Abstract
Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) is an anadromous fish of considerable conservation concern, following its long history of overfishing and exposure to a variety of other anthropogenic stressors. Though reaching a large size, population abundances (Nc) of Atlantic sturgeon are not easily surveyed using traditional fishery methods because of their relative scarcity and their many age classes, often exhibiting extensive and differential movements that render them not readily amenable to traditional sampling efforts. A metric of their vulnerability to inbreeding and a possible alternative to Nc for abundance monitoring is effective population size (Ne). We surveyed 14 Atlantic sturgeon populations across their range using a suite of DNA microsatellite markers. Contemporary levels of Ne ranged from 1 in the St. Marys River, Florida–Georgia, to 156 in the Hudson River, New York. Historical Ne estimates averaged about 11.5 × higher than contemporary estimates, with a high of 927 for the Kennebec River population. Because of a mix of life history characteristics that make traditional Ne estimates based on imperfectly mixed year classes of juveniles questionable, we propose and provide results of a new, more robust and diagnostic approach based on sequential cohorts, termed Ne Max. Although contemporary Ne values obtained were at levels that might be considered troublesome, we suggest that the unique life history factors of this species may render it more resistant than other taxa to inbreeding effects. Because of the many differences among populations in size, age, and sex ratio and in harvest histories, we do not believe that a reliable extrapolation factor between Ne and Nc is yet possible. However, ongoing monitoring of Ne and Nc in populations might be sensitive to population changes and could form the basis of determining a relationship between Ne and Nc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reductions in the populations of the Atlantic sturgeon subspecies (Acipenser oxyrinchus oxyrinchus) and its increased danger of extinction resulted in its inclusion in 2012 on the U.S. federal Endangered Species Act list (ESA). Based largely on genetic results, distinct population segments (DPS) of Atlantic sturgeon labeled as New York Bight, Chesapeake Bay, Carolina, and South Atlantic also were adopted and then designated as endangered, whereas the Gulf of Maine DPS was listed as threatened (Federal Register 2012a). The ESA listings follow decades of concern over declining numbers of Atlantic sturgeon. In 1998, a 40-year fishing moratorium was enacted to protect 20 year-classes of females (ASMFC 1998). Prior to the moratorium, the Atlantic States Marine Fisheries Commission (ASMFC) developed a fishery management plan outlining conservation and restoration measures to achieve population levels that would support harvests at 10% of the historical peak landings (ASMFC 1990). Although the Saint John River and the St. Lawrence River populations in Canada are designated as Threatened (COSEWIC 2011), Atlantic sturgeon is the subject of regulated fisheries in Canada (Dadswell 2006). However, Atlantic sturgeon is not listed under Canada’s Species at Risk Act (Hilton et al. 2016).
As for most Atlantic Coast anadromous fish species, Atlantic sturgeon has endured centuries of human-induced population reductions (Limburg and Waldman 2009). An intensive caviar-focused fishery in the Delaware River in the late 1800s produced as much as 2000 metric tons of adult Atlantic sturgeon annually; these harvests ended in the early 1900s (Secor and Waldman 1999), with few adults or juveniles seen in the Delaware until the early twenty-first century (Wirgin et al. 2015b). Other populations were also overfished for caviar or sturgeon meat, with additional reductions stemming from fishery bycatch, poor water quality, blockage of migrations by dams, habitat degradation, chemical pollution and even ship strikes (Smith and Clugston 1997; ASSRT 2007).
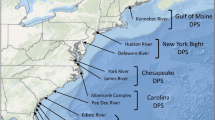
Today, there is strong interest in restoring Atlantic sturgeon populations. Historically, Atlantic sturgeon spawned in 38 rivers along the East Coast of North America from the St. Lawrence, Quebec, to the St. Marys River, Florida (ASSRT 2007). A recent assessment (ASMFC 2017) concluded that contemporary spawning is confirmed or highly likely in 19 of these rivers and suspected in 6 additional rivers. Both microsatellite and mitochondrial DNA (mtDNA) studies have indicated that each spawning river of Atlantic sturgeon surveyed to date contains genetically distinguishable populations (e.g., King et al. 2001; Grunwald et al. 2008; Wirgin et al. 2015a, b; Savoy et al. 2017).
Estimation of population census sizes (Nc) is a fundamental aspect of managing extant populations of endangered and threatened species. However, Atlantic sturgeon abundances are difficult to quantify for a number of reasons, many related to complexities of their life history. Young-of-the-year are challenging to survey because they tend to be widely dispersed and not easily caught in sampling gear (Farrae et al. 2009). Unknown proportions of young may leave their natal rivers as early as age-2 to occupy coastal waters for extended durations. Others remain in natal rivers for as many as 8 more years (Caron and Tremblay 1999); however, southern juveniles may remain in natal rivers for fewer years than northern individuals (Schueller and Peterson 2010). While ranging through coastal waters Atlantic sturgeon may seasonally occupy non-natal estuaries, further confounding river-specific population estimates (Waldman et al. 2013; Wirgin et al. 2018).
Census estimates are further complicated by reproductive maturation that is earlier for males than females and that individual adults do not spawn every year. Also, males may remain in rivers longer and occupy a broader area than females when spawning (Caron et al. 2002). Another complication is that Atlantic sturgeon, in addition to having long-recognized spring spawning runs across the northern and central portions of their range also have more recently defined fall spawning runs in the center (Balazik et al. 2012; Hager et al. 2014; Smith et al. 2015) and southern portions (Ingram and Peterson 2016). Finally, it is hard to obtain sufficiently robust sample sizes for census estimates of a species with relatively few individuals dispersed over wide geographic ranges. Thus, it is likely that any Atlantic sturgeon population is both thinly and widely dispersed at any given time, and sampling any particular life stage at any single location has considerable vagaries as to what proportion of the population it represents, making extrapolation problematic. However, at the pooled-populations level, Kocik et al. (2013) estimated the that mean abundance of Atlantic sturgeon in oceanic waters (excluding any that reside year-round in rivers and estuaries) off the Northeast coast of the U.S. during 2006–2011 was 417,934 individuals, with a 95% confidence interval of 165,381–744,597.
One alternative measure of population size is genetically-based effective population size (Ne). Effective population size is the size of an ideal population experiencing the same rate of random genetic change over time as the actual population under consideration (Wright 1938). Ne is typically much smaller than census population size (Nc) because of the influences of life history and reproductive biology characteristics, such as fluctuating population size, unequal sex ratio, overlapping generations, and variance in reproductive success among individuals (Frankham 1995; Palstra and Ruzzante 2008). Ne, therefore, can be diagnostic of ongoing or impending genetic stress, such as inbreeding, and assessment of such vulnerability is usually why Ne estimates are made. Some conservation geneticists have advocated the 50/500 rule proposed by Franklin (1980) in which animal populations must be above Ne = 50 to avoid inbreeding depression in the short-term and above Ne = 500 to retain the ability to evolve in perpetuity. Evidence accumulated since 1980 shows that Ne = 50 is inadequate for preventing inbreeding depression over five generations in the wild, with Ne ≥ 100 being required to limit loss in total fitness to ≤ 10% (Frankham et al. 2014). Further, even Ne = 500 may be too low for retaining evolutionary potential for fitness in perpetuity, with Ne ≥ 1000 being a better approximation (Frankham et al. 2014).
However, beyond its importance as an indicator of genetic vulnerability to inbreeding effects, Ne estimates are also a form of population census, albeit indirect and imperfect as a direct measure of census size, and with many caveats in their interpretation, such as the effects of sampling biases and the influences of historical effects of demography and migration on genetic characteristics. For instance, Ne can be as much as five orders of magnitude lower than census sizes in marine broadcast spawners that have large variances in reproductive success (O’Leary et al. 2013). A recent analysis of the effective number of breeders Nb and the number of spawners (Na; developed from mark-recapture studies) in the Atlantic sturgeon population of the Altamaha River, Georgia, however, showed a more even relationship with Nc, with Nb at levels 7–45% lower than Nc (Moyer et al. 2012).
Despite this lack of direct correspondence between Ne and Nc, Ne offers a useful, rigorous, and integrative means with which to compare population-specific information that is at least somewhat reflective of, if not perfectly correlated, with population census sizes. Simulations suggest that contemporary single-sample methods to estimate Ne often can detect population trends as accurately as classical ecological methods (Tallmon et al. 2010). For instance, Portnoy et al. (2009) suggested that for sandbar sharks (Carcharhinus plumbeus), the magnitude of Ne appeared to be strongly coupled with, and roughly half the size of Nc. Development of an accepted quantitative relationship between Ne and Nc for Atlantic sturgeon would be valuable for such a species with difficult-to-estimate census sizes.
Several life history characteristics have been demonstrated to potentially impact the ability to accurately estimate Ne in populations of species with overlapping generations (Waples et al. 2014). Two of the most prominent, maximum life expectancy and age at initial maturity (Waples et al. 2013), are problematic to define in Atlantic sturgeon. That is because of the dramatic differences among DPS, and even populations within DPS, for these characteristics. Atlantic sturgeon are potentially very long-lived with a maximum life expectancy of over 60 years (Dadswell 2006) reported for the Saint John River, Canada; however, it is likely that extreme longevity does not apply to most other populations because of the significantly slower growth rates of northern compared to southern populations. Moreover, there are latitudinal differences among populations, and even genders within populations, in age at reproduction. For example, initial spawning ranges between 5 and 32 years (Smith and Clugston 1997), occurring in southern rivers at younger ages than in northern populations. Furthermore, males achieve sexual maturity earlier than females, e.g., in the James River initial spawning occurs at age-10 for males and age-15 for females (Balazik 2012).
To assess reduced genetic variability, to provide a metric of relative population sizes for Atlantic sturgeon, and to explore the relationship between Ne (both historical and contemporary) and Nc, we used a suite of DNA microsatellite markers to estimate Ne and Nb in Atlantic sturgeon populations ranging from Canada to the Georgia–Florida border. Although several alternative methods to estimate Ne are available, we used the linkage-disequilibrium approach, as recommended by Waples and Do (2009). To better understand the effects of declines of Atlantic sturgeon on their population genetics, we also estimated historical values of Ne for the same populations using a coalescent-based method that incorporates migration between populations. However, because of the many asymmetric aspects of their life histories at the individual, male vs. female, and population levels and because of possible biases inherent in the sampling of Atlantic sturgeon, we also analyzed contemporary Ne on a year-class basis for selected populations for which collections of juveniles were available for multiple years. These annual calculations of Ne based on individual year-classes were then examined in a cumulative fashion over several consecutive years to obtain a more robust assessment of Ne for the entire population, what we termed Ne Max. By then comparing our calculations of historical Ne values with Ne and Ne Max obtained from contemporary samples, our results provide a novel genetic assessment regarding the current status of several populations.
Methods
Atlantic Sturgeon collections
Atlantic sturgeon were collected from across their range from June 1980 to July 2017, for 43 individual collections. In total, 2030 Atlantic sturgeon were analyzed from 14 extant reference spawning populations (Fig. 1). The number of individual collections made within spawning rivers (Table 1) ranged from 1 (Connecticut) to 9 (Hudson). These specimens were either juveniles (defined as ≤ 54 cm TL) or adults (defined as ≥ 127 cm TL), except for those from the St. Lawrence River collected in 1992, which were subadult males of unknown length and 5 two-year old specimens collected from the St. Marys River in 2015. Because of their differential availabilities in individual estuaries, collections are composed of either juveniles or adults, except for the Edisto, James, and Hudson Rivers which contain both life stages. Fin clips were taken from all specimens except for those from the St. Lawrence River in 1992, from which livers were removed and immediately frozen, and from the Kennebec River in 1980, from which DNA was isolated from dried fin spines. Fin clips were stored in 95% ethanol prior to DNA isolations. Except for the Connecticut River, all rivers were sampled over two or more years.
DNA isolations
Fin clips were washed with phosphate-buffered saline and incubated in cetyltrimethyl ammonium bromide (C-Tab) buffer (Saghai-Maroof et al. 1984) and digested at 65 °C with proteinase K (Roche Diagnostics, Indianapolis, IN). Genomic DNA was purified using phenol–chloroform extractions, alcohol precipitations, air drying and resuspension in Tris–EDTA buffer. Concentrations and purities of DNAs were evaluated using a Nanodrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). DNA concentrations were adjusted to 50 ng/μl for standardization of subsequent analyses.
Microsatellite analysis
Eleven microsatellite loci were scored that were previously shown to be effective in distinguishing spawning populations of Atlantic sturgeon and that behaved in a codominant pattern in pedigree analysis (King et al. 2001; Wirgin et al. 2015a, b). These included LS19, LS39, LS54, LS68 (May et al. 1997), Aox23, AoxD45 (King et al. 2001), and Aox44, AoxD165, AoxD170, AoxD188, AoxD24 (Henderson-Arzapalo and King 2002). PCR amplifications followed the procedure described in Wirgin et al. (2015a).
Microsatellite genotypes were determined using a Beckman Coulter CEQ8000™ capillary-based DNA sequencer (Beckman Coulter Fullerton, CA). Individual PCR reactions were multi-pooled, diluted up to 1:3 with Sample Loading Solution (Beckman Coulter), 0.5–2.0 µl of reactions were loaded onto 96 well plates along with 0.5 µl of CEQ DNA Size Standard-400 (Beckman Coulter) and 40 µl of Sample Loading Solution (Beckman Coulter), and run with the FRAG 1 program (Beckman Coulter).
Microsatellite data analysis
Microsatellite data were initially examined using MicroChecker (Van Oosterhout et al. 2004) for the presence of null alleles, scoring errors, and large allele drop-out. Exact tests in GENEPOP (version 4.2) (Raymond and Rousset 1995; Rousset 2007) were used to test the genotypes at each locus and in each collection for their conformity to Hardy Weinberg equilibrium (HWE). Linkage disequilibrium (LD) was tested for all pairs of loci in each population using contingency tables in GENEPOP. All tests of HWE and LD used default Markov chain parameters in GENEPOP. Significance levels for HWE and LD tests were adjusted using sequential Bonferroni correction (Rice 1989). Microsatellite allelic diversity at all loci and in all collections was quantified in GenAlEx (Peakall and Smouse 2006, 2012), FSTAT version 2.9.3 (Goudet 2001), and HP Rare 1.0 (Kalinowski 2005); measures presented (Supplementary Table 1) include mean number of alleles, effective number of alleles, allelic richness, expected heterozygosity, and observed heterozygosity.
Contemporary effective population size and effective number of breeders in their parental generation were estimated for population-specific cohorts of juveniles and adults using the bias-corrected version of the linkage disequilibrium (LDNe) methods of Waples and Do (2008) implemented in NeEstimator v2 (Do et al. 2014). The LDNe method has been shown empirically to outperform other approaches in accurately estimating Ne (Gilbert and Whitlock 2015; Wang et al. 2016). Low frequency alleles were screened out with a P crit. of 0.02 (Waples and Do 2008). For each population except that in the Connecticut River, two or more years of collections were pooled to calculate estimates of Ne.
Calculation of Ne max
Because only juvenile specimens were available from rivers in the South Atlantic DPS (except the Edisto River), we were interested in empirically determining how many years of juvenile cohort collections were needed to accurately portray their effective population sizes. This would guide future work with newly discovered Atlantic sturgeon populations from which only juvenile collections were available and other species in which only cohorts of young life-stages are available for analysis. We first identified those populations where tissue samples had been previously collected from juveniles in each of at least 3 years (usually consecutive). These populations included the Altamaha (5 years, n = 37–64/year, mean = 49), Savannah (3 years, n = 50–61/year, mean = 54), Ogeechee (4 years; n = 15–67/year, mean = 44) and Hudson (4 years; n = 30–46/year, mean 36). After estimating the annual Nb for the initial cohort on each river system, we sequentially combined individual cohorts and then recalculated their Ne. We then constructed a simple plot of cumulative Ne, for each year, by combining the samples in each successive year. The resulting plots were then used to identify the Ne Max for each population based on the equation of the parabolic function depicted in each of the plots.
Estimating historical Ne and gene flow
To compare current Ne estimates with long-term (historical) Ne, we used a Bayesian framework implemented in the program MIGRATE-n version 3.6.11 (Beerli and Felsenstein 2001; Beerli 2006). MIGRATE-n uses coalescent modeling to jointly estimate long-term effective population size (θ = 4Neµ) and gene flow (M = m/µ) over approximately 4Ne generations. For these analyses, we used 13 populations, excluding the Connecticut as this population is believed to have been founded in modern times. All samples and loci were included in the analysis, which used a Bayesian analysis strategy (details below) and a microsatellite (Brownian motion) model of evolution. First, we compared three migration models in MIGRATE-N using the strategy outlined in Beerli and Palczewski (2010): (1) full migration model (migration estimated for all possible binary combinations among 13 populations); (2) DPS model (migration estimated between populations within each DPS) (between populations within each DPS; populations grouped into the 5 DPS and the 2 individual populations in Canada); (3) stepping-stone migration (migration between adjacent populations).
In all models, asymmetric and bidirectional migration was allowed. We determined the best-fitting migration model via log marginal likelihood using Bezier scores from MIGRATE-N runs with four heated chains in a Bayesian framework (Beerli and Palczewski 2010). Two shorter initial MIGRATE runs were performed to determine whether parameter space was being captured by the MCMC (four runs sampling 100,000 generations). We then performed a final run including three replicate chains of 10,000,000 steps (burn-in of 1,000,000 steps) for 50,000 recorded steps. A static heating scheme was implemented with the following four chain temperatures: 1.00, 1.50, 3.00, 1 × 107. We used uniform prior distributions (min, max, delta): Θ = (0.001, 100, 10); and M (0, 1000, 10). We calculated the effective number of migrants (Nm) as (Θ × M/4) where M is the mutation-scaled immigration rate (Beerli and Palczewski 2010). To convert θ to Ne estimates, we used a mutation rate (µ) of 4.15 × 10−4 (CI 1.45 × 10−3 to 4.15 × 10−4) mutations per locus per generation based on a recent genealogy-based study of microsatellite mutation rate in Atlantic sturgeon (Panagiotopoulou et al. 2017). We incorporated uncertainty in these estimates by creating a script in R (version 3.5.1) (R Core Team 2018) that performed bootstrap resampling 100,000 times from the published 95% confidence intervals of µ given above and the 95% highest posterior density (HPD) intervals of Θ produced by Migrate-n for each population.
Results
Contemporary and historical Ne estimates
Estimates of contemporary Ne ranged from a low of 1 for the St. Marys population to a high of 156 for the Hudson River population (Table 2). Three of the five DPS had only a single population represented within it: the Ne for the Kennebec River population (the only one from the Gulf of Maine DPS) was 67; the Ne for the James River (the only one from the Chesapeake DPS) was 32; and the Ne for Albemarle Sound (lone representative of the Carolina DPS) was 19. The mean Ne for the Connecticut, Hudson, and Delaware Rivers of the New York Bight DPS was 66; the mean Ne for the Edisto, Savannah, Ogeechee, Altamaha, Satilla, and St. Marys Rivers of the Southeast DPS was 63. Furthermore, the mean Ne for the two rivers in Canada, the St. Lawrence and the Saint John, was 67. The mean contemporary Ne for all 14 populations was 58.4.
The long-term migration model that was best supported in Migrate-n was the stepping stone model, with a model likelihood > 84.9%, with the second-ranked model being the DPS model. Mean estimates of historical Ne ranged from a low of 468 for the St Marys River population to a high of 927 for the Kennebec River population (the Connecticut River was excluded as it is believed to be recently recolonized) (Table 2). Estimates of historical Ne were higher than contemporary Ne for all populations. The percentage of contemporary to historical Ne estimates ranged from less than 1% for the St. Mary’s population to 21.5% for the Altamaha population (Table 2). The mean percentage of contemporary to historical Ne across all populations was 9.2% (with Connecticut River population excluded).
Plots of cumulative Ne from juveniles for each of the four populations yielded Ne Max values near or higher than traditional Ne estimates, varying from a low of 32.2 for the Ogeechee River (vs. 26) to 116.3 for the Savannah River (vs. 123) to 145.9 for the Altamaha River (vs. 149), with a large difference for the Hudson (294.6 vs. 156) (Fig. 2). In all of the southern populations, Ne Max was obtained at 3–4 years of collections, while in the Hudson, Ne Max was still increasing after the 4th consecutive collection.
Cumulative effective population sizes (Ne) of Atlantic sturgeon in the Ogeechee, Savannah, Altamaha, and Hudson rivers. Juvenile cohorts sampled from each river include Hudson (2013, 2014, 2015, 2016), Savannah (2013, 2014, 2017), Ogeechee (2014, 2015, 2016, 2017), and Altamaha (2005, 2011, 2014, 2015, 2016). Maximum Ne was calculated using the equation of the line depicting cumulative Ne
Estimates of the long-term effective number of migrants per generation (4Nem) varied across population pairs, but were generally high, with the exception of low historic gene flow between the St. Lawrence and the Saint John and from the Ogeechee to the Altamaha (Table 3). The highest values were from Hudson to the Kennebec (61.7) and the Savannah to the Ogeechee (60.7).
Discussion
Implications for conservation genetics
As Ne decreases, genetic drift erodes genetic variation, elevates the probability of fixation of deleterious alleles, and reduces the effectiveness of selection, all of which reduce overall population fitness and limit adaptive responses. These genetic changes can directly or indirectly drive a threatened population closer to extirpation through interactions with population dynamics that create an extinction vortex (Hare et al. 2011). Estimated contemporary Ne values for individual Atlantic sturgeon populations examined in this study fell both within and below the suggested safety zone of 50–500 (Frankham et al. 2014). Even Ne values for populations within this range, nonetheless, were on its lower end, with the highest estimate at 156 for the Hudson River population. Ne values also were somewhat higher at both ends of the distribution of the Atlantic sturgeon, i.e., Canada and the South Atlantic DPS. In fact, the highest regional mean Ne value, for the South Atlantic DPS (63), was about double that of the lowest, the single-river Chesapeake Bay DPS (32).
Following the criteria of Frankham et al. (2014), our results suggest that the 8 of 14 Atlantic sturgeon populations with Ne estimates of 50 or less are in danger of inbreeding and genetic drift. However, concern for these should be tempered by the varied history and status of these populations. For example, the extremely low Ne for the Connecticut River population appears to be the result of a recent recolonization by a small number of founders and, as such, would be expected to have a low Ne (Savoy et al. 2017). In contrast, the James River population, with a Ne of 32, now shows evidence of ongoing recruitment failure, perhaps due to predation from blue catfish Ictalurus furcatus, thermal pollution at major nursery areas, or power plant impingement (Pipkin 2017).
Contemporary effective population size versus population status and trends
Differences among populations in our contemporary Ne estimates were largely in keeping with surveys and anecdotal observations of population status. The St. Lawrence River population, at Ne = 35, has supported commercial fishing for Atlantic sturgeon for centuries. Since 2000, size regulations and an annual quota of 60 metric tons of subadults only have resulted in a stable population roughly estimated by Dadswell (2006) as 500 + spawning adults.
The other Canadian river included in our study, the Saint John (Ne = 88), has also supported a long-term commercial fishery, but with limited participation in recent years and an annual quota imposed since 2009 of 350 individuals or 125 females, whichever comes first. Dadswell (2006) estimated that approximately 200 to 300 adults were captured each year in the fishery (though it is possible that other populations may have contributed very low numbers of individuals) (e.g., 3% of adults; Savoy et al. 2017). Recent analysis of contemporary and historical fishery data by Dadswell et al. (2017) estimated the virgin population in 1880, prior to prosecution of commercial fisheries at 14,200–23,700 individuals. Bradford et al. (2016), using mark-recapture techniques concluded the Saint John River population currently includes 1000 to 3050 spawners, whereas Dadswell et al. (2017) estimated the mean annual population abundance between 2011 and 2016 as 18,000–20,800 adults. Our estimate of Ne = 88.4 for the Saint John River population is lower than that of Bradford et al. (2016) who reported an average Ne of 110 using the linkage disequilibrium method as we did, but with an expanded set of microsatellite loci that were analyzed in 232 adult specimens collected over multiple years. They combined their Ne = 110 with their empirically determined Nc values of 3050 adults in 2009 and 950 in 2012 to obtain Ne/Nc ratios of 0.04 and 0.12, respectively, for the Saint John population.
Atlantic sturgeon were historically abundant in the Kennebec River and its tributaries, including the Androscoggin and Sheepscot rivers. Sporadic captures since the 1990s suggest a spawning population persists in the watershed (ASSRT 2007), which was validated by the fisheries-independent collection of 32 adults in June of 2010 and 2011 and August of 2011 from the Kennebec River and 8 adults in June 2011 from the Androscoggin. Spawning was confirmed in the Kennebec by the collection in July 2011 of two 15-cm young-of-the year. Our Ne estimate for the Kennebec population (Ne = 67) was near the middle of the overall range found for the 14 populations.
The second lowest value obtained, for the Connecticut River (Ne = 2), reflects what appears to be a recent recolonization but presently low abundance (Savoy et al. 2017). Atlantic sturgeon were speared in the river in Massachusetts in the 1700s (Judd 1905) and a limited commercial fishery for adults centered at Cromwell, CT, was reported (Galligan 1960), but access to waters beyond northern Connecticut was cut off by construction of the Enfield Dam in 1827. Substantial numbers of subadult Atlantic sturgeon were surveyed in the lower Connecticut River from 1988 to 2014 (Savoy et al. 2017); however, genetic analysis indicated they were primarily of Hudson River origin (Waldman et al. 2013). There were other occasional catches and sightings in the river but no evidence of reproduction reported by ASSRT (2007) and its authors believed them to be extirpated. However, recent net surveys and genetic analysis indicate that the Connecticut River was recolonized via spawning in 2013 by individuals chiefly from the Chesapeake Bay and the South Atlantic DPS (Savoy et al. 2017). It remains to be determined if successful reproduction occurred in subsequent years.
The Hudson River population showed the highest contemporary Ne, at 156. Stock composition analysis of Atlantic sturgeon in the New York Bight in 1993 and 1994 (Waldman et al. 1996), off the Delaware coast during the early spring migratory run (Wirgin et al. 2015a), and of coastal bycatch from the Gulf of Maine to North Carolina obtained in NOAA’s Observer program (Wirgin et al. 2015b) indicated that the Hudson River population was the overwhelming contributor (97.2%, 44% and 46%, respectively), suggesting a relatively robust abundance for that population. However, Kahnle et al. (2007) believed there was substantial overfishing before and during the burgeoning caviar fishery in the 1990s which contributed for a low mean annual spawning stock size between 1986 and 1995 of 863 (596 males and 267 females). Tighter harvest regulations in New York followed by the species-wide closure in 1998 may have stemmed further population decline as indicated by increasing numbers of juveniles collected in recent fishery independent surveys (ASMFC 2017).
Hatchery augmentation is another theoretical contributor to low Ne. The only population among those we sampled that had been stocked was the Hudson River, which received 4929 young-of-the-year in 1994 (Waldman and Wirgin 1998). Sampling in the Hudson River from 1995 to 1997 showed that those hatchery-produced individuals comprised 35–53% of the total juvenile catch (Peterson et al. 2000). Despite this, the Hudson population provided the highest contemporary Ne estimate.
The Delaware River may have hosted the largest Atlantic sturgeon population on the Atlantic Coast before it was overfished in the late 1800’s (Secor and Waldman 1999). Following this decline, there was uncertainty as to whether a relict Delaware population persisted, due to the almost complete absence of adult or juvenile specimens over many decades of sampling in the river. Genetic analysis of subadults collected between 1991 and 2004 suggested the presence of a relict stock (Wirgin et al. 2007) and successful reproduction there was confirmed beginning in 2009 (and continuing in 2011 and 2014) with the collection of modest numbers of pre-migratory juveniles at, or near, the presumed nursery grounds. A subsequent mark-recapture estimate of age-0 and age-1 juveniles made in 2014 (Hale et al. 2016) indicated a population size of 3656 (95% CI 1935–33041). The moderate Ne estimated for the current Delaware population, 40, may reflect a balance between the pervasive genetic signal from an extremely large historical abundance and a contemporary relict population that experienced a severe bottleneck.
Our Ne estimate of 32 for Atlantic sturgeon from the James River is in the lower range among the populations we examined. However, until recently there was uncertainty as to whether its population remained extant. Grogan and Boreman (1998) used a time series of incidental capture records during annual trawl surveys in the James conducted between 1964 and 1994 to yield probability estimates that the species was extirpated of 0.856 and 0.995%. Nonetheless, a subsequent reward program for commercial fishers demonstrated population persistence, with genetic analysis indicating the presence of a relict stock (Wirgin et al. 2007), and young sturgeon captured in the James in 1996 and 1997 showed that reproduction was occurring there. Additionally, between 2007 and 2011, Balazik et al. (2012) captured nearly 150 spawning adults in the tidal-freshwater portion of the James River during spawning runs. Balazik and Musick (2015) estimated a Nc for spawning males in the James of 3399 (± 575). Recently, however, the stock appears to be declining (Blankenship 2016).
Albemarle Sound and the Roanoke River have long supported a small commercial fishery for Atlantic sturgeon. Its Ne estimate of 19 is consistent with a relatively small census size. Observations of young individuals indicate that spawning still occurs there, but catch records suggest declining recruitment after year 2000 (ASSRT 2007). In 2010, N-mixture and distance models with data acquired from side-scan sonar surveys were used to quantify abundance of sturgeon in 5 rivers in North Carolina and South Carolina. These results were then used to estimate abundances of sturgeon > 1 m TL in the Carolina DPS as 2031 using a count model and 1912 via a distance model (Flowers and Hightower 2015).
The Edisto River appears to support a relatively robust Atlantic sturgeon population, reflected in its Ne estimate of 60 and also that surveys reveal both young and mature individuals (ASSRT 2007). However, the Edisto enters the ACE Basin, which also includes the Ashepoo and Combahee Rivers, and it may be that individuals captured in the Edisto are part of a single population that spawns in 2 or all 3 of the ACE Basin rivers (Grunwald et al. 2008). In fact, comparisons of mtDNA haplotype and microsatellite allelic frequencies in adults from the Edisto and Combahee did not show significant genetic differentiation between these populations (Grunwald et al. 2008; Wirgin unpublished data).
The Savannah River supports a reproducing Atlantic sturgeon population, with a relatively high Ne value of 123. Mark-recapture analysis by Bahr and Peterson (2016) indicated age-1 cohort sizes of between 528 and 597 individuals from 2013 to 2015. The authors concluded that the Savannah population of Atlantic sturgeon is the second largest within the South Atlantic DPS, a relative estimate that is consistent with our derived Ne value.
We found a relatively low Ne of 26 for the Ogeechee River population. Its only recent census estimate was 450 (CI 203–1125) juveniles (defined as age-1 and age-2 combined) in 2007 (Farrae et al. 2009). The Ne estimate is consistent with the characterization of this population by ASSRT (2007) as having abundant juveniles only rarely, with high interannual variability.
In contrast, Atlantic sturgeon is abundant in the Altamaha River, reflected in its Ne estimate of 149, the second highest for all populations characterized. During the summers of 2004–2007, Schueller and Peterson (2010) performed mark–recapture of age-1 juveniles in the Altamaha and derived annual abundance estimates that ranged from 1072 to 2033 individuals. The authors reported that age-1 and age-2 individuals comprised greater than 87% of the total juvenile population in each year of their study.
A low Ne value of 21 was obtained for the Satilla River population. Only 4 spawning adults and young-of-the-year, all used for genetic analysis (Ong et al. 1996), were collected from this river between 1995 and publication of ASSRT (2007). However, 61 ages-0 to -1 juveniles were collected in the Satilla in summer of 2010. Fritts et al. (2016) speculated that these juvenile specimens were the offspring of a small contingent of “cryptic” subadults that remained in coastal waters since enactment of the 1998 harvest moratorium and only re-entered the Satilla to initially spawn in 2009.
ASSRT (2007) stated that the lack of Atlantic sturgeon captures in either scientific sampling or as bycatch in other fisheries in the St. Mary’s River indicates that the river neither supports a spawning subpopulation nor serves as a nursery ground for Atlantic sturgeon, though no directed sampling surveys had been conducted in years immediately prior. However, genetic samples were collected from 9 pre-migratory juveniles in 2014 (mean TL = 36.0 cm) and tissues were taken in 2015 from 5 pre-migratory juveniles spawned in 2014 (mean TL = 66.2 cm). Collection of juveniles over 2 years from a single cohort confirmed the presence of contemporary spawning in the St. Marys River (Fox et al. In Press). The St. Mary’s collection yielded a Ne estimate of 1, the lowest for all populations characterized, a value likely reduced by its derivation from only 1 year class.
Contemporary versus historical effective population sizes
There were two main findings in the comparison of the contemporary and historical effective population sizes obtained. First, contemporary values for Ne across all 14 populations (range 1–156; x̄ = 58.5) were very low by conservation biology criteria, and well below the Ne rule of 500 recommended to retain long-term genetic viability of populations. Even when both the Connecticut and St. Marys populations are excluded the mean Ne is only 68.0. Secondly, historical analysis suggests Ne levels were higher in the past (mean across all populations, excluding Connecticut River = 672.9) or about 11.5 × contemporary estimates (also excluding Connecticut River). Thus, contemporary Ne estimates are on average considerably lower and historical Ne estimates somewhat higher than the Ne rule of 500 recommended for retaining long-term genetic viability of populations.
Reduced contemporary Ne values may most readily be explained by more than a century of overfishing across almost all populations as well as other stressors that led to a 40-year moratorium on their harvest and then to listing of Atlantic sturgeon under the ESA. Thus, it is not surprising that contemporary Ne estimates are lower than their associated historical Ne estimates. These stressors, while impacting all populations, may have been particularly intense on some populations such as that in the Delaware, which went from what was likely the largest to one of the smallest due to rampant historic overharvest and more recently, low dissolved oxygen concentrations in nursery areas (Kahn and Fisher 2012), reduced suitable freshwater spawning and nursery habitats (Moberg and DeLucia 2016) because of dredging, and a relatively large numbers of vessel strikes (Brown and Murphy 2010).
Moyer et al. (2012) obtained a Ne value of 125 for Atlantic sturgeon of the Altamaha River, which is similar to our estimate of 149, the second highest for all populations. However, the historical estimate of Ne by Moyer et al. was an order of magnitude more (1100–5500) than their contemporary Ne estimate, whereas our historical Ne estimate (Ne = 764) was only 5.1 × that of our contemporary estimate. Why did Moyer et al. (2012) estimate a higher historical Ne for the Altamaha River population compared with our results? A likely possibility is the mutation rate applied for their historical analyses. We used a recently determined, empirically-derived, species-specific microsatellite mutation rate for Atlantic sturgeon (Panagiotopoulou et al. 2017) based on analysis of mutations between parents and their offspring of Atlantic sturgeon, whereas Moyer et al. (2012) used a rate 4.15 × higher (Jarne and Lagoda 1996), which would help account for their higher historical Ne estimate.
Furthermore, the method employed here to estimate historical Ne (Migrate-n), assumes migration-drift equilibrium; violations of this assumption from processes above can result in underestimated Ne values (Beerli 2009). While Migrate-n and other coalescent methods have been applied to many species with overlapping generations (e.g., Shrimpton and Heath 2003; Hughes et al. 2012; Moore et al. 2013; Bracken et al. 2015), it is important to recognize that the effect of high variance in reproductive success, e.g., of a small number of individuals having many more offspring than others can result in a smaller effective population size (Eldon and Wakeley 2006) and a non-linear relationship between genetic diversity and population size (Wakely and Sargsyan 2009). Additional simulation studies, while outside the scope of the present work, are needed to determine the behavior of coalescent-based methods such as Migrate-n using realistic life history parameters. Moreover, these estimates of historical Ne will be reduced if populations experienced dramatic demographic events such as prolonged bottlenecks, near-extirpations, or extirpation-recolonization events, either due to recent overharvest or earlier climatic events such as glaciations. Geological history indicates that all Atlantic sturgeon populations from the Hudson River northward were derived following deglaciation, i.e., within ~ 15,000 years; this hypothesis is corroborated by mtDNA analysis (Waldman et al. 2002) that shows a pattern of sequential decreases in haplotype diversity in populations from the Hudson to the St. Lawrence.
The low contemporary Ne estimates across populations (and relative to large historical catches in rivers such as the Delaware, obtained from known large populations), may reflect several factors known to reduce Ne. First, the particular life history demographics of Atlantic sturgeon likely serve to reduce Ne far below census sizes, perhaps more so than for most other species. These include uneven sex ratios among breeders, overlapping generations, fluctuating population sizes, variation among individuals in mating success, migration among spawning populations, and several factors which severely limit the number of actual breeders at any given spawning cycle from the overall potential pool. Among these are non-annual spawning of sexes, discrete spring and fall spawning in some rivers in the middle and perhaps southern portion of their range with consequent subdivision of river populations, and the late age of initial spawning, particularly for northern populations.
It has been shown that unequal sex ratios reduce Ne by an average of 36% (Frankham 1995). Sex ratios have not been measured in all populations of Atlantic sturgeon but do exist for a small subset that hosted fisheries. These studies have demonstrated that sex ratios vary considerably among populations with males tending to outnumber females, sometimes by considerable margins. For example, male:female sex ratios ranged from 1.2:1.0 in the Saint John River (Dadswell et al. 2017), 3.0:1.0 in the Edisto and Combahee rivers (Collins et al. 2000), 4.0:1.0 in the Hudson River (Dadswell and Nack 2012), 4.9:1.0 in the St. Lawrence River (Caron et al. 2002), and 60:1 in the James River (Balazik et al. 2012). However, it is possible that these sex ratios are skewed by differential catchability of the sexes based on the gear type employed in these fisheries or research efforts (Hilton et al. 2016). This is particularly true for estimates based on fishery-dependent data as reported for the Saint John, St. Lawrence and Hudson Rivers. Additionally, males may spend more time on the spawning grounds than females, thereby biasing sex ratios (Bain 1997; Whippelhauser et al. 2017).
Given the longevity of Atlantic sturgeon, particularly of northern populations, it is conceivable that individuals spawned across several decades may reproduce in any given year, a factor which lessens estimates of Ne (Felsenstein 1971). Moreover, most Ne estimation methods assume discrete generations and produce a per-generation estimate; however, in species with overlapping generations, it is difficult to define (and challenging to collect) a random sample from an entire generation (Hare et al. 2011). Generally, mixed-age adult samples such as those collected from northern populations in our study (St. Lawrence, Saint John, Kennebec, Hudson, and James Rivers) produce downwardly biased estimates of Ne, which can be attributed to a two-locus Wahlund effect (mixture LD) caused by combining parents from different cohorts in a single sample (Waples et al. 2014).
Variation in mating success among spawners and subsequent variation in family size is known to greatly decrease Ne, but to our knowledge, there are no empirical studies that have addressed this phenomenon in sturgeon populations. However, it is likely that this occurs, particularly because of the differential age at first spawning for the two sexes. Hauser and Carvalho (2008) believed this to the most important factor in reducing Ne among marine fishes. With modern bioinformatic approaches to analyze molecular data sets such as ours, estimation of variation in spawning success and family size should now be addressed.
Intermittent spawning is another factor likely decreasing Ne relative to Nc in Atlantic sturgeon populations. This behavior severely reduces the number of mature adults engaging in breeding in any given year. Intermittent spawning likely occurs in all populations, but its frequency varies between genders and among populations. For example, the spawning interval in females was reported at 2–5 years in South Carolina rivers (Smith 1985) and 3–5 years in the Hudson River (Bain 1997). Intervals between spawnings in males is reported to be shorter, occurring at 1–5 years (Smith 1985) or maybe every year in some southern populations (Hilton et al. 2016).
Spawning in both the spring and fall, recently suggested for some populations of Atlantic sturgeon, is another phenomenon that may serve to reduce Ne relative to Nc. Rivers where dual spawning has been reported include the James (Balazik et al. 2012) where significant genetic differentiation at microsatellite loci was reported between two spawning cohorts of adult males collected in the spring and fall (Balazik et al. 2017). We anticipate that the potential dual spawning in this and perhaps other populations serve to reduce their Ne to Nc ratios by limiting the opportunities for mating among complete adult gene pools within the populations. The decreased Ne in these populations should be reflected when calculating Ne from their juvenile aggregations, which will be comprised of offspring from both spawning cycles.
Variable migration rates among some sturgeon populations is another factor that may significantly impact estimates of Ne. Waples and England (2011) showed that pulse migration of genetically divergent individuals can decrease Ne in a local population. Conversely, low rates of migration between populations differentiated by low Fst values serve to increase estimates of Ne. Historical rates of migration differ among our 14 populations of Atlantic sturgeon, from very low between the Canadian populations to high in the South Atlantic DPS, and between the Gulf of Maine and NY Bight DPS. Two of the populations with the highest migration rates leading to them lay near the middle of their zoogeographic provinces, the Kennebec in the Acadian, and the Ogeechee in the Carolinian. We speculate that such sitings may make these rivers a crossroads for migrating sturgeon, some of which may stray reproductively. Similarly, mean Fst among populations within the South Atlantic DPS (Fst = 0.049) is lower than among more northern populations, suggesting greater contemporary migration among these rivers. That may be a function of the greater proximity of contemporarily active spawning rivers in the South Atlantic DPS through the intracoastal waterway. Thus, estimates of Ne among southern populations may be deflated by the extensive migration among some populations. While these estimates cannot capture the complex and variable history of source-sink dynamics and pulse migrations in the past, they may provide a useful perspective on relative migration rates across populations.
Year class-specific contemporary Ne analyses
Although derivations of Ne Max may not be necessary or desirable for most other fishes, our calculations suggest that Ne Max may provide a more robust measure of effective size for some Atlantic sturgeon populations than traditional methods of calculating contemporary Ne. For many populations in the Carolina and South Atlantic DPS, only juveniles are available for genetic analysis. Because of its intermittent spawning, collections of single year classes of Atlantic sturgeon juveniles may only harbor a small fraction of the total gene pool in any given population; therefore, Ne Max may provide a more comprehensive view of their actual Ne. In our study, final Ne Max values were not far from the contemporary Ne values calculated across year classes in 3 of 4 populations (Fig. 2). However, in 2 of these (Savannah and Altamaha), a Ne estimate based on a single year class would have yielded substantially lower values (Fig. 2). Moreover, there was a large difference between Ne Max and Ne for the Hudson (294.9 vs. 156), with the cumulative Ne Max still ascending at the fourth consecutive cohort. The number of years required to reach Ne Max, likely reflects the “average” spawning interval for both sexes in each population. Hence, the reason that the Hudson curve is still ascending may be because in northern climates, the spawning interval is longer for both sexes compared to southern populations. This is potentially important because it provides an important indicator of how many years of sampling juveniles is required to reach a good estimate of Ne Max.
The Ne calculation should, intuitively “peak” at Ne Max, at which point juvenile cohorts in the population have been fully represented. The decline in Ne after Ne Max, occurs because additional sample years begin to increase the number of juveniles within the total sample that were produced from repeat spawners. Essentially, these juveniles (from repeat spawners) effectively add partial siblings to the total cumulative juvenile sample population. This addition of partial siblings causes the resulting Ne estimate to decline after Ne Max. Hence the parabolic function was not only the best fitting function we observed, but it best represents the reproductive cycle of Atlantic sturgeon with respect to how each result cohort is produced and how the genetic diversity of the adult population is propagated throughout that reproductive cycle.
Additionally, the use of river-resident juveniles for calculation of Ne Max allows researchers to include small populations in these comparisons, populations where spawning adults are rare or not easily sampled. Inclusion of small populations in genetic analyses of Ne is especially important within the context of species recovery because Ne is of particular concern for smaller populations. Unfortunately, no standardized sampling approach has yet been established for assessment of Ne among Atlantic sturgeon populations; Ne has been calculated from spawning adults in some rivers while river-resident juveniles or combinations of adults and juveniles have been used in one other, the Hudson River. Given the protracted and variable spawning period that has been well documented for Atlantic sturgeon, the calculation of Ne Max provides a less variable estimate of Ne, one that is standardized among populations and hence, may provide a more accurate comparison of the genetic composition of different populations throughout the range. Future studies are needed to examine the differences in Ne calculated from single and multiple years of adult and river-resident juvenile collections to identify the best sampling method for monitoring Ne as one important measure of species recovery.
Does polyploidy play a protective role in preventing inbreeding effects at low effective population sizes?
A possible, as yet unexplored, but potentially important question for Acipenseriformes, including Atlantic sturgeon, is whether their polyploidy exerts an ameliorating effect on what may appear to be low Ne levels for some populations. Sturgeon species are allopolyploid and their chromosome numbers range from around 120 (tetraploid) in multiple species, including Atlantic sturgeon (Hilton et al. 2016), to 372 (dodecaploid) in shortnose sturgeon (Acipenser brevirostrum) (Vasil’ev 2009).
One benefit of allopolyploidy involves the fixing of divergent parental genomes. Whereas heterozygosity and heterosis decay in the progeny of a diploid F1 hybrid (at each generation half the heterozygous loci become homozygous), the enforced pairing of homologous chromosomes in allopolyploids prevents intergenomic recombination, effectively maintaining the same level of heterozygosity through the generations (Comai 2005). Another advantage of polypoidy is gene redundancy. Gene redundancy can mask recessive alleles by dominant wild-type alleles (Comai 2005). This effect can act at two life stages, one of which is the haploid stage. Although this form of the organism has reduced complexity, its function requires the activity of many genes. This exposes the egg to the action of lethal and deleterious loss-of-function mutations. In the second, 2N phase, polyploidy can reduce the incidence of homozygous recessives.
Comai (2005) speculated that the protective effect of polyploidy against deleterious recessive mutations might be important when isolated and severely bottlenecked populations are forced to inbreed, at a time when the purging of deleterious alleles is made difficult by the reduced number of breeding individuals. Such a protective response would have value for organisms such as sturgeons, which because of the large sizes of some species, would not likely maintain highly abundant populations, particularly for potentially more food limited landlocked forms and, thus, would be subject to inbreeding risks. Furthermore, Yi and Streelman (2005) concluded that genome size in ray-finned fish is negatively correlated with Ne and that reduced Ne underlies the evolution of larger and more complex genomes. If polyploidy is an ancestral trait in sturgeons, it would be expected to appear in all species, as in fact, it does. Additional research is needed as to whether any dangers from the low Ne seen in Atlantic sturgeon are modulated by their polyploidy.
Is effective population size predictive of census size for Atlantic sturgeon?
Given the challenges of conducting census estimates of Atlantic sturgeon, estimation of Ne may be a way to at least crudely estimate census sizes, but only if there is reasonable confidence in the accuracy of extrapolation factors from Ne to Nc. Theory suggests that Ne/Nc ratios for animals in the wild should be ≥ 0.10 (Hare et al. 2011). In most fish species, Ne is ~ 0.05–0.10 the census population size (Hare et al. 2011) but estimated Ne/Nc ratios in marine species are often as low as 10−5 (Hauser and Carvalho 2008; Palstra and Ruzzante 2008). A complication is that the relationship between Ne and Nc is not linear but rather, a power function in which the ratio decreases with increasing population size.
Bradford et al. (2016) obtained Ne/Nc ratios of 0.04 and 0.12 for two collection years of spawning adults for the Saint John River Atlantic sturgeon population, values near the 0.5–0.10 proposed by Hare et al. (2011). The Saint John is the only population in which there are whole-population Nc estimates with which to make comparison with contemporary Ne. For the Hudson, our Ne estimate of 156 compares with an estimate of mean annual adult spawners of 863, for a ratio of 0.18. However, we are unable to address the generality of their estimate to other populations. Additional whole-population Nc estimates are needed to attempt to develop an extrapolation factor between Ne and Nc.
It may be that tracking individual populations using Ne would be more reliable than applying an extrapolation factor between Ne and Nc. However, it would be important to monitor both Ne and Nc for periods long enough to determine how sensitive Ne is to changes in Nc and over what time frame.
Conclusions
Atlantic sturgeon show low contemporary Ne values; these may be of concern given current conservation biology guidelines for vertebrates. However, four factors temper this notion: (1) to date there is no evidence of any genetically-based problems, e.g., alarmingly low estimates of genetic diversity or morphological abnormalities for Atlantic sturgeon populations, (2) historical Ne values, while higher than contemporary values, are also considerably lower than might be expected based on historical catch records, suggesting that the particular life history attributes of the subspecies result in low Ne levels, (3) calculations of Ne Max suggest that some estimates of Ne are likely biased low because of inadequate representation of the population, and (4), although speculative, polyploidy may provide a buffering effect for low Ne in Atlantic sturgeon populations.
Additionally, Ne may be a useful monitoring tool as a surrogate for Nc, but there is not yet enough information to propose a reliable adjustment factor. To achieve that goal, there needs to be more research on Ne, Ne Max, and Nc simultaneously on individual populations across a sufficient number of year classes to achieve a flattening of the curve of Ne Max.
References
ASMFC (Atlantic States Marine Fisheries Commission) (1990) Fishery management plan for Atlantic sturgeon. Fishery Management Report Number 17. Washington DC
ASMFC (Atlantic States Marine Fisheries Commission) (1998) Amendment 1 to the interstate fishery management plan for Atlantic sturgeon. Fishery Management Report 31. Washington DC
ASMFC (Atlantic States Marine Fisheries Commission) (2017) 2017 Atlantic Sturgeon Benchmark Stock Assessment and Peer Review Report. Washington DC
ASSRT (Atlantic Sturgeon Status Review Team) (2007) Status Review of Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus). Report to National Marine Fisheries Service, Northeast Regional Office
Bahr DL, Peterson DL (2016) Recruitment of juvenile Atlantic sturgeon in the Savannah River, Georgia. Trans Am Fish Soc 145:1171–1178
Bain MB (1997) Atlantic and shortnose sturgeon of the Hudson River: common and divergent life history attributes. Environ Biol Fishes 48:347–358
Balazik M (2012) Life history analysis of James River Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) with implications for management and recovery of the species. Ph.D. Thesis Virginia Commonwealth University
Balazik MT, Musick JA (2015) Dual annual spawning races in Atlantic sturgeon. PLoS ONE. https://doi.org/10.1371/journal.pone.0128234
Balazik MT, Garman GC, Van Eenennaam JC, Mohler J, Woods LC III (2012) Empirical evidence of fall spawning by Atlantic sturgeon in the James River, Virginia, USA. Trans Am Fish Soc 141:1465–1471
Balazik MT, Farrae DJ, Darden TL, Garman GC (2017) Genetic differentiation of spring-spawning and fall-spawning male Atlantic sturgeon in the James River, Virginia. PLoS ONE. https://doi.org/10.1371/journal.pone.0179661
Beerli P (2006) Comparison of Bayesian and maximum likelihood inference of population genetic parameters. Bioinformatics 22:341–345
Beerli PE (2009) How to use MIGRATE or why are Markov chain Monte Carlo programs difficult to use. In: Bertorelle G, Bruford MW, Hauffe HC, Rizzoli A, Vernesi C (eds) Population genetics for animal conservation, vol 17 of Conservation Biology. Cambridge University Press, Cambridge, pp 42–79
Beerli P, Felsenstein J (2001) Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc Natl Acad Sci USA 98:4563–4568
Beerli P, Palczewski M (2010) Unified framework to evaluate panmixia and migration direction among multiple sampling locations. Genetics 185:313–326
Blankenship K (2016) Biologists alarmed over lack of young Atlantic sturgeon in surveys. Bay J 26(5)
Bracken FS, Hoelzel AR, Hume JB, Lucas MC (2015) Contrasting population genetic structure among freshwater-resident and anadromous lampreys: the role of demographic history, differential dispersal and anthropogenic barriers to movement. Mol Ecol 24:1188–1204
Bradford RG, Bentzen P, Ceapa C, Cook AM, Curry A, LeBlanc P, Stokesbury M (2016) Status of Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) in the Saint John River, New Brunswick. DFO Can Sci Advis Sec Res Doc 2016/072
Brown JJ, Murphy GW (2010) Atlantic sturgeon vessel-strike mortalities in the Delaware Estuary. Fisheries 35:72–83
Caron F, Tremblay S (1999) Structure and management of an exploited population of Atlantic sturgeon (Acipenser oxyrinchus) in the St. Lawrence estuary, Quebec, Canada. J Appl Ichthy 15:153–156
Caron F, Hatin D, Fortin R (2002) Biological characteristics of adult Atlantic sturgeon (Acipenser oxyrinchus) in the Saint Lawrence River estuary and the effectiveness of management rules. J Appl Ichthy 18:580–585
Collins MR, Smith TIJ, Post WC, Pashuk O (2000) Habitat utilization and biological characteristics of adult Atlantic sturgeon in two South Carolina rivers. Trans Am Fish Soc 129:982–988
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
COSEWIC (Committee on the Status of Endangered Wildlife in Canada) (2011) COSEWIC assessment and status report on the Atlantic Sturgeon Acipenser oxyrinchus in Canada, Ottawa, Canada
Dadswell MJ (2006) A review of the status of Atlantic sturgeon in Canada, with comparisons to populations in the United States and Europe. Fisheries 31:218–229
Dadswell MJ, Nack S (2012) An analytical critique of the scientific data used in the NOAA/NMFS 2012 listing of the USA Atlantic coast Atlantic sturgeon population as endangered. 2012 Annual Meeting Atlantic States Marine Fisheries Commission
Dadswell MJ, Ceapa C, Spares AD, Stewart ND, Curry RA, Bradford RG, Stokesbury MW (2017) Population characteristics of adult Atlantic sturgeon captured by the commercial fishery in the Saint John River Estuary, New Brunswick. Trans Am Fish Soc 146:318–330
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resources 14:209–214
Eldon B, Wakeley J (2006) Coalescent processes when the distribution of offspring number among individuals is highly skewed. Genetics 172:2621–2633
Farrae DJ, Schueller PM, Peterson DL (2009) Abundance of juvenile Atlantic sturgeon in the Ogeechee River, Georgia. In: Proceedings of the Annual Conference Southeast Association of Fish and Wildlife Agencies, vol 63, pp 172–176
Federal Register (2012a) Endangered and threatened wildlife and plants; final listing determinations for distinct population segments of Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus) in the Northeast region. Fed Reg 77:5880–5912
Federal Register (2012b) Endangered and threatened wildlife and plants; final listing determinations for two distinct population segments of Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus) in the Southeast. Fed Reg 77:5914–5982
Felsenstein J (1971) Inbreeding and variance effective numbers in populations with overlapping generations. Genet Res 68:581–597
Flowers HJ, Hightower JE (2015) Estimating sturgeon abundance in the Carolinas using side-scan sonar. Mar Coast Fish 7:1–9
Fox AG, Wirgin I, Peterson DL (In Press). Occurrence of Atlantic sturgeon in the St. Marys River, Georgia. Mar Coastal Fish
Frankham R (1995) Effective population size/adult population size ratios in wildlife: a review. Genet Res 66:95–107
Frankham R, Bradshaw CJA, Brook BW (2014) Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol Conserv 170:56–63
Franklin IR (1980) Evolutionary change in small populations. In: Soulé ME, Wilcox BA (eds) Conservation biology: an evolutionary–ecological perspective. Sinauer Associates, Sunderland, pp 135–150
Fritts MW, Grunwald C, Wirgin I, King TL, Peterson DL (2016) Status and genetic character of Atlantic sturgeon in the Satilla River, Georgia. Trans Am Fish Soc 145:69–82
Galligan JP (1960) History of the Connecticut River sturgeon fishery. Wildl Conserv Bull 6:1–6
Gilbert KJ, Whitlock MC (2015) Evaluating methods for estimating local effective population size with and without migration. Evolution 69:2154–2166
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm
Grogan CS, Boreman J (1998) Estimating the probability that historical populations of fish species are extirpated. N Am J Fish Manag 18:522–529
Grunwald C, Maceda L, Waldman J, Stabile J, Wirgin I (2008) Conservation of Atlantic sturgeon Acipenser oxyrinchus oxyrinchus: delineation of stock structure and distinct population segments. Conserv Genet 9:1111–1124
Hager C, Kahn J, Watterson C, Russo J, Hartman K (2014) Evidence of Atlantic sturgeon spawning in the York River system. Trans Am Fish Soc 143:1217–1219
Hale EA, Park IA, Fisher MT, Wong RA, Stangl MJ, Clark JH (2016) Abundance estimate for and habitat use by early juvenile Atlantic sturgeon within the Delaware River Estuary. Trans Am Fish Soc 145:1193–1201
Hare MP, Nunney L, Schwartz MK, Ruzzante DE, Burford M, Waples RS, Ruegg K, Palstra F (2011) Understanding and estimating effective population size for practical application in marine species management. Conserv Biol 25:438–449
Hauser L, Carvalho GR (2008) Paradigm shifts in marine fisheries genetics: ugly hypotheses slain by beautiful facts. Fish Fisheries 9:333–362
Henderson-Arzapalo A, King TL (2002) Novel microsatellite markers for Atlantic sturgeon population delineation and broodstock management. Mol Ecol Notes 2:437–439
Hilton EJ, Kynard B, Balazik MT, Horodysky AZ, Dillman CB (2016) Review of the biology, fisheries, and conservation status of the Atlantic sturgeon, (Acipenser oxyrinchus oxyrinchus Mitchill, 1815). J Appl Ichthy 32(Suppl. 1):30–66
Hughes JM, Real KM, Marshall JC, Schmidt DJ (2012) Extreme genetic structure in a small-bodied freshwater fish, the purple spotted gudgeon, Mogurnda adspersa (Eleotridae). PLoS ONE 7:e40546. https://doi.org/10.1371/journal.pone.0040546
Ingram EC, Peterson DL (2016) Annual spawning migrations of adult Atlantic sturgeon in the Altamaha River. Mar Coast Fish 8:595–606
Jarne P, Lagoda PGL (1996) Microsatellites, from molecules to populations and back. Trends Ecol Evol 11:424–429
Judd S (1905) History of Hadley: Including the Early History of Hatfield, South Hadley, Amherst and Granby, Massachusetts. H.R. Hunting and Company, Springfield
Kahn DM, Fisher M (2012) Endangered Atlantic sturgeon in the Delaware River require higher standards for dissolved oxygen. Delaware Division of Fish and Wildlife, Dover
Kahnle AW, Hattala KA, McKown K (2007) Status of Atlantic sturgeon (Acipenser oxyrinchus) of the Hudson River Estuary, New York, USA. Am Fish Soc Symp 56:347–364
Kalinowski ST (2005) HP-RARE 1.0: A computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5:187–189
Kalinowski ST, Manlove KR, Taper ML (2008) ONCOR: software for genetic stock identification. http://www.montana.edu/kalinowski/Software.htm
King TL, Lubinski BA, Spidle AP (2001) Microsatellite DNA variation in Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) and cross-species amplification in the Acipenseridae. Conserv Genet 2:103–119
Kocik J, Lipsky C, Miller T, Rago P, Shepherd G (2013) An Atlantic Sturgeon Population Index for ESA Management Analysis. U.S. Dept Commerce, Northeast Fish Sci Cent Ref Doc, 13–06
Limburg K, Waldman JR (2009) Dramatic declines in North Atlantic diadromous fishes. BioSci 59:955–965
May B, Krueger CC, Kincaid HL (1997) Genetic variation at microsatellite loci in sturgeon: primer sequence homology in Acipenser and Scaphirhynchus. Can J Fish Aquat Sci 54:1542–1547
Moberg T, DeLucia M (2016) Potential impacts of dissolved oxygen, salinity and flow on successful recruitment of Atlantic sturgeon in the Delaware River. The Nature Conservancy, Philadelphia
Moore JS, Harris LN, Tallman RF, Taylor EB (2013) The interplay between dispersal and gene flow in anadromous Arctic char (Salvelinus alpinus): implications for potential for local adaptation. Can J Fish Aquat Sci 70:1327–1338
Moyer GR, Sweka JA, Peterson DL (2012) Past and present processes influencing genetic diversity and effective population size in a natural population of Atlantic Sturgeon. Trans Am Fish Soc 141:56–67
NMFS (National Marine Fisheries Service) (2012) Endangered and threatened wildlife and plants; threatened and endangered status for distinct population segments of Atlantic sturgeon in the northeast region. Federal Register 77:24(6 February 2012):5880–5912
O’Leary SJ, Hice LA, Feldheim KA, Frisk MG, McElroy AE, Fast MD, Chapman DD (2013) Severe inbreeding and small effective number of breeders in a formerly abundant marine fish. PLoS ONE 8:e66126. https://doi.org/10.1371/journal.pone.0066126
Ong T-L, Stabile J, Wirgin I, Waldman JR (1996) Genetic divergence between Acipenser oxyrinchus oxyrinchus and A. o. desotoi as assessed by mitochondrial DNA sequencing analysis. Copeia 1996:464–469
Palstra FP, Ruzzante DE (2008) Genetic estimates of contemporary effective population size: what can they tell us about the importance of genetic stochasticity for wild population persistence? Mol Ecol 17:3428–3447
Panagiotopoulou H, Austin JD, Zalewska K, Gonciarz M, Czarnogórska K, Gawor J, Weglenski P, Popović D (2017) Microsatellite mutation rate in Atlantic sturgeon (Acipenser oxyrinchus). J Hered 108:686–692
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol 6:288–295
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research an—update. Bioinformatics 28:2537–2539
Peterson D, Haley N, Bain M (2000) Evidence of declining recruitment of Atlantic Sturgeon in the Hudson River. N Am J Fish Manag 20:231–238
Peterson D, Schueller P, Devries R, Fleming J, Wirgin I, Grunwald C (2008) Annual run size and genetic characteristics of Atlantic Sturgeon in the Altamaha River, Georgia. Trans Am Fish Soc 137:393–401
Pipkin W (2017) Endangered sturgeon may run into trouble at Virginia power station. Chesapeake Bay Magazine. https://www.chesapeakebaymagazine.com/baybulletin/2017/11/19/endangered-sturgeon-may-run-into-trouble-at-virginia-power-station. Accessed 19 Nov 2017
Portnoy DS, McDowell JR, Musick JA, Graves JE (2009) Effective size closely approximates the census size in the heavily exploited western Atlantic population of the sandbar shark, Carcharhinus plumbeus. Conserv Genet 10:1697–1705
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice WW (1989) Analyzing tables of statistical tests. Evol 43:223–225
Rousset F (2007) Genepop’007: a complete re-implementation of the GENEPOP software for Window and Linux. Mol Ecol Res 8:103–106
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Salisbury SJ, McCracken GR, Keefe D, Perry R, Ruzzante DE (2016) A portrait of a sucker using landscape genetics: how colonization and life history undermine the idealized dendritic metapopulation. Mol Ecol 25:4126–4145
Savoy T, Maceda L, Roy NK, Peterson D, Wirgin I (2017) Evidence of natural reproduction of Atlantic sturgeon in the Connecticut River from unlikely sources. PLoS ONE 12(4):e0175085. https://doi.org/10.1371/journal.pone.0175085
Schueller P, Peterson D (2010) Abundance and recruitment of juvenile Atlantic sturgeon in the Altamaha River, Georgia. Trans Am Fish Soc 139:1526–1535
Secor DH, Waldman JR (1999) Historical abundance of Delaware Bay Atlantic sturgeon and potential rate of recovery. Am Fish Soc Symp 23:203–216
Shrimpton JM, DD Heath (2003) Census vs. effective population size in chinook salmon: large-and small-scale environmental perturbation effects. Mol Ecol 12:2571–2583
Smith TIJ (1985) The fishery, biology, and management of Atlantic sturgeon, Acipenser oxyrhynchus, in North America. Environ Biol Fishes 14:61–72
Smith TIJ, Clugston JP (1997) Status and management of Atlantic sturgeon, Acipenser oxyrinchus, in North America. Environ Biol Fishes 48:335–346
Smith JA, Flowers HJ, Hightower JE (2015) Fall spawning of Atlantic sturgeon in the Roanoke River, North Carolina. Trans Am Fish Soc 144:48–54
Tallmon DA, Gregovich D, Waples RS, Baker CS, Jackson J, Taylor BL, Archer E, Martien KK, Allendorf FW, Schwartz MK (2010) When are genetic methods useful for estimating contemporary abundance and detecting population trends? Mol Ecol Resour 10:684–692
Vaisl’ev VP (2009) Mechanisms of polyploid evolution in fish: polyploidy in sturgeons. In: Carmona R, Domezain A, Garcia-Gallego M, Hernando JA (eds) Biology, conservation, and sustainable development of sturgeon. Springer, Berlin, pp 97–117
van Oosterhout C, Hutchinson WF, Wills DMP (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellites data. Mol Ecol Notes 4:535–538
Wakely J, Sargsyan O (2009) Extensions of the coalescent effective population size. Genetics 181:341–345
Waldman JR, Wirgin II (1998) Status and restoration options for Atlantic sturgeon in North America. Conserv Biol 12:631–638
Waldman JR, Hart JT, Wirgin II (1996) Stock composition of the New York Bight Atlantic sturgeon fishery based on analysis of mitochondrial DNA. Trans Am Fish Soc 125:364–371
Waldman JR, Grunwald C, Stabile J, Wirgin I (2002) Impacts of life history and biogeography on genetic stock structure in Atlantic Sturgeon, Acipenser oxyrinchus oxyrinchus, Gulf sturgeon A. oxyrinchus desotoi, and shortnose sturgeon, A. brevirostrum. J Appl Ichthy 18:509–518
Waldman JR, King T, Savoy T, Maceda L, Grunwald C, Wirgin I (2013) Stock origins of subadult and adult Atlantic sturgeon, Acipenser oxyrinchus, in a non-natal estuary, Long Island Sound. Estuar Coasts 36:257–267
Wang J, Santiago E, Caballero A (2016) Prediction and estimation of effective population size. Heredity 117:193–206
Waples R, Do C (2008) ldne a program for estimating effective population size from data on linkage disequilibrium. Mol Ecol Res 8:753–756
Waples RS, Do C (2009) Linkage disequilibrium estimates of contemporary Ne using highly variable genetic markers: a largely untapped resource for applied conservation and evolution. Evol Appl 3:244–262. https://doi.org/10.1111/j.1752-4571.2009.00104.x
Waples RS, England PR (2011) Estimating contemporary effective population size on the basis of linkage disequilibrium in the face of migration. Genetics 189:633–644
Waples RS, Luikart G, Faulkner JR, Tallman DA (2013) Simple life-history traits explain key effective size ratios across diverse taxa. Proc R Soc B 280:20131339
Waples RS, Antao T, Luikart G (2014) Effects of overlapping generations on linkage disequilibrium estimates of effective population size. Genetics 197:769–780
Whippelhauser GS, Sulikowski J, Zydlewski GB, Altenritter MA, Kieffer M, Kinnison MT (2017) Movements of Atlantic Sturgeon of the Gulf of Maine inside and outside of the geographically defined distinct population segment. Mar Coast Fish 9:93–107
Wirgin I, Grunwald C, Stabile J, Waldman J (2007) Genetic evidence for relict Atlantic sturgeon stocks along the Mid-Atlantic coast of the USA. N Am J Fish Manag 27:1214–1229
Wirgin I, Maceda L, Grunwald C, King T (2015a) Population origin of Atlantic sturgeon bycaught in U.S. Atlantic coast fisheries. J Fish Biol 85:1251–1270
Wirgin I, Breece MW, Fox DA, Maceda L, Wark KW, King T (2015b) Origin of Atlantic sturgeon Acipenser oxyrinchus oxyrinchus collected off the Delaware Coast during spring months. N Am J Fish Manag 35:20–30
Wirgin I, Roy NK, Maceda L, Mattson M (2018) DPS and population origin of subadult Atlantic sturgeon in the Hudson River. Fish Res 207:165–170
Wright S (1938) Size of population and breeding structure in relation to evolution. Science 87:430–431
Yi S, Streelman JT (2005) Genome size is negatively correlated with effective population size in ray-finned fish. Trends Genet 21:643–646
Acknowledgements
We thank all of the students, scientists, and resource managers for their collections of Atlantic sturgeon that were the target of this study. We are also grateful for helpful reviews by Robin Waples and two anonymous reviewers. We also acknowledge a NOAA Fisheries Endangered Species Act Section 6 program grant for its support and the use of the Molecular Facilities Core of the NYU NIEHS Center Grant ES000260.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Waldman, J., Alter, S.E., Peterson, D. et al. Contemporary and historical effective population sizes of Atlantic sturgeon Acipenser oxyrinchus oxyrinchus. Conserv Genet 20, 167–184 (2019). https://doi.org/10.1007/s10592-018-1121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-018-1121-4