, Mazzoni CJ, Moraes-Barros N, Morgante JS, Sommer S (2015) Spatial pattern of adaptive and neutral genetic diversity across different biomes in the lesser anteater (Tamandua tetradactyla). Ecol Evol 5:4932–4948
Dobrynin P, Liu S, Tamazian G, Xiong Z, Yurchenko AA, Krasheninnikova K, Kliver S, Schmidt-Küntzel A, Koepfli K-P, Johnson W, Kuderna LFK, García-Pérez R, Manuel MD, Godinez R, Komissarov A, Makunin A, Brukhin V, Qiu W, Zhou L, Li F, Yi J, Driscoll C, Antunes A, Oleksyk TK, Eizirik E, Perelman P, Roelke M, Wildt D, Diekhans M, Marques-Bonet T, Marker L, Bhak J, Wang J, Zhang G, O’Brien SJ (2015) Genomic legacy of the African cheetah, Acinonyx jubatus. Genome Biol 16:277
Doherty PC, Zinkernagel RM (1975) Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature 256:50–52
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Durant SM, Mitchell N, Groom R, Pettorelli N, Ipavec A, Jacobson AP, Woodroffe R, Böhm M, Hunter LTB, Becker MS, Broekhuis F, Bashir S, Andresen L, Aschenborn O, Beddiaf M, Belbachir F, Belbachir-Bazi A, Berbash A, Brandao de Matos Machado I, Breitenmoser C, Chege M, Cilliers D, Davies-Mostert H, Dickman AJ, Ezekiel F, Farhadinia MS, Funston P, Henschel P, Horgan J, de Iongh HH, Jowkar H, Klein R, Lindsey PA, Marker L, Marnewick K, Melzheimer J, Merkle J, M’soka J, Msuha M, O’Neill H, Parker M, Purchase G, Sahailou S, Saidu Y, Samna A, Schmidt-Küntzel A, Selebatso E, Sogbohossou EA, Soultan A, Stone E, van der Meer E, van Vuuren R, Wykstra M, Young-Overton K (2017) The global decline of cheetah Acinonyx jubatus and what it means for conservation. Proc Natl Acad Sci 114:528–533
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Edwards SV, Hedrick PW (1998) Evolution and ecology of MHC molecules: from genomics to sexual selection. Trends Ecol Evol 13:305–311
Ellegren H, Parsch J (2007) The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8:689–698
Ellison A, Allainguillaume J, Girdwood S, Pachebat J, Peat KM, Wright P, Consuegra S (2012) Maintaining functional major histocompatibility complex diversity under inbreeding: the case of a selfing vertebrate. Proc R Soc Lond B 279:5004–5013
Ende Z, Deymier MJ, Claiborne DT, Prince JL, Mónaco DC, Kilembe W, Allen SA, Hunter E (2018) HLA class I downregulation by HIV-1 variants from subtype C transmission pairs. J Virol. https://doi.org/10.1128/JVI.01633-17
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Fu M, Waldman B (2017) Major histocompatibility complex variation and the evolution of resistance to amphibian chytridiomycosis. Immunogenetics 69:529–536
Graffelman J (2015) Exploring diallelic genetic markers: the HardyWeinberg package. J Stat Softw 64:1–23
Greene JM, Wiseman RW, Lank SM, Bimber BN, Karl JA, Burwitz BJ, Lhost JJ, Hawkins OE, Kunstman KJ, Broman KW, Wolinsky SM, Hildebrand WH, O’Connor DH (2011) Differential MHC class I expression in distinct leukocyte subsets. BMC Immunol 12:39
Handunnetthi L, Ramagopalan SV, Ebers GC, Knight JC (2010) Regulation of MHC class II gene expression, genetic variation and disease. Genes Immun 11:99–112
Heinrich SK, Hofer H, Courtiol A, Melzheimer J, Dehnhard M, Czirják G, Wachter B (2017) Cheetahs have a stronger constitutive innate immunity than leopards. Sci Rep 7, 44837
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hughes AL, Nei M (1992) Maintenance of MHC polymorphism. Nature 355:402–403
IUCN (2017) The IUCN Red List of Threatened Species. Version 2017-2. http://www.iucnredlist.org. Accessed 14 Sep 2017.
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Kaufman J, Salomonsen J (1997) The “minimal essential MHC” revisited: both peptide-binding and cell surface expression level of MHC molecules are polymorphisms selected by pathogens in chickens. Hereditas 127:67–73
Kaufman JIM, VöLk H, Wallny H-J (1995) A “minimal essential MHC” and an “unrecognized MHC”: Two extremes in selection for polymorphism. Immunol Rev 143:63–88
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621
Lenz TL (2011) Computational prediction of MHC II-antgen binding supports divergent allele advantage and explains trans-species polymorphism. Evolution 65:2380–2390
Lighten J, Papadopulos AST, Mohammed RS, Ward BJ, Paterson G, Baillie I, Bradbury L, Hendry IR, Bentzen AP, van Oosterhout P C (2017) Evolutionary genetics of immunological supertypes reveals two faces of the Red Queen. Nat Commun 8:1294
Mazerolle MJ (2016) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.0–4. http://CRAN.R-project.org/package=MuMIn
Meyer-Lucht Y, Sommer S (2009) Number of MHC alleles is related to parasite loads in natural populations of yellow necked mice, Apodemus flavicollis. Evol Ecol Res 11:1085–1097
Munson L, Terio KA, Worley M, Jago M, Bagot-Smith A, Marker L (2005) Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J Wildl Dis 41:542–548
Murphy K, Weaver C (2017) Janeway’s immunobiology, 9th Edition
O’Brien SJ, Evermann JF (1988) Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol Evol 3:254–259
O’Brien SJ, Roelke ME, Marker L, Newman A, Winkler CA, Meltzer D, Colly L, Evermann JF, Bush M, Wildt DE (1985) Genetic basis for species vulnerability in the cheetah, 227. Science, New York, pp 1428–1434
O’Brien SJ, Wildt DE, Bush M (1986) The cheetah in genetic peril. Sci Am 254:84–95
Pohlert T (2014) The pairwise multiple comparison of mean ranks package (PMCMR). R package. http://CRAN.R-project.org/package=PMCMR
R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing., Vienna, Austria. http://www.R-project.org/
Radwan J, Biedrzycka A, Babik W (2010) Does reduced MHC diversity decrease viability of vertebrate populations? Biol Conserv 143:537–544
Richman AD (2000) Evolution of balanced genetic polymorphism. Mol Ecol 9:1953–1963
Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucl Acids Res 37(6):e45
Ruijter JM, Lorenz P, Tuomi JM, Hecker M, van den Hoff MJB (2014) Fluorescent-increase kinetics of different fluorescent reporters used for qPCR depend on monitoring chemistry, targeted sequence, type of DNA input and PCR efficiency. Mikrochim Acta 181(13–14):1689–1696
Sachdev M, Sankaranarayanan R, Reddanna P, Thangaraj K, Singh L (2005) Major histocompatibility complex class I polymorphism in Asiatic lions. Tissue Antigens 66:9–18
Sandberg M, Eriksson L, Jonsson J, Sjöström M, Wold S (1998) New chemical descriptors relevant for the design of biologically active peptides. A multivariate characterization of 87 amino acids. J Med Chem 41:2481–2491
Santos PSC, Michler F-U, Sommer S (2017) Can MHC-assortative partner choice promote offspring diversity? A new combination of MHC-dependent behaviours among sexes in a highly successful invasive mammal. Mol Ecol 26:2392–2404
Schuster AC, Herde A, Mazzoni CJ, Eccard JA, Sommer S (2016) Evidence for selection maintaining MHC diversity in a rodent species despite strong density fluctuations. Immunogenetics 68:429–437
Schwensow N, Fietz J, Dausmann K, Sommer S (2007) Neutral versus adaptive variation in parasite resistance: importance of MHC-supertypes in a free-ranging primate. Heredity 99:265–277
Schwensow N, Mazzoni CJ, Marmesat E, Fickel J, Peacock D, Kovaliski J, Sinclair R, Cassey P, Cooke B, Sommer S (2017) High adaptive variability and virus-driven selection on major histocompatibility complex (MHC) genes in invasive wild rabbits in Australia. Biol Invasions 19:1255–1271
Sengupta S, Naz S, Das I, Ahad A, Padhi A, Naik SK, Ganguli G, Pattanaik KP, Raghav SK, Nandicoori VK, Sonawane A (2017) Mycobacterium tuberculosis EsxL inhibits MHC-II expression by promoting hypermethylation in class-II transactivator loci in macrophages. J Biol Chem 292:6855–6868
Sepil I, Lachish S, Hinks AE, Sheldon BC (2013) MHC supertypes confer both qualitative and quantitative resistance to avian malaria infections in a wild bird population. Proc R Soc B 280:20130327
Siddle HV, Marzec J, Cheng Y, Jones M, Belov K (2010) MHC gene copy number variation in Tasmanian devils: implications for the spread of a contagious cancer. Proc R Soc Lond B 277:2001–2006
Sidney J, Grey HM, Kubo RT, Sette A (1996) Practical, biochemical and evolutionary implications of the discovery of HLA class I supermotifs. Immunol Today 17:261–266
Sommer S (2005) The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool 2:16
Sommer S, Courtiol A, Mazzoni C (2013) MHC genotyping of non-model organisms using next-generation sequencing: a new methodology to deal with artefacts and allelic dropout. BMC Genom 14:542
Takahata N, Nei M (1990) Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics 124:967–978
Thalwitzer S, Wachter B, Robert N, Wibbelt G, Müller T, Lonzer J, Meli ML, Bay G, Hofer H, Lutz H (2010) Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clin Vaccine Immunol 17:232–238
Thomas R, Apps R, Qi Y, Gao X, Male V, O’hUigin C, O’Connor G, Ge D, Fellay J, Martin JN, Margolick J, Goedert JJ, Buchbinder S, Kirk GD, Martin MP, Telenti A, Deeks SG, Walker BD, Goldstein D, McVicar DW, Moffett A, Carrington M (2009) HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet 41:1290–1294
Tourdot S, Gould KG (2002) Competition between MHC class I alleles for cell surface expression alters CTL responses to influenza A virus. J Immunol 169:5615–5621
Trachtenberg E, Korber B, Sollars C, Kepler TB, Hraber PT, Hayes E, Funkhouser R, Fugate M, Theiler J, Hsu YS, Kunstman K, Wu S, Phair J, Erlich H, Wolinsky S (2003) Advantage of rare HLA supertype in HIV disease progression. Nat Med 9:928–935
Tuomi JM, Voorbraak F, Jones DL, Ruijter JM (2010) Bias in the Cq value observed with hydrolysis probe based quantitative PCR can be corrected with the estimated PCR efficiency value. Methods 50(4):313–322
van Oosterhout C (2009) A new theory of MHC evolution: beyond selection on the immune genes. Proc R Soc B 276:657–665
van Oosterhout C (2013) Maintenance of major histocompatibility supertype variation in selfing vertebrate is no evidence for overdominant selection. Proc R Soc B 280:20122501
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12
Wakeland EK, Boehme S, She JX, Lu CC, McIndoe RA, Cheng I, Ye Y, Potts WK (1990) Ancestral polymorphism of MHC class II genes: divergent allele advantage. Immunol Res 9:115–122
Wang S, Guo L, Liu D, Liu W, Wu Y (2016) HLAsupE: an integrated database of HLA supertype-specific epitopes to aid in the development of vaccines with broad coverage of the human population. BMC Immunol 17:17
Wang S, Liu C, Wilson AB, Zhao N, Li X, Zhu W, Gao X, Liu X, Li Y (2017) Pathogen richness and abundance predict patterns of adaptive major histocompatibility complex variation in insular amphibians. Mol Ecol 26:4671–4685
Wildt DE, Bush M, Howard JG, O’Brien SJ, Meltzer D, Van Dyk A (1983) Unique seminal quality in the South African cheetah and a comparative evaluation in the domestic cat. Biol Reprod 29:1019–1025
Acknowledgements
We thank the German Research Foundation (DFG; SO 428/10-1), the Leibniz Institute for Zoo and Wildlife Research (IZW) in Germany and the Messerli Foundation in Switzerland for funding this study. We also thank the Namibian Ministry of Environment and Tourism for permission to conduct the research, the Namibian farmers for their support and collaboration and the team members of the IZW cheetah research project, especially Jörg Melzheimer and Annika Weigold, for valuable assistance in the field. We are grateful to Anke Schmidt for providing help in the laboratory and Theresa Jones for language editing. Two anonymous reviewers provided very useful comments on a former draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schwensow, N., Castro-Prieto, A., Wachter, B. et al. Immunological MHC supertypes and allelic expression: how low is the functional MHC diversity in free-ranging Namibian cheetahs?. Conserv Genet 20, 65–80 (2019). https://doi.org/10.1007/s10592-019-01143-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-019-01143-x