Abstract
In desert streams, fishes and other organisms that depend on surface water are predicted to inhabit smaller and more isolated wetted reaches, while the frequency and severity of disturbance is expected to increase under most climate change models. Together, these factors should reduce population genetic diversity and persistence probabilities. In this study, our goal was to understand genetic responses of stream fish populations to disturbance in an intermittent stream network. This network is occupied by Rio Grande sucker (Pantosteus plebeius) that is native to highland desert streams in North America. Sample localities in upland perennial reaches were connected by moderate to high levels of gene flow even when separated by up to a 30-km intermittent reach. However, drier and lower-elevation reaches were significant barriers to gene flow. Effects of genetic drift (lower allelic diversity and higher levels of inbreeding) were more pronounced in the watershed with fewest wetted reaches. Temporal analysis of genetic diversity indicated that streams with several spatially distinct wetted reaches were more genetically resistant to wildfire-induced demographic bottlenecks than a stream with only one wetted reach. Maintenance of multiple wetted reaches within streams and facilitated gene flow among watersheds could slow losses of genetic diversity in upland desert stream fishes, and will be important strategies for conserving stream biodiversity in the face of habitat fragmentation and disturbance related to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stream fishes in arid regions of the world face multiple threats from climate change. First, nearly all climate models predict increases in maximum air and water temperatures, thus fishes must either relocate or cope with local physiological stress imposed by an altered thermal regime (Kennedy et al. 2009; Isaak et al. 2010). Second, surface water in high desert regions, like those in the southwestern US, may become scarcer as climate-induced drying and increased water extraction diminishes surface and ground water supplies (Gutzler 2013; Jaeger et al. 2014). Third, the frequency of severe disturbances due to wildfire and scouring are expected to increase as conditions become drier and rainfall events become more intense and sporadic (Westerling et al. 2006; Seager et al. 2007). Thus, climate change is predicted to simultaneously alter local conditions for fishes and restrict their ability to move to more suitable patches (Allan and Flecker 1993), consequently, many stream fishes and other obligate stream organisms are expected to become confined to smaller and less suitable habitat patches.
Theory indicates that extirpation becomes more probable as populations become small and isolated. This is because small populations are subject to environmentally-induced and stochastic demographic fluctuations that can force them below threshold densities for recovery (Lande 1988), and speed the rate of accumulation of deleterious mutations, genetic load, and inbreeding effects on viability (Higgins and Lynch 2001). In very small populations, demographic and genetic processes are expected to depress local populations synergistically in a process known as the ‘extinction vortex’ (Gilpin and Soulé 1986). Despite these expectations, there are a number of examples of desert fishes that have persisted for many (>100) generations in small and isolated, but environmentally stable habitats like springs (e.g. spring pupfishes [Cyprinodon] – Echelle et al. 2005; Brown and Feldmeth 1971; Hoagstrom et al. 2011; hardyhead [Craterocephalus dalhousiensis]; desert gobies [Chlamydogobius], Dalhousie catfish [Neosilurus gloveri] – Kodric-Brown and Brown 1993). Much less is known about demographic and genetic dynamics of fishes restricted to small, intermittent stream reaches where environmental conditions fluctuate dramatically across seasons and years.
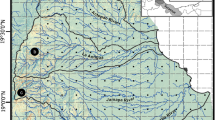
Here, we evaluated spatial and temporal genetic variation of Rio Grande sucker, Pantosteus plebeius, a species endemic to the southwestern United States (US) and northwestern Mexico (Sublette et al. 1990; Miller et al. 2005; Unmack et al. 2014). This fish occurs in three intermittent catchments at the southern-most boundary of the species’ range in the Rio Grande Basin. These streams are tributaries of the Rio Grande, but perennial flow is restricted to bedrock-confined sections on the east slope of the Black Mountain Range. Perennial reaches are sporadically connected by high flow events (a few days to months during wet cycles) that usually follow summer monsoon rains and less frequent snowmelt runoff. Like other watersheds in the region, they are also subject to major disturbances like wildfires and scouring floods that can have negative effects on abundance, movement, and distribution of aquatic biota.
We characterized genetic diversity of adult fishes within perennial stream segments of Palomas, Seco, and Las Animas creeks in 2003 and 2012. The intervening time period between samples encompassed roughly three sucker generations (generation time ≈3 years; McPhee 2007). Wildfires burned upland reaches of Palomas and Seco creeks, but not Las Animas Creek, one month after sampling concluded in 2003. We thus used pre- and post-fire demographic surveys and genetic data together to ask whether local genetic diversity, population structuring, and levels of gene flow remained stable, increased, or declined over the study period. These analyses allowed us to quantify genetic responses of fishes in intermittent streams and evaluate alternative population models for the entire system. Results pointed to general management strategies that should mitigate risks of local extirpation from genetic and demographic factors in intermittent high desert streams.
Methods
Genetic sampling
In 2003, five sites in Palomas, Seco, and Las Animas creeks were selected for genetic study of Rio Grande sucker (Fig. 1; Table 1). Two additional sites were sampled for genetic assay in 2012; one in Palomas Creek (Lower Palomas site), and one in Las Animas Creek (Lower Animas site) (Fig. 1). At each site, fish were captured in dip nets with the aid of a backpack electrofisher. Fishes were sampled from the entire wetted reach to minimize the possibility of sampling groups of siblings. After capture, fish were anesthetized in MS-222, and biopsied for a small (>5 mm2) clip of the caudal fin that was preserved in 95 % ethanol. Fish were allowed to recover and released at the point of capture unless preserved as voucher specimens. Voucher specimens are held at the Museum of Southwestern Biology at the University of New Mexico catalog numbers MSB 44632 (Palomas Creek) MSB 18490 (Seco Creek), and MSB 18481 (Las Animas Creek).
Molecular methods
Genomic DNA was extracted from fin clips using standard proteinase-K digestion and standard phenol/chloroform methods (Hillis et al. 1996). Individual samples were screened for variation at nine microsatellite loci (Tranah et al. 2001; Turner et al. 2009). Microsatellites were amplified in 10 μL reaction volumes, containing one μL diluted DNA, 1X Colorless GoTaq® Flexi Buffer, 2 mM MgCl2 solution, 125 µM dinucleotide triphosphates (dNTPs), 0.4 µM of both forward and reverse primers, and 0.375 units of GoTaq® DNA Polymerase. PCR was conducted with initial denaturation at 90 °C for 2 min, followed by 30 cycles of denaturation at 90 °C for 20–30 s, annealing at 49 °C (Xte11) or 50 °C (Dlu209, Dlu230, Xte10) or 54 °C (Dlu456) or 57 °C (Xte5) or 58 °C (Dlu229, Dlu4184, Dlu233) or for 20–30 s, extension at 72 °C for 30 s, and ending with a final extension at 72 °C for 30 min. One microliter of PCR product was mixed with 10 µl formamide and 0.35 µl HD400 size standard, and then denatured at 90 °C for five minutes. All samples were run on an automated ABI 3130 DNA sequencer and microsatellite sizes were obtained using Genemapper software and verified by direct examination of chromatographs.
Data analyses
Genetic variability and population structure
The computer program FSTAT Version 2.9.3.2 (Goudet 1995) was used to calculate Nei’s unbiased gene diversity (Nei 1987), allelic richness, inbreeding coefficients (F IS ) and observed heterozygosity for microsatellites. In cases where F IS was significantly different than zero for a particular site or locus, we used the program MICROCHECKER Version 2.2.3 (Van Oosterhout et al. 2004) to evaluate the probable cause of deviation (e.g., null alleles, stuttering, mis-scoring, etc.). Allelic richness was calculated by rarefaction (Petit et al. 1998) in FSTAT. This method allows allelic diversity to be compared among samples that differ in size (Leberg 1992) and is based upon the smallest number of individuals per sample genotyped for any locus. GENEPOP Version 3.1 (Raymond and Rousset 1995) was used to conduct the modified exact test to determine whether observed genotype frequencies conformed to Hardy–Weinberg expectations and to conduct a global test for linkage disequilibrium among loci. Measures of genetic variability were also estimated for each watershed by pooling sites within watershed (when multiple sites existed) and repeating calculations above.
To determine the extent and nature of spatial genetic structure among Rio Grande sucker populations, a measure of genetic distance, F ST , was calculated between each pair of localities using GENEPOP. Bonferroni correction was applied to assess statistical significance of p values obtained in pairwise analysis. Pairwise F ST values were plotted by the stream-course distance (km) that separated each population pair (distances estimated in GoogleEarth). We used ordinary least squares regression and visual examination of bivariate plots and residuals to determine the likely underlying model of genetic divergence on the landscape (Hutchison and Templeton 1999). We considered isolation-by-distance (IBD), hierarchical (e.g., Kalinowski et al. 2008), and n-island models (Slatkin 1985) of population structure. Metapopulation models were evaluated through estimation and analysis of effective population size, N e (see below).
Hierarchical analysis of molecular variance (AMOVA) was conducted in the program ARLEQUIN (Schneider et al. 2000; Excoffier et al. 2005) to estimate genetic variance attributable to differences among individuals due to inbreeding within localities (F IS ), differences among localities within a watershed (F SC ), differences across watersheds (F CT ), and differences attributable to non-random mating across the entire collection of localities (F IT ). Confidence intervals and p values were estimated via bootstrapping in ARLEQUIN. Hierarchical F-statistics were estimated for each sample year, and then compared across years to assess whether population structure remained temporally stable.
To gain additional insight into divergence and gene flow in the system, we conducted analyses of population structure using multi-locus microsatellite DNA data (rather than averaging across loci as in AMOVA) and the program STRUCTURE Vers. 3.1 (Pritchard et al. 2000). STRUCTURE uses a Bayesian approach to assign individuals to K clusters based on multilocus genotypes. The program was run with the admixture model and assuming independent allele frequencies for each population. K was set to range from 1 to 7 for 2003 samples and 1 to 9 for 2012 samples, and corresponded to the number of distinct sampling localities in each year with two added to account for the possibility of genetic structure within populations. The ‘burn-in’ length for each run was 50,000 and data was collected over 500,000 iterations. Five runs were conducted for each value of K to ensure stability across runs. Results were visualized using the program DISTRUCT (Rosenberg 2004). To identify the most likely value of K, Evanno’s ΔK (Evanno et al. 2005) statistic was calculated using the program STRUCTURE HARVESTER v. 0.9.93 (Earl and von Holdt 2012).
Demographic analysis
In summer 2003, two separate fires (unnamed because of their relatively small size) burned 2,266 and 1,817 hectares in the headwaters of Seco and South Fork Palomas creeks, respectively. Although the area immediately surrounding the wetted reaches we studied was not burned, a series of ash- and sediment-flow events attributed to upland fires affected water quality in both Seco and Palomas creeks (excluding the Upper Palomas site located on the North Fork Palomas Creek). Six flow events that mobilized ash and sediment through the Seco site were documented between 27 June and 7 October 2003 and it is likely that there were similar occurrences in affected areas of Palomas Creek.
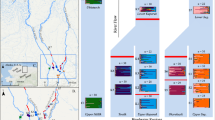
To evaluate fire-related effects on local fish abundance, demographic sampling of Seco and Palomas creeks was conducted separately from genetic sampling (but at the same sites), and aimed at estimating fish density and census size within these wetted reaches. Three-pass electrofishing surveys were done in defined 50-m sampling areas that were distributed throughout Seco, Palomas, and Lower Palomas sites. The number of fish captured was divided by the total area sampled to estimate catch-per-unit-effort (CPUE), which is reported as the mean number of fish m−2. Demographic sampling was conducted in Seco Creek in May, June 2003 (pre-fire) and in July and October 2003, July 2004, June 2008, and June 2012 (post-fire); and in Palomas Creek in July 2003 (pre-fire), and in July 2004, June 2006, 2007, 2008, 2012 (post-fire). Las Animas Creek was unaffected by fires and was not sampled. Additional details regarding sampling methods, including sampling efficiencies, are available upon request (from CGK).
Genetic effective size (N e )
Variance genetic effective size, N eV , was estimated from changes in microsatellite allele frequencies across three generations (~9 years) using the method of Jorde and Ryman (2007). N eV was estimated for each site separately, and for sites pooled within watersheds in Palomas and Las Animas Creeks. Total metapopulation effective size, meta-N eV , was estimated similarly by pooling all samples across sites within years. Samples from Lower Palomas and Lower Animas sites were excluded in calculations of N eV at all hierarchical levels (sites, watershed, meta-N eV ) because these were not sampled in 2003. Inbreeding effective size, N eD , was estimated via the linkage disequilibrium method (Hill 1981) as implemented in the program LDNE (Waples and Do 2008). All calculations were conducted using the program NeESTIMATOR v. 2 (Do et al. 2014). Alleles that occurred at a frequency less than or equal to 0.03 were excluded in calculations of N eD as suggested by Waples and Do (2008) when samples sizes are small.
A number of theoretical papers have evaluated how genetic diversity is maintained in a metapopulation with alternative models that allow for variation in local population size, gene flow, and local productivity. We substituted observed values of N e and hierarchical F-statistics into five alternative models (summarized in Table 2 of Gomez-Uchida et al. 2013), and used results as an heuristic tool to evaluate management options for Rio Grande sucker (and similar organisms) in intermittent streams.
Results
Genetic variability
Nine microsatellite loci were identified as polymorphic in Rio Grande sucker, with levels of variability ranging from three (Xte5) to 32 alleles (Dlu4184). Six of nine loci conformed to Hardy–Weinberg expectations in all populations. Of 110 total locus-by-site comparisons, 10 deviated from Hardy–Weinberg expectations at Bonferroni-corrected alpha-values. Significant deviations were caused by homozygote excess and involved three loci (Dlu209, Dlu456, Dlu4184) that exhibited the highest levels of polymorphism observed across loci (allele count = 12, 20, and 32, respectively), and analysis with MICROCHECKER indicated the presence of null alleles at these loci. Four of 10 deviations were identified in Seco Creek in both 2003 and 2012. No statistically significant linkage disequilibrium was detected.
Nei’s unbiased gene diversity, observed heterozygosity and allelic richness were markedly lower in Seco Creek compared to all other localities (Table 1). Palomas and Las Animas creeks had similar levels of genetic diversity at all metrics both within and across sample years. Site-specific inbreeding coefficients were significantly positive (i.e., F IS > 0 at 6 of 9 loci) and of similar magnitude at Seco Creek for both 2003 and 2012 (Table 1), indicating an excess of homozygotes at this locality. Values of F IS were near zero at the Animas site in 2012 but otherwise ranged from slightly to moderately negative at all other sample sites, which indicated an excess of heterozygotes at these localities in both 2003 and 2012 (Table 1). Analysis of diversity in samples pooled by watershed indicated that rates of change of genetic diversity over time varied depending on the total number of wetted reaches within the watershed, but not local density. Seco Creek experienced the greatest loss of heterozygosity from 2003 to 2012, but no decline of allelic richness (Table 2). Palomas and Las Animas watersheds had lower proportional losses of heterozygosity compared to Seco Creek, but a modest decline in allelic richness was noted in Palomas Creek from 2003 to 2012 (Table 2).
Population structure
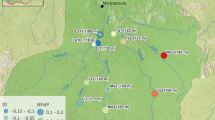
Analysis of pairwise F ST indicated relatively low levels of divergence among sites within streams, and moderate (e.g., Palomas Creek vs. Las Animas Creek sites) to high (e.g., Seco Creek vs. all others) levels of divergence among sites located in different watersheds (Table 3; Fig. 2). A plot of pairwise F ST on stream-course distance is consistent with a scenario where gene flow and genetic drift affect regional population structure differently; gene flow is an important structuring force within watersheds (across wetted reaches within highland stream habitats), and genetic drift is most important across watersheds separated by lowland (desert) stream habitats (Case IV in Hutchison and Templeton 1999). This plot and AMOVA results presented below indicate that a hierarchical model of divergence best fits observed patterns of genetic diversity within and among these isolated watersheds compared to isolation-by-distance or n-island models of population structure.
A biplot of pairwise values of F ST versus stream distance in kilometers. Highest pairwise values were observed in comparisons between Seco Creek and other watersheds, where F ST -values uniformly increased between 2003 and 2012. Pairwise values of F ST remained relatively unchanged over time both within and among sites in Palomas and Las Animas creeks
Results from hierarchical AMOVA were qualitatively consistent when compared between 2003 and 2012. Similarities include an observation that F IS values (averaged across loci and sites) were significantly negative in 2003 (F IS = −0.059, p = 0.003) and in 2012 (F IS = −0.092, p < 0.001) in Palomas and Las Animas Creeks, but significantly positive population-specific F IS values in Seco Creek were identified in both sample years (Table 1). A small, but significant portion of spatial genetic variance was attributable to differences across sampling localities within streams in 2003 (F SC = 0.041, p < 0.001), and was lower in 2012 (F SC = 0.017, p < 0.001). The among-watershed component (F CT ) accounted for more genetic variance than any other hierarchical level in both sample years, and this value increased from F CT = 0.167 (p = 0.069) in 2003 to F CT = 0.281 (p = 0.014) in 2012. Marginal non-significance of F CT in 2003 may be attributable to relatively small sample sizes in that year. Finally, F IT was equal to 0.156 (p < 0.001) in 2003 and 0.230 (p < 0.001) in 2012.
Additional support for the hierarchical model of population structure was provided by STRUCTURE analysis. Calculations of Evanno’s ΔK indicated that the highest peak was at K = 3 in 2003 and in 2012, with groups corresponding geographically to the three watersheds (Fig. 3a, b). The absence of appreciable population structure within drainages, despite the intermittent nature of these streams, suggested that gene flow among sites within watersheds was high (Fig. 3a, b).
Plots from the program STRUCTURE, where bars represent the probability of assignment of each individual to one of three groups (i.e., K = 3 determined via the Evanno method). Groups correspond to each of three intermittent watersheds in south-central New Mexico. There is some suggestion of infrequent gene flow events among watersheds as indicated by some individuals with ambiguous assignment within groups
Demographic responses
Sampling conducted before wildfires and ash-flow events indicated that average density (measured as CPUE) was 1.22 fish m−2 at Seco Creek, and 0.6 fish m−2 at Palomas Creek. Following fires and ash-flow events, mean density declined 50-fold to 0.025 fish m−2 in Seco Creek (Fig. 4). Similar declines were observed in Palomas Creek where fish density (0.011 fish m−2) remained 50-fold lower than pre-fire values. By June 2008, suckers recovered to pre-fire densities in both watersheds (Fig. 4).
Genetic effective size
Estimates of variance genetic effective size (N eV ) are presented in Table 4. Values for each site (excluding Lower Palomas and Lower Animas sites because they were not sampled in 2003) ranged from 21 at Seco Creek to just over 100 at the Animas site, and the sum of N eV across sites was 237 (Table 4). Values of N eV for samples pooled by watershed ranged from 21 in Seco Creek to 103 in Palomas Creek and the sum of N eV across watersheds was 188. Finally, meta-N eV (all sites pooled within year) was estimated as 186 (Table 4). Estimates of N eD based on the linkage disequilibrium method (LDNE) were much less precise than estimates of N eV . Estimates of N eD are reported by site in Supplemental Table 1.
Of five metapopulation models presented in Gomez-Uchida et al. (2013), only the interdemic model of Nunney (1999) predicted meta-N eV < ∑ N eV across subpopulations. We checked the observed estimate of meta-N eV by substituting ∑ N eV and F IT (from the hierarchical AMOVA = 0.230 in 2012) into Nunney’s (1999—Eq. 16) model to obtain an expected value of meta-N eV = 193, which is very close to the observed value of 186 (Table 4).
Discussion
Like many native fishes in the southwestern US (Minckley and Marsh 2009; Clarkson et al. 2012) and other high desert regions of the world, Rio Grande sucker has generally declined in abundance and geographic range over the last century (Rinne 1995). Earlier assessments of the species’ status identified stream impoundment, alteration of the natural flow regime, land use changes, and hybridization with non-native fishes as the most important threats to species persistence (Sublette et al. 1990; Swift-Miller et al. 1999; Calamusso et al. 2002). These threats continue to be important, but regional climate models also predict higher stream temperatures, more sporadic and diminished rainfall and snowpack, and increased chances for long-term drought (Hurd and Coonrod 2007) that are all expected to negatively impact Rio Grande sucker and other aquatic biota in desert streams. Moreover, large-scale disturbances like wildfires are also expected to become more frequent, and compound existing and future threats (Westerling et al. 2006).
If climate in the western US warms and dries as predicted, it is likely that intermittent stream systems will represent a greater proportion of available stream habitat for fishes (Jaeger et al. 2014). Nonetheless, there are little empirical data on ecological and genetic dynamics of surface water-obligate species in such systems (Dunham et al. 2003). Thus, our study system in southern New Mexico represents a test case for the likely effects of climate change and associated changes in disturbance regime and their impacts on fishes in broader western US landscapes.
Rio Grande sucker populations in the southerly portion of the range we studied share recent common ancestry with populations in the Upper Rio Grande in northern New Mexico and southern Colorado (McPhee et al. 2008). The most common mitochondrial DNA haplotype identified at Palomas Creek was shared with all six other localities sampled in the Upper Rio Grande Basin. Las Animas and Seco creeks were not sampled by McPhee et al. (2008), but these populations also probably exchanged genes with Upper Rio Grande populations in the past. However, these streams are now demographically and genetically isolated from Upper Rio Grande populations.
Our analysis shows that population structure within and among intermittent watersheds is determined by a balance of gene flow and genetic drift at differing spatial scales. A plot of pairwise F ST against stream-course distance indicated little support for an isolation-by-distance model, but instead revealed a pattern consistent with a hierarchical model of genetic structure (Kalinowski et al. 2008) across watersheds. In this model, gene flow is the predominant evolutionary force within watersheds (where multiple wetted reaches exist) but is limited among watersheds. Accordingly, we observed low pairwise values of F ST , and inferred relatively high gene flow across localities within Palomas and Las Animas creeks that were separated by up to 30 km with intervening dry reaches. Gene flow probably involves downstream movement of larval fishes during spring runoff and upstream movement of juveniles and/or adults during high-flow events later in the water year (e.g., summer monsoon rains—see also Faulks et al. 2010 for a description of the importance of seasonal rains in intermittent watersheds in Australia).
Analysis of pairwise F ST -values and AMOVA indicated that gene flow appears to be nearly completely restricted by lower-elevation reaches that flow intermittently to the Rio Grande mainstem. Rio Grande sucker is rarely found in high-order and lower elevation streams, even when wetted dispersal corridors from tributaries exist, such as in the upper Rio Grande Basin (McPhee et al. 2008). McPhee et al. (2008) reported hierarchical F CT = 0.14 (from AMOVA) across eight Upper Rio Grande localities separated by up to 210 km of perennial stream habitat. Values reported in McPhee et al. (2008) are similar in magnitude to the F CT -value we observed in 2003 (F CT = 0.167), although shorter stream distances separate watersheds in our study (maximum pairwise stream-course distance is 100 km). Results of STRUCTURE analysis were also consistent with limited gene flow across watersheds, but did suggest the intriguing possibility that rare, long-distance gene flow events once occurred but are likely now limited by downstream dewatering, physical obstructions, and mainstem reservoirs. Such events may be tied to infrequent large precipitation events that restore flow and suitable water temperatures to low-elevation reaches. Nonetheless, in this intermittent stream network, genetic drift is the overwhelming force that shapes allelic diversity within watersheds, and each stream should be considered demographically independent for the purposes of management.
Genetic responses to a fire-induced demographic bottleneck
Wildfires have direct and indirect impacts on abundance and distribution of stream fishes (Lyon and O’Connor 2008; Isaak et al. 2010). Most relevant for our study are effects of ash and sediment flows. Low dissolved oxygen levels and elevated ammonia concentrations associated with ash flows are acutely toxic to fish, and can result in high (>95 %) mortality (Lyon and O’Connor 2008). Accordingly, we observed a ~98 % reduction in CPUE after fires burned upland areas of Palomas and Seco Creeks. Fish densities returned to pre-fire levels by 2008 (and perhaps before), suggesting demographic recovery and ecological resilience. It is also possible that effects of ash-laden flows on mortality were variable among localities within watershed, and that recovery was facilitated by movement of fishes from refugia. In any event, large demographic bottlenecks like these can alter processes like migration and gene flow and significantly reduce genetic variation in affected populations despite rapid ecological recovery (Frankham et al. 2009).
For Rio Grande sucker, patterns of population structure and levels of genetic diversity were qualitatively similar before and after wildfires in the study area. For example, a hierarchical model of structure best explained the demographic and evolutionary relationships within and across watersheds in both 2003 and 2012. However, we observed three important properties of this population over time: (i) higher levels of among-watershed (F CT ) divergence in 2012 compared to 2003, (ii) lower levels of genetic divergence and higher levels of inferred gene flow across sites within creeks in 2012, and (iii) remarkable stability of allelic diversity suggesting ‘genetic resistance’ to fire-induced bottlenecks. We will consider each of these observations in turn below.
Between 2003 and 2012, much of the observed increase in among-watershed values of genetic divergence (F CT –from the hierarchical model) is directly attributable to genetic drift at Seco Creek, an effect that is seen by examining values of pairwise F ST for Seco Creek versus all other sampling localities (Fig. 2). Genetic isolation and genetic drift is pronounced at Seco Creek because there is only a single occupied wetted reach, and within-watershed gene flow opportunities are lacking. In the absence or near absence of gene flow, the rate of genetic drift is determined exclusively by the genetic effective population size, N e (Frankham et al. 2009). Among-watershed pairwise values of F ST were nearly identical in 2003 and 2012 between Palomas and Las Animas Creeks, suggesting no change in response to fire effects in Palomas Creek.
Unlike Seco Creek, Palomas and Las Animas creeks are characterized by at least three occupied wetted reaches within each watershed. Levels of genetic divergence were lower and gene flow more pronounced among perennial reaches within watersheds in 2012 compared to 2003. It is tempting to ascribe enhanced gene flow in Palomas Creek to a response to fire-related demographic shifts. Under this scenario, gene flow could be facilitated by movement of fishes from unaffected areas (i.e., the Upper Palomas site in the North Fork) to those with depressed fish densities after fire-related mortality (i.e., Palomas and Lower Palomas). However, reduced divergence and higher gene flow was also noted among sites in Las Animas Creek that was not affected by wildfires during the study period. It is possible that other events, like increased precipitation or snow melt may have facilitated gene flow across wetted reaches that are usually separated by dry stretches, but we were unable to test this directly.
Since these watersheds are essentially now isolated from one another, it is reasonable to expect that within-watershed genetic diversity (gene diversity and allelic richness) should be proportional to local N e and inversely proportional to the magnitude of the bottleneck induced by fire. Decreases in expected and observed heterozygosity from 2003 to 2012 (summarized for the entire watershed) were most pronounced in Seco Creek, less so in Palomas Creek, and least pronounced in Las Animas Creek. If local abundance were the only determinant of N e , we might expect each watershed to have roughly equal changes in genetic diversity over the study period. Equivalence is expected because wetted area in Seco Creek (4.0 km) is under half that of Palomas Creek (9.8 km), but pre- and post-fire densities in Seco are over twice those observed at Palomas sites, and thus census size might be roughly equal. Although not estimated in this study, fish densities in Las Animas Creek (7.5 km total wetted area) are probably similar to those observed in Palomas (Carter Kruse, personal observation). Thus, greater reductions in heterozygosity in Seco and Palomas Creeks are consistent with fire-related reductions in density and N e in these watersheds, all else being equal. However, maintenance of allelic richness across time, despite demographic change in Seco and Palomas creeks, suggests resistance to fire-induced demographic bottlenecks.
Additional information pertinent to management can be obtained from analysis of N e in a metapopulation context. As expected, per-site estimates of N eV were smallest for Seco Creek, intermediate for Palomas Creek, and largest for Las Animas Creek. Comparison of the sum of N eV across sites (∑N eV ) to meta-N eV , allowed additional insight into the nature of population structure, genetic drift, and its overall effect on genetic diversity for the entire collection of populations studied here. In this case, ∑N eV exceeded meta-N eV , and only the interdemic model of Nunney (1999) predicted this result. This model explicitly accounts for effects of variance in productivity among subpopulations, and predicts that meta-N e can never exceed ∑ N e when habitats vary in size or quality that affects production of offspring. A practical implication of this model is that increasing the number of occupied wetted reaches in Seco Creek (if possible) would potentially increase genetic diversity for the entire metapopulation. A similar result could be accomplished by facilitating gene flow across watersheds by physically transferring fish to recover diversity and diminish inbreeding in this population to increase persistence probabilities. However, risks to viability from outbreeding depression should be assessed prior to implementing cross-watershed translocations (e.g., Frankham et al. 2011).
Single-sample estimates of N eD based on LDNE had lowered precision, and possibly lowered accuracy in our study compared to N eV based on temporal-method estimates. For example, values of N eD for Seco Creek were much larger than expected based on our observation of high inbreeding coefficients and small values of N eV . One possible explanation for overestimation of N eD in Seco Creek is that most loci are near fixation, and it is known that very low- or high-frequency alleles tend to overestimate N eD (Waples and Do 2010). Conversely, meta-N eD values were much smaller than expected and it is possible that admixture of divergent populations created an effect of inflated linkage disequilibrium coefficients and downwardly biased estimates of meta-N eD . A recent study (Neel et al. 2013) suggested caution using single-sample estimators under some models of population structure (i.e., isolation-by-distance). It may also be that highly structured populations (where some are subject to intense genetic drift) may also pose problems for N eD estimation via LDNE. Nonetheless, the observation that ∑N eD > > meta-N eD (see Supplemental Table) also supports the Nunney model of metapopulation dynamics.
Conclusions
There are daunting challenges to effective conservation of desert stream organisms in the face of climate change. This is because fishes that inhabit mountain streams in arid environments are not likely to respond to warming and drying in the same manner as terrestrial animals. For example, mammals responded to past warming cycles by retreating to higher-elevation “sky islands” (Heald 1967; Brown 1971). However, because of predicted water scarcity in upstream reaches, stream-obligate taxa will have limited ways to respond to climate change. Our study shows that local populations are somewhat resistant to demographic bottlenecks, particularly if there are multiple localities that exchange genes periodically. Nonetheless, small effective population sizes and effects of genetic drift were evident in all watersheds, suggesting high vulnerability to sustained drought and future demographic bottlenecks.
When considered as a metapopulation, our results show that small and isolated populations can decrease genetic diversity for the entire metapopulation by increasing variance in productivity among populations. Thus, in this case, management efforts should focus on establishing additional localities in Seco Creek that can hold fish. At a larger scale, it will be important to maintain integrity of riparian habitat and protect ground and surface water in all perennial reaches. Finally, if outbreeding depression is not a factor, then this system may also benefit from facilitated gene flow from Palomas and/or Las Animas creeks to Seco Creek to recover genetic diversity of this population. Implementation of conservation measures like these could provide a level of insurance against extirpation due to one-time or infrequent catastrophic events, and increase chances of local persistence if surface water becomes more limited with climate change.
We have shown that the Rio Grande sucker exhibits considerable ecological resilience and some resistance to acute fire-related disturbance, suggesting that conservation and management attention in this species needs to be focused on chronic effects of global climate change. These include diminishing surface water supplies and increases in water temperature. Ultimately, population persistence in intermittent streams will require active management to maintain genetic diversity, and renewed attention to conservation of watershed resources. Grazing, diversion, and ground-water pumping practices must be adjusted to accommodate changes in surface water availability. Exploitation at current rates will reduce the number and quality of wetted reaches in intermittent stream systems and thus hasten demise of unique high desert fauna.
References
Allan JD, Flecker AS (1993) Biodiversity conservation in running waters. Bioscience 43:32–43
Brown JH (1971) Mammals on mountaintops: nonequilibrium insular biogeography. Am Nat 105:467–478
Brown JH, Feldmeth CR (1971) Evolution in constant and fluctuating environments: thermal tolerances of desert pupfish (Cyprinodon). Evolution 25:390–398
Calamusso B, Rinne JR, Turner PR (2002) Distribution and abundance of the Rio Grande sucker in the Carson and Santa Fe National forests, New Mexico. Southwest Nat, 47:182–186
Clarkson RW, Marsh PC, Dowling TE (2012) Population prioritization for conservation of imperiled warmwater fishes in an arid-region drainage. Aquat Conserv Mar Freshw Ecosyst 22:498–510
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Dunham JB, Young MK, Gresswell RE, Rieman BE (2003) Effects of fire on fish populations: landscape perspectives on persistence of native fishes and nonnative fish invasions. For Ecol Manag 178:183–196
Earl DA, Von Holdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Echelle AA, Carson EW, Echelle AF, Van Den Bussche RA, Dowling TE, Meyer A (2005) Historical biogeography of the new-world pupfish genus Cyprinodon (Teleostei: Cyprinodontidae). Copeia 2005:320–339
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Faulks LK, Gilligan DM, Beheregaray LB (2010) Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua). Mol Ecol 19:4723–4737
Frankham R, Ballou JD, Briscoe DA (2009) Introduction to conservation genetics, 2nd edn. Cambridge University Press, Cambridge
Frankham R, Ballou JD, Eldridge MDB, Lacy RC, Ralls K, Dudash MR, Fenster CB (2011) Predicting the probability of outbreeding depression. Conserv Biol 25:465–475
Gilpin ME, Soulé ME (1986) Minimum viable populations: processes of species extinctions. In: Soulé ME (ed) Conservation biology–the science of scarcity and diversity. Sinauer Associates, Sunderland, pp 19–34
Gomez-Uchida D, Palstra FP, Knight TW, Ruzzante DE (2013) Contemporary effective population and metapopulation size (N e and meta-N e ): comparison among three salmonids inhabiting a fragmented system and differing in gene flow and its asymmetries. Ecol Evol 3:569–580
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Gutzler DS (2013) Regional climatic considerations for borderlands sustainability. Ecosphere. doi:10.1890/ES12-00283.1
Heald WF (1967) Sky island. Van Nostren, Princeton
Higgins K, Lynch M (2001) Metapopulation extinction caused by mutation accumulation. Proc Natl Acad Sci USA 98:2928–2933
Hill WG (1981) Estimation of effective population size from data on linkage disequilibrium. Genet Res 38:209–216
Hillis D, Moritz C, Mable B (1996) Molecular systematics. Sinauer, Sunderland
Hoagstrom CW, Brooks JE, Davenport SR (2011) A large-scale conservation perspective considering endemic fishes of the North American plains. Biol Conserv 144:21–34
Hurd BH, Coonrod J (2007) Climate change and its implications for New Mexico’s water resources and economic opportunities. National Commission on Energy Policy. New Mexico State University, Las Cruces
Hutchison DW, Templeton AR (1999) Correlation of pairwise genetic and geographic distance measures: inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution 53:1898–1914
Isaak DJ, Luce CH, Rieman BE, Nagel DE, Peterson EE, Horan DL, Parkes S, Chandler GL (2010) Effects of climate change and wildfire on stream temperatures and salmonid thermal habitat in a mountain river network. Ecol Appl 20:1350–1371
Jaeger KL, Olden JD, Pelland ND (2014) Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc Natl Acad Sci USA. doi:10.1073/pnas.1320890111
Jorde PE, Ryman N (2007) Unbiased estimator for genetic drift and effective population size. Genetics 177:927–935
Kalinowski ST, Meeuwig MH, Narum SR, Taper ML (2008) Stream trees: a statistical method for mapping genetic differences between populations of freshwater organisms to the sections of streams that connect them. Can J Fish Aquat Sci 65:2752–2760
Kennedy TL, Gutzler DS, Leung RL (2009) Predicting future threats to the long-term survival of Gila trout using a high-resolution simulation of climate change. Clim Change 94:503–515
Kodric-Brown A, Brown JH (1993) Highly structured fish communities in Australian desert springs. Ecology 74:1847–1855
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Leberg PL (1992) Effects of population bottlenecks on genetic diversity as measured by allozyme electrophoresis. Evolution 46:477–494
Lyon JP, O’Connor JP (2008) Smoke on the water: can riverine fish populations recover following a catastrophic fire-related sediment slug? Austral Ecol 33:794–806
McPhee MV (2007) Age, growth and life history comparisons between the invasive white sucker (Catostomus commersoni) and native Rio Grande sucker (C. plebeius). Southwest Nat 52:15–25
McPhee MV, Osborne MJ, Turner TF (2008) Genetic diversity, population structure, and demographic history of the Rio Grande Sucker, Catostomus (Pantosteus) plebeius, in New Mexico. Copeia 2008:191–199
Miller RR, Minckley WL, Norris SM (2005) Freshwater fishes of Mexico. University of Chicago Press, Chicago
Minckley WL, Marsh PC (2009) Inland fishes of the greater Southwest: chronicle of a vanishing biota. University of Arizona Press, Tucson
Neel MC, McKelvey K, Ryman N, Lloyd MW, Short Bull R, Allendorf FW, Schwartz MK, Waples RS (2013) Estimation of effective population size in continuously distributed populations: there goes the neighborhood. Heredity 111:189–199
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nunney L (1999) The effective size of a hierarchically structured population. Evolution 53:1–10
Petit RJ, El Mousadik A, Pons P (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol 12:844–855
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multi-locus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) GENEPOP Version 1.2: population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rinne JN (1995) Reproductive biology of the Rio Grande sucker Catostomus plebeius (Cypiniformes), in a montane stream, New Mexico. Southwest Nat 40:237–241
Rosenberg NA (2004) DISTRUCT: a program for the graphical display of population structure. Mol Ecol Notes 4:137–138
Schneider S, Roessli D, Excoffier L (2000) Arlequin: a software for population genetics data analysis. Ver 2.000. Genetics and Biometry Laboratory, Department of Anthropology, University of Geneva
Seager R, Ting M, Held I, Kushnir Y, Lu J et al (2007) Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316:1181–1183
Slatkin M (1985) Gene flow in natural populations. Annu Rev Ecol Syst 16:393–430
Sublette EJ, Hatch DM, Sublette M (1990) The fishes of New Mexico. University of New Mexico Press, Albuquerque
Swift-Miller SM, Johnson BM, Muth RT, Langlois D (1999) Distribution, abundance, and habitat use of Rio Grande sucker (Catostomus plebeius) in Hot Creek, Colorado. Southwest Nat 44:42–48
Tranah GJ, Agresti JJ, May B (2001) New microsatellite loci for suckers (Catostomidae): primer homology in Catostomus, Chasmistes, and Deltistes. Mol Ecol Notes 1:55–60
Turner TF, Osborne MJ, Dowling TE, McPhee MV, Broughton RE, Gold JR (2009) Microsatellite markers for the endangered razorback sucker, Xyrauchen texanus, are widely applicable to genetic studies of other catostomine fishes. Conser Genet 10:551–553
Unmack PJ, Dowling TE, Laitinen NJ, Secor CL, Mayden RL, Shiozawa DK, Smith GR (2014) Influence of introgression and geological processes on phylogenetic relationships of western North American mountain suckers. (Pantosteus, Catostomidae). PLoS One 9:e90061. doi:10.1371/journal.pone.0090061
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Waples RS (2005) Genetic estimates of contemporary effective population size: to what time periods do the estimates apply? Mol Ecol 14:3335–3352
Waples RS, Do C (2008) LDNE: a program for estimating effective population size from data on linkage disequilibrium. Mol Ecol Resour 8:753–756
Waples RS, Do C (2010) Linkage disequilibrium estimates of contemporary N e using highly variable genetic markers: a largely untapped resource for applied conservation and evolution. Evol Appl 3:244–262
Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW (2006) Warming and earlier spring increase western U.S. forest wildfire activity. Science 313:940–943
Acknowledgments
We thank Heather Johnson, Eric Leinonen, and Michael Konsmo for field assistance. Krista Leibensperger, Tyler Pilger, Hailey Conover, and George Rosenberg provided assistance in the laboratory. Genotyping was done in the UNM Molecular Biology Facility supported, in part, by NIH grant number P20GM103452. David Propst, Tyler Pilger, John Carlos Garza, and an anonymous reviewer made valuable comments and suggestions that greatly improved the manuscript. Field collections were made under New Mexico Department of Game and Fish Authorization for Taking Protected Wildlife For Scientific and Educational Purposes Permit # 3261 and UNM IACUC Protocol # 10-100492-MCC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Turner, T.F., Osborne, M.J., McPhee, M.V. et al. High and dry: intermittent watersheds provide a test case for genetic response of desert fishes to climate change. Conserv Genet 16, 399–410 (2015). https://doi.org/10.1007/s10592-014-0666-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-014-0666-0