Abstract
This paper is a cross fertilization of ideas about the importance of molecular aspects of breast cancer metastasis by basic scientists, a pathologist, and clinical oncologists at the Henry Ford Health symposium. We address four major topics: (i) the complex roles of lymphatic endothelial cells and the molecules that stimulate them to enhance lymph node and systemic metastasis and influence the anti-tumor immunity that might inhibit metastasis; (ii) the interaction of molecules and cells when breast cancer spreads to bone, and how bone metastases may themselves spread to internal viscera; (iii) how molecular expression and morphologic subtypes of breast cancer assist clinicians in determining which patients to treat with more or less aggressive therapies; (iv) how the outcomes of patients with oligometastases in breast cancer are different from those with multiple metastases and how that could justify the aggressive treatment of these patients with the hope of cure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S. David Nathanson
The ultimate goal of both basic and clinical scientists studying breast cancer (BC) metastases should be to cure the disease that kills about 44,000 women per year in the United States [1]. Until the latter part of the twentieth century, doctors could only stand by and watch the devastation of stage IV BC. There was little in their arsenal to treat these patients. Although the situation began to improve with more accurate, appropriate, and less drastic surgical and radiotherapeutic procedures, the truly major advances have evolved only as research uncovered disease mechanisms at the genetic, molecular, and cellular levels. A better understanding of an individual tumor’s molecular genetics guides decisions about prevention, diagnosis, and treatment of BC. By characterizing each BC by microscopic subtypes and their associated molecular fingerprints, we are better able to keep more patients from developing systemic metastases and to treat stage IV BC more effectively.
Breast surgery (lumpectomy or mastectomy plus axillary lymph node (LN) biopsy/dissection), often with adjuvant locoregional radiation and well-tolerated hormonal manipulation in estrogen receptor positive (ER+) patients cures at least three quarters of early BC patients [2]. The decision to use more toxic therapies, such as systemic chemotherapy, is dependent upon evolving demographic, pathologic, molecular, and genetic subtypes. The important combined information in every BC patient promotes a more precise classification that helps determine which BC patients are likely to remain metastasis-free without adjuvant chemotherapy and other systemic therapies. In the ideal situation, we would select only those patients likely to develop metastases to receive adjuvant, or neo-adjuvant, chemo- and targeted therapy. While there have been significant advances in this selection process, we are, unfortunately, not perfect yet.
What prompts BC cells to metastasize to sentinel lymph nodes (SLNs) and distant sites? This important question, addressed by scientists and clinicians in the past, has excited the interest of the authors of this paper. The more we know about the mechanisms of BC metastases, the more likely we are to find better ways to treat BC. Reynaud and Dieterich summarize and discuss the interaction of BC cells with lymphatic endothelial cells (LECs), molecular excitatory and inhibitory molecules, in the breast primary tumor leading to invasion of neighboring lymphatic vessels, and then LECs and parenchyma in SLNs to which they have metastasized. BC cells in SLNs may gain access to the systemic circulation via nodal blood vessels, finally colonizing organs primed to receive these wandering cells by the creation of a pre-metastatic niche (PMN). Wu and Zhang focus on bone, one of the more common sites of BC systemic metastases, explaining their important observations of molecular and cellular events at that site using a unique animal model and in vitro techniques. They also show how bone metastases may themselves metastasize to other organs. Chitale highlights the association of certain molecular characteristics with pathologic morphologic variants of BC, vital information for clinicians wishing to treat patients in the most appropriate ways. Ríos-Hoyo and Pusztai make the case that stage IV BC patients with synchronous oligometastases might justify more aggressive treatment than the average patient with recurrent metastases, expecting that they might be cured of the disease.
Multifaceted roles of the lymphatic system in BC metastasis
Emma Reynaud, Lothar C. Dieterich
Lymphatic remodeling in BC
The lymphatic vasculature is a unidirectional transport system essential for recycling interstitial fluids, provision of immunosurveillance and absorption of dietary lipids [3]. Lymphatic vessels are composed of a single layer of LECs with distinct characteristics depending on their localization along the lymphatic tree. LECs from peripheral capillary lymphatic vessels are connected by discontinuous junctions that allow immune cells and interstitial fluid to cross the endothelial layer into the lymphatic lumen. LECs from collecting lymphatic vessels form tight junctions and valves to efficiently transport the lymph to LNs and the bloodstream [3].
Lymphangiogenesis, the growth of new lymphatic vessels, either by new growth or expansion of pre-existing ones [3,4,5], generally does not occur in a healthy, full-grown organism, but can be induced in pathologic conditions, including cancer [4]. The most prominent drivers of this process, secreted by tumor cells, stromal and immune components of the tumor microenvironment (TME), are vascular endothelial growth factor-C (VEGF-C) and -D (VEGF-D) that bind to the receptor tyrosine kinase VEGF receptor-3 (VEGFR-3) on LECs. Multiple additional lymphangiogenesis-stimulating molecules have been identified, including angiopoietin 2, basic fibroblast growth factor, hepatocyte growth factor, adrenomedullin and sphingosine-1-phosphate (reviewed in [3]).
In contrast to many other solid cancer types, the extent of lymphangiogenesis and lymphatic expansion in BC has been controversial [6]. Several studies evaluating lymphatic vascular density and LEC proliferation using double staining for a lymphatic and a proliferation marker concluded that tumor lymphangiogenesis may be absent or rare in BC [7,8,9], whereas others found clear evidence of proliferating LECs in BC [10]. These divergent results probably reflect technical aspects (varying use of molecular markers and quantification methods) but also biological differences between BC subtypes and stages. For example, lymphatic vascular density tends to be elevated in inflammatory BC [11, 12], postpartum BC [13], human epidermal growth factor receptor 2 (HER2/neu+) and triple-negative BC [14, 15]. Lymphangiogenesis can be observed at distant metastatic sites as well, such as in the SLN and in the lung in a mouse model of triple-negative BC [16,17,18]. Lymphangiogenesis in experimental cancer models may even precede metastatic colonization in SLNs and systemic sites, indicating that an increased lymphatic vascular network is a constituent of the PMN and may promote metastatic growth and secondary metastases [16, 19,20,21,22]. While the precise signals that induce PMN lymphangiogenesis in BC are not known, growth factors such as VEGFs and midkine as well as tumor cell derived extracellular vesicles (EVs) have been identified as drivers of this process in various other cancer models [20, 22,23,24,25].
Lymphatic metastases in BC (see Fig 1 )
The first site of BC metastases is usually the SLNs in the axilla. While molecular and cellular changes in the primary site in the breast can be associated with SLN metastases, BC cells must invade lymphatics at the primary site to gain access to the SLNs. Lymphovascular invasion, visible as tumor cells in the lumens of lymphatic capillaries at the primary tumor site, is a crucial step within the metastatic cascade. Correspondingly, lymphovascular invasion is associated with an increase in SLN and systemic metastases in clinical studies of BC [26, 27] and with poor progression-free and overall survival (Table 1) [28,29,30,31,32,33,34]. BC cells may invade vessels as single cells or cell clusters, potentially reflecting distinct molecular and cellular invasion mechanisms (reviewed in [35]). For example, transforming growth factor-β-induced upregulation of CCR7 can direct invading BC cells into lymphatic vessels [36]. Inflammatory cytokines and growth factors released in the TME have been found to reduce junctional vascular endothelial-cadherin in lymphatic vessels in BC models [37, 38], thus making these vessels more permissive for cellular intravasation, whereas expression of the lipoxygenase ALOX15 in BC cells has been reported to induce lymphatic defects allowing invasion of entire cell clusters [39]. Of note, in a rat BC model, lymphatic vessels predominantly contained tumor cell clusters that had a high metastatic capacity [40].
While the extent of tumor-associated lymphangiogenesis in the various subtypes of BC is still not clear, its relevance for metastases has been demonstrated in cancer models where tumor cell overexpression of the lymphangiogenic growth factors VEGF-C and -D is sufficient to increase lymphatic metastases [37, 38, 41]. Clinical support in several (but not all) comprehensive meta-analyses representing thousands of BC cases (Table 1) observed tumor lymphatic vascular density and the expression of VEGF-C (and -D) to correlate with poor outcome in BC.
The presence of SLN metastases is valuable in BC staging [42, 43]. While the contribution of different routes to systemic BC metastases is still debated [44], animal, genetic, and human studies seem to favor an orderly anatomic route of LN metastases leading to systemic dissemination. Animal models have demonstrated that cancer cells in the LN can enter directly into the nodal blood circulation and subsequently seed to distant sites, such as the lung [43, 45]. Genetic analyses of human prostate and colorectal cancer metastases support a direct contribution of LN metastases-derived cell clones to systemic spread [42, 46,47,48]. Recently, similar findings have been reported in a small cohort of ER+ BC cases [49], whereas clinical studies suggest that many, if not most, primary BCs gain access to the visceral blood vasculature by way of SLNs [26, 27].
The lymphatic system as conduit and barrier for tumor derived EVs
Circulating, tumor derived EVs have been shown to induce PMN formation in various body sites and to regulate tumor immunity in melanoma and other cancer types as they interact with distant cells and tissues [50,51,52,53]. Tumor derived EVs may follow interstitial fluid flow away from blood vessels and towards lymphatic vessels where specific button-like LEC junctions at initial lymphatic vessels promote intravasation of particles 20–100 nm in diameter [3]. EVs derived from cultured cancer cells are rapidly taken up by lymphatic vessels upon interstitial injection in mice [50, 51]. Similarly, EVs released by primary tumors specifically homed to SLNs in mice [52] where they interacted with LN LECs and macrophages, mediated at least in part by integrins on the EV surface. The resulting PMN formation via induction of VEGF-C and nerve growth factor receptor increased lymphatic metastases [23, 25, 54]. On the other hand, if SLNs act as efficient barriers for EVs, they would likely limit their access to the blood circulation and, thus, metastasis-inducing functions in potential metastatic sites. Most of these studies were done using melanoma and colorectal carcinoma animal models. We do not yet know if tumor derived EVs similarly drain via the lymphatic system and act in SLNs in BC patients.
The lymphatic system in BC immunity
Besides its well-documented role in BC metastases, the lymphatic vascular system is highly relevant for the generation of endogenous and therapy-induced, cancer-specific immune responses. The somatic mutational burden of BC is moderate compared to other cancer types [55], which has been linked to a limited pool of neo-antigens, low immunogenicity, and immunotherapy responsiveness in BC. Nonetheless, tumor-infiltrating lymphocytes and immune-associated gene expression signatures have prognostic value in BC, particularly in triple-negative BC and BC with a high rate of proliferation [56, 57]. Recently, the US Food and Drug Administration approved pembrolizumab (a PD-1-blocking antibody) in combination with chemotherapy for locally recurrent, unresectable, or metastatic PD-L1 + triple-negative BC and for Stage II-III triple negative breast cancer as neoadjuvant (preoperative) therapy concurrent with chemotherapy regardless of PD-L1 status [54]. After neoadjuvant PD-1 blockade in BC [58], irrespective of the subtype, one-third of the patients exhibited expansion of T cell clones that could be detected within the TME even before treatment. Expanded CD8 + T cells showed expression of effector (granzyme B) and exhaustion markers (PD-1, LAG-3) [58]. These data indicate that tumor cells are subject to immune surveillance and that immune-inhibitory checkpoint pathways are active in BC.
LNs are essential secondary lymphoid organs that support and orchestrate interactions between antigen-presenting cells and lymphocytes, resulting in the generation (or inhibition) of adaptive immune responses. To achieve this goal, LNs are highly organized spatially, with distinct zones or structures for entry, activation and exit of T- and B-lymphocytes, various types of dendritic cells and macrophages, as well as stromal cells such as lymphatic and blood endothelial cells and fibroblastic reticular cells. All these cell types need to interact optimally to enable activation of adaptive immune responses. SLNs are one of the first sites receiving tumor antigens and immune cells from the TME, implying that they exert a key role in anti-tumor immunity [59]. Naïve, tumor-specific CD8 + T cells are indeed primed and activated in SLNs acquiring an activated, memory and stem-like phenotype (TCF1 + TOX- TTSM) [60,61,62]. These cells have the capacity to undergo massive proliferation, display true memory capacity and may turn into exhausted progenitor T cells (TCF1 + TOX + TPEX) within SLNs. Upon migration to the tumor, co-stimulatory signals finally drive these cells into effector T cells with a terminally exhausted phenotype (TCF1- TOX + TEX) [60, 62].
SLNs are not only sites where tumor-specific T cells are activated but also where active immune suppression takes place. PD-L1/PD-1 interactions occur frequently in SLNs and correlate with checkpoint blockade responsiveness in cancer patients [59]. Similarly, local checkpoint blockade therapy in SLNs enhanced the migration of tumor-specific T cells to the TME and was at least equally efficient in controlling tumor growth as systemic therapy in several cancer models, including BC [59,60,61, 63]. Moreover, lymphadenectomy impaired the efficiency of checkpoint blockade, demonstrating the importance of SLNs in tumor immunity by providing a reservoir of CD8 + TTSM cells that directly respond to PD-L1/PD-1 blocking therapies [60].
Multiple cell types within the SLN environment may provide inhibitory signals such as PD-L1 to tumor-specific T cells, and we currently do not know the relative contribution of individual cell types and the precise stage at which T cells are sensitive to those. For example, antigen-presenting cells are known to upregulate PD-L1 expression upon activation and may thereby inhibit T cells already during the priming phase [59]. Additionally, LN stromal cells, including LECs, have been implicated in the regulation of T cell responses [64]. LN LECs not only present peripheral tissue self-antigens but also scavenge and cross-present exogenous antigens from the lymph on MHC-I [65, 66]. In a melanoma mouse model, these included tumor-derived antigens, which can be transferred directly from primary tumor cells to LN LECs via tumor cell derived EVs [25, 67]. Antigen-presenting LECs induce a dysfunctional, apoptotic state in CD8 + T cells recognizing these antigens upon co-culture ex vivo, suggesting these cells are by default T cell-inhibitory and tolerogenic [65, 66]. However, it is not entirely clear how exactly LN LECs restrain T cell responses. In steady-state, LN LECs lack co-stimulatory molecules and constitutively express PD-L1, particularly in LECs populating the subcapsular sinus floor (fLECs) and medullary sinuses (mLECs) [68,69,70]. This expression pattern of PD-L1 is maintained in SLNs in melanoma and BC models in mice [19, 71, 72] and is conserved in humans [73]. In addition, deletion of lymphatic PD-L1 resulted in reduced apoptosis of tumor-specific CD8 + T cells with a memory phenotype in SLNs [74], a finding that is consistent with LN LECs acting as inhibitors of TTSM, most likely of those exiting the LN microenvironment via medullary sinuses and efferent lymphatic vessels. Further studies are needed to better understand the molecular interactions between LN LECs and tumor-specific T cells and their impact on tumor immunity and immunotherapy to precisely define the role of lymphatic PD-L1 and any other potential immune-inhibitory signals during the process of tumor-specific T cell activation.
Finally, LECs within the TME in tumor models have also been shown to inhibit T cell-mediated, tumor-specific immune responses. While peripheral LECs typically do not express PD-L1 at steady-state, its expression is induced within the TME by interferon-γ [71, 75]. Furthermore, tumor associated LECs may induce regulatory T cells [76] and mediate egress of effector T cells from the TME, all of which facilitate tumor immune evasion.
Conclusion
The lymphatic system exerts multifaceted functions that may both enhance and impair BC progression. Lymphatic vessels are conduits for regional and systemic BC metastases, but also for tumor cell derived EVs and for tumor antigens that are essential for the activation of TTSM cells in SLNs. At the same time, LN and TME-associated LECs appear to restrain tumor immunity through multiple direct and indirect mechanisms. Consequently, therapeutic targeting of specific, tumor progression-promoting functions of LECs could be synergistic with current targeted and immunotherapeutic approaches in BC and other cancer types.
Bone metastases: from disseminated Tumor cells to the source of further dissemination
Yi-Hsuan Wu, Xiang H.-F. Zhang
Introduction
Bone is one of the most common sites of cancer systemic metastases in several cancer types, including breast, prostate, lung, thyroid, and kidney cancer. The distinctive microenvironment within bone offers an ideal habitat for the proliferation of tumor cells. In the advanced stage of bone metastases, tumor growth is driven by a vicious cycle. These tumor cells secrete parathyroid hormone-related peptide and stimulate the expression of receptor activator of nuclear factor-kB ligand (RANKL) which leads to the proliferation of osteoclasts and bone resorption. This process also releases additional growth factors [77]. Moreover, osteoblasts also secrete pro-tumorigenic growth factors in osteoblastic lesions [78]. The reciprocal interaction between cancer and bone cells causes a positive-feedback cycle that facilitates tumor expansion. To date, treatments for bone metastases mainly focus on reducing osteoclast activity in the vicious cycle, such as with bisphosphonate [79] and denosumab [80]. Unfortunately, these therapeutic interventions often have limited efficacy in late stage metastases and only modest impact on reducing bone metastases in early stage disease with adjuvant therapy [81]. Consequently, there is an urgent need to create therapeutics that specifically target early micro-metastases and prevent the development of overt bone metastases.
A clinically appropriate bone metastasis model
Intra-cardiac and intra-tibial injections are the commonly used preclinical models for bone metastasis research [82, 83]; however, they both have limitations and drawbacks. The intra-cardiac injection model leads to tumor cells disseminating throughout various organs in the mouse, but only a restricted number of cancer cells reach the bone. On the other hand, the intra-tibial injection model can effectively deliver most cells to the bone, but it can result in localized damage and inflammation of bone tissue. Since our research primarily focuses on the early stage of bone metastases, these models do not adequately meet the conditions for a clinically valid model. Therefore, neither model is appropriate for our study. To mimic the early dissemination of bone metastases, we have developed an intra-iliac artery injection model, which specifically delivers tumor cells to the hind limb bones without causing harm to the local tissue. Using the bloodstream to transport the cells to bone, we can more accurately replicate the process by which disseminated tumor cells (DTCs) colonize bone [84]. Besides in vivo models, we have evolved an ex vivo bone-in-culture array platform, where we coculture bone pieces with tumor cells. This allows us to mimic the growth of tumor cells in the bone microenvironment (BME) to study the mechanisms of tumor cell colonization and conduct rapid drug screening [85, 86].
Dormancy, DTCs and NG2+ cells in BC metastases to bone
Metastases start from single cells or cell clusters that separate from the original tumor and migrate to distant areas. In most cases of BC, the dissemination of tumor cells to other tissues occurs early on, concomitant with the progression of the primary tumor [87, 88]. Despite this, it is possible for DTCs to remain inactive for many years or even decades before visible metastases emerge. The perivascular niche (PN) serves as a habitat for DTCs and controls the state of micro-metastases, whether dormant or active [89, 90] (Fig. 2a). We have demonstrated that the PN and osteogenic niche work together to trigger the activation of DTCs, leading to the development of osteolytic bone metastases [91]. Recent studies have shown that bone formation is coupled with angiogenesis, which occurs at the PN. Several cell types with distinct functional roles are involved in the PN, and these cells play important roles regulating hematopoiesis, osteogenesis, and vascular homeostasis [92]. Among these cell types, neuroglial antigen-2 positive (NG2+) bone mesenchymal stem/stromal cells (MSCs) have been found to be important participants in metastasis progression. It has been indicated that dormant DTCs colocalized with NG2+ cells in the PN [89, 93].
Schematic diagram for the BC bone colonization and micro-metastases. [a] Tumor cells remaining dormant in the perivascular niche. [b] NG2+ MSCs and tumor cells would be co-recruited to the pathologic fracture sites and initiate micro-metastases. [c] Tumor cells adhering to osteoblasts and migrate with them via a “migration-and-tethering” mechanism. [d] The interaction of hAJs triggering the proliferation of tumor cells in the bone by inducing mTOR and calcium influx. [e] The upregulation of EZH2 by hAJs, causing transient loss of ER and resistance to endocrine therapy, and [f] leading to an increase in the stemness and plasticity of the tumor cells, facilitating secondary metastases. Abbreviations: BC, breast cancer; ER, estrogen receptor; EZH2, enhancer of zeste homolog 2; hAJs, heterotypic adherens junctions; metastases, metastasis; MSCs, mTOR.
Our study confirmed that NG2+ cells participate in osteogenic differentiation and play an important role in bone remodeling, as supported by lineage-tracing experiments. Under the homeostatic condition, depletion of NG2+ cells resulted in a significant decrease in osteoblast and osteoclast activities and in the rate of new bone formation. On the other hand, NG2+ cells are recruited to the site of injury when there is a pathologic fracture, and they take part in the production of new bone. In short, the involvement of NG2+ cells is crucial in both maintaining homeostasis and wound healing in bone tissue. We provided evidence suggesting the existence of N-cadherin-mediated cell-cell direct contact between DTCs and NG2+ cells, which may facilitate the proliferation and movement of cancer cells toward osteogenic signals. NG2+ MSCs and DTCs could co-migrate from the PN to the osteogenic niche. Moreover, DTCs would be recruited to the pathologic fracture sites, leading to increased metastases colonization by the surrounding area of the injury (Fig. 2b). Upon depleting NG2+ cells or knocking out N-cadherin, we noticed that the metastatic colonization in bone was diminished. This study revealed how the outgrowth of DTCs occurs due to pathologic repair and elucidated the role of NG2+ MSCs in this process [91].
Co-colonization of molecules and cells in bone metastases
Several of our previous studies showed evidence to support that cancer cells might colocalize with MSCs. We observed that DTCs in BME would relocate between different niches via migration-by-tethering. The physical contact between cancer cells and osteogenic cells is facilitated by a dendritic spine-like structure of the cancer cells, mediating this distinct migration process [94] (Fig. 2c). We discovered an osteogenic niche, mediated by heterotypic adherens junctions (hAJs)-involving cancer-derived E-cadherin and osteogenic N-cadherin in the early stages of BC bone metastases. The hAJs activate the mTOR pathway in cancer cells, which leads to the progression of DTCs to micro-metastases (Fig. 2d). In addition, disrupting this hAJs showed inhibition on bone metastasis outgrowth [95]. Besides the mTOR pathway, calcium signaling cooperates to mediate the osteogenic niche’s metastasis promoting effects. Due to the low efficiency of calcium uptake from the BME by cancer cells, they rely on direct interaction with osteogenic cells to obtain a sufficient influx of calcium [96] (Fig. 2d). Based on these studies, we know the mechanisms of how the cancer cells travel from PN to osteogenic niches together with MSCs, and hAJs in the osteogenic niches promote micro-metastases by several pathways. Furthermore, we provide ideas for potential therapeutics for early metastases to prevent tumor progression to osteolytic metastases.
When DTCs arrive at the bone, the BME boosts their plasticity, leading to the reprogramming of ER+ breast tumor cells. Specifically, we found that the osteogenic niche drives the enhancer of zeste homolog 2 (EZH2)- mediated epigenetic reprogramming process, which changes the cancer cells to a stem-like and ER-dependent state. During this process, tumors that are ER+ would experience a temporary loss of ER expression, resulting in resistance to endocrine therapy (Fig. 2e). The partial restoration of ER expression in cancer cells was observed in bone-in-culture array when either gap junction or calcium signaling was suppressed. We observed the highest fold-change in the FGFR and PDGFR pathways when comparing bone-entrained to un-entrained cells and determined that these pathways are epigenetically regulated and are responsive to EZH2 inhibition. Treating tumors with an EZH2 inhibitor can restore endocrine sensitivity and even prevent spontaneous bone metastases [97]. Most of all, EZH2 inhibitors are currently available for clinical use, which could facilitate their application in future therapeutic approaches.
Secondary metastases from bone
The common perception is that metastases represent the final stage of tumor progression in clinics. However, our study challenges this notion by showing that certain BC metastases may originate from the metastasized tumor, which means those metastasized tumors can further produce secondary metastases [98] (Fig. 2f). This is consistent with clinical observations, which show that the bone is the most common site for the initial occurrence of metastases in BC and that more than two-thirds of these cases will end up with metastases to multiple different organ sites [99]. In this study, we used an evolving barcode system along with parabiosis to demonstrate that the BME can facilitate metastases-to-metastases seeding [98]. Based on another study showing that BME upregulates EZH2 expression in ER+ cancer cells and enhances their stem-like properties [97], we also observed ER− breast tumors exhibited similar upregulated stem-like properties as ER+ cancer cells. We hypothesized that EZH2 might be the key factor responsible for facilitating tumor cells’ secondary metastases in both ER+ and ER− tumors. We show that using EZH2 inhibitors or inducible knockdown could affect downstream expression of stem cell markers. It is important to note that inhibiting EZH2 expression would only reduce secondary metastases, without any effect on the primary bone lesions. Taken together, this suggests that bone may function as a launch site of secondary metastases rather than merely a destination in the metastatic cascade [98].
Potential targeting of early-stage BC metastases
The findings described above encouraged us to develop antibody-based therapeutics that specifically target early-stage BC bone metastases. However, systemic treatments face a significant obstacle in that only a small quantity of the antibody can reach the bone to target tumor cells. To address this limitation, we have conjugated the HER2/neu antibody, Herceptin, with bone-targeting molecules-Alendronate. Our research demonstrates that the bone-targeting Herceptin is more effective than the wildtype Herceptin antibody at treating bone metastases. Moreover, we observed a reduced incidence of secondary metastases [100]. This study provides a great therapeutic strategy. For example, generating antibody drug conjugates with a bone-targeting mechanism, targeting the crucial factors promoting metastases in the bone DTCs or micro-metastases. This might eliminate the bone metastases before entering the osteolytic stage. Bone-targeting treatments would enhance the site-specificity of the drugs, reducing the risk of adverse off-target effects. Furthermore, by enriching the concentration of antibody drug conjugates in the bone, we could potentially reduce the required dosage of the drug.
Future directions
Although some of the pathways and mechanisms of BC bone metastases are being defined, our understanding of this topic remains incomplete. To gain a better insight into bone metastases, we need to address some missing links. First, the presence of pre-metastatic effects in the BME of mammary tumors remains unclear. Studies support the concept that EVs released from primary BC transfer miR-21 to osteoclasts, creating an environment that facilitates bone metastasis progression [101]. Gaining a better understanding of the underlying molecular mechanisms of PMNs could potentially benefit BC patients in preventing bone metastases. Second, studies have shown that the niches harbor cancer cells with distinct cell fates, as the PN shelters dormant cells while the osteogenic niche contains tumor cells with a high proliferation rate [89, 95]. However, other studies suggest that osteoblasts in the osteogenic niche may be involved in cancer cell dormancy [102]. Therefore, the role of different niches might vary depending on the cancer types. Specifically, we should explore the distinct functions and connections between the perivascular and osteogenic niches across various cancers. Third, it is crucial to elucidate the progression from micro-metastases to osteolytic bone metastases. Currently, little is understood about the factors that trigger cancer cells to exit the dormant phase and initiate the vicious osteolytic cycle. Patients with BC frequently experience a delayed onset of bone metastases, which can occur even decades after the removal of the primary tumor [103]. Understanding the molecular mechanisms might create novel therapeutics targeting DTCs or micro-metastases. Last but not least, after uncovering the presence of secondary metastases, some questions still need to be answered [98]. For example, considering that BC metastasizes relatively early and remains dormant in the bone, the mechanisms that initiate secondary metastases and the timing of their onset both hold importance as clinical references for medication.
Anatomic and molecular characteristics Associated with BC Metastases
Dhananjay A. Chitale
Malignant tumor cells acquire the hallmarks of cancer from a premalignant transformed state to a widely metastatic disease through a multistep process [104]. These neoplasms are a mixture of heterogeneous cells that start from ‘clonal’ progeny of a single cell that acquires, or less commonly inherits, mutations, thus gaining survival advantage through natural selection.
Microscopic features, including nuclear size, shape, pleomorphism, mitosis, necrosis, infiltration, lymphatic, and vascular invasion, often predict tumor aggressiveness when correlated with clinical outcomes. When combining grade (tubule formation, nuclear pleomorphism, and mitotic counts) and more than 20 histologic subtypes, it is clear that BC is highly heterogeneous. Modern staging classifications require an accurate histologic diagnosis before any molecular classification can be of use in predicting metastases [105].
This section describes correlations of complex anatomic features of BC with multifaceted molecular underpinnings. Standard anatomic histopathologic features and hormone receptor status can be associated with cancer metastases in BC subtypes. We also describe the biological, biochemical, and molecular associations between tumor growth, as described under the framework of hallmarks of cancer by Hanahan and Weinberg [104, 106, 107], as they relate to BC metastases.
Anatomic pathologic characteristics of BC metastases
Clinical oncologists depend highly on synoptic pathologic checklists for invasive BC based on core biopsies and surgical specimens. Early-stage BC has 10-year survival rates of over 90%; selecting the minority who are at risk for recurrence should be the aim for more aggressive treatment and is a continued prognostic challenge. Approximately 7% of early BC patients have stage IV BC at presentation, and ~ 30% of women with early-stage BC at diagnosis eventually develop metastatic BC. With 5-year relative survival rates of ~ 25-30% of metastatic BC and a median overall survival of ~ 2–4 years depending on molecular subtype, patients at high risk for recurrence must be identified and treated early with the appropriate systemic and local therapies to avoid development of metastatic disease [105, 108].
Specific pathologic characteristics are typically associated with a higher likelihood of local recurrence and metastases to regional LNs and distant sites. These include larger tumor size, higher histologic grade, certain histological subtypes (such as inflammatory carcinoma -T4D), lymphovascular invasion, margin status, infiltration of local structures (such as chest wall-T4a, skin-T4b), and presence of extensive ductal carcinoma in-situ. Importantly, patients with favorable clinical pathologic features might safely avoid toxic therapies.
The metastatic potential of invasive BCs-no special type (IDC-NST), correlates with tumor grade and other microanatomic findings and includes the accurate diagnosis of cancer, histologic grade, and type. The histologic diagnosis of invasive BC involves a combination of architectural and cytological features of the tumor cells, stroma, TME, and lack of basal myoepithelial cells in the infiltrating epithelial cells (in most cases).
The Nottingham grading system determines tumor differentiation and includes the degree of tubule or gland formation, nuclear pleomorphism, and mitotic rate. Multivariate analyses showed that histologic grade independently predict BC-specific and disease-free survival in operable BCs [109].
Some histologic types of BC often independently predict a low likelihood of metastases. For example, pure low-grade adenosquamous carcinoma, fibromatosis-like metaplastic carcinoma, mucoepidermoid carcinoma, adenoid cystic carcinoma, and secretory carcinoma, despite all being triple negative (negative ER/PR/HER2/neu) are very indolent [110]. A special morphological pattern under IDC-NST included medullary/medullary-like carcinomas, glycogen-rich, lipid-rich, sebaceous, and oncocytic carcinomas, as their biological behavior correlated with the grade and stage of IDC-NST [111].
The patterns of metastases in invasive lobular carcinoma (ILC) are highly dependent upon histologic variations. The classic ILCs are typically grade 2, and their metastatic potential is like IDC-NST when matched with grade and stage. However, the pleomorphic ILC variant has high-grade cytological features and a poor prognosis. In contrast, the alveolar and tubulo-lobular variants of ILC have a good prognosis [112].
Molecular characteristics that predict BC metastases
Morphologic characteristics of BC in predicting locoregional and distant metastases have limitations, especially in the early-stage tumors where there is no evidence of local or distant spread of the tumor. It is often difficult to predict the biological potential of the malignant tumor based purely on histologic and radiologic parameters. Therefore, the knowledge of molecular programs of these tumors is fundamental to predicting the potential of cancer metastases and thus guiding therapies. The current transformation of the staging checklists from purely anatomic pathology-driven items to a combination of anatomic and molecular biomarkers (prognostic and predictive) introduced in the recent 8th AJCC staging classifications to further fine-tune tumor stages directed to targeted therapeutics, is a testament to that concept. These stratification approaches help separate more aggressive tumors within the same anatomic stage for optimal treatments and outcomes. BC AJCC staging [113, 114] now includes biomarker status such as ER, PR, HER2/neu expression status along with multigene signature panels such as the 21-gene Oncotype Dx Recurrence Score, 70-gene MammaPrint, the 12-gene EndoPredict, or the PAM50 test making its way into clinical decision-making.
Molecular classification of BCs
In a seminal article on molecular portraits of BC, Perou et al. [115] described 5 distinct intrinsic molecular subgroups using complementary DNA-based microarray messenger RNA expression profiling of BCs. After further study, the ER+ normal breast-like subgroup was removed, leaving 4. Luminal BC constituting 50-65% of cases, displays a pattern of gene expression dominated by genes that are regulated by estrogen. Morphologically, this group has the most variable distribution of histologic grades. Out of this group, luminal-B BC [~ 15-20%] have lower ER expression and higher expression of proliferation-associated genes and are associated with higher histologic grade and metastatic potential. HER2/neu+ tumors typically are of high histological grade, high proliferation rate, apocrine morphology in some, and infiltrative growth pattern. They are frequently associated with background high-grade comedo ductal carcinoma in-situ. Basal-like BC (~ 15%), named as such due to their gene expression profile related to basal myoepithelial cells, have a high mitotic rate, and often present as a large tumor with distinct, geographic, central cellular zones and large zones of necrosis. These tumors also have high tumor-infiltrating lymphocytes. Basal-like BCs express high molecular weight cytokeratins (CK), such as CK5 or CK5/6, CK14, CK17, and epidermal growth factor receptor, CKIT, P63, P-Cadherin, and SMA, as the profile characteristic of this tumor and are most often triple-negative [116]. Both HER2/neu and basal-like BC have higher rates of locoregional recurrence and distant metastases, with basal-like BC having the worst overall and disease-specific survival [111, 117].
Based on the expression profiles, many prognostic and predictive multigene classifier signature assays are offered commercially using technologies such as complementary DNA microarray, real-time polymerase chain reaction, immunohistochemistry, and DNA single nucleotide polymorphism array-based gene copy number. These typically stratify tumors into low- vs. high-risk groups predicting locoregional and distant recurrence; some are part of national guidelines and are used in clinical decision-making, especially in small-localized tumors.
Despite advances in understanding BC at the genomic level, gene signatures have not replaced the currently used prognostic and predictive factors in BC management. They provide valuable complementary information to traditional clinicopathologic parameters in the clinically intermediate risk group of patients, particularly those with ER+, HER2/neu−, early-stage BC. The prognostic value of these tests in ER− and HER2/neu+ BC remains limited [105].
Hallmarks of cancer metastases and histopathologic correlates
The past 4-plus decades of cancer research have given deep insights into the molecular characteristics and underpinnings that drive tumor biology and the propensity toward metastases. This large body of knowledge further underscores the heterogeneity and complexity of BCs, characterized as a disease driven by dynamic changes in the genome through a sequential acquisition of large numerical or structural changes to the chromosomal complement or subtle point mutations in oncogenes leading to gain of function and tumor suppressor genes with loss of function [118].
Malignant breast tumors are mixtures of different types of cells, including cancer, immune, stem, and supporting stromal cells, blood and lymphatic vessels, and the stromal matrix, all working together as an autonomous organ. Defined as the ‘Hallmarks of Cancer,‘ Hanahan and Weinberg [104, 106] described ten biological components that cancer cells and the TME acquire and maintain during growth and metastases. The key hallmarks include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, reprogramming of energy metabolism, and evading immune destruction. The enabling characteristics included genomic instability and mutation, tumor-promoting inflammation, deregulating cellular metabolism, and avoiding immune destruction. Unlocking phenotypic plasticity and senescence were added to the hallmarks of cancer, and non-mutational epigenetic reprogramming and polymorphic microbiomes as enabling characteristics were recently added to the list [107].
The following paragraphs summarize how these hallmarks correlate with morphologic features of BC and cancer invasion, metastases, phenotypic plasticity, and disrupted differentiation.
Invasion and metastasis
One of the most aggressive properties of cancer cells is the invasion and destruction of local tissues and the development of metastases. Stephen Paget’s “seed and soil” hypothesis [119, 120] identified patterns of BC metastases, suggesting a propensity for cancer to spread only to host organs (the ‘soil’) supportive of their growth. More than a century later, extensive research has upheld this concept with the “seed” now identified as the cancer stem cell [121]. This hallmark of cancer occurs through a complex progression, the invasion-metastasis cascade.
The major steps in this process include invasion of the extracellular matrix, angiolymphatic dissemination, tissue homing and extravasation at a metastatic site, and colonization. Noninvasive BC cells first breach the supporting basement membrane and infiltrate surrounding connective tissues. In many high-grade ductal carcinoma in-situ, microinvasion is noted as an outpouching or budding of the tumor cells or single cells, often cuffed by a dense desmoplastic stroma and inflammatory cells (Fig. 3).
Invasive BC cells move through connective tissues and access lymphovascular spaces. This is facilitated by the loss of adhesion molecule E-cadherin encoded by the CDH1 gene through epithelial-mesenchymal transition (EMT), similar to that observed in ILC. EMT, considered an integral part of metastases, especially in BCs, is controlled by a combination of Snail, Slug, Twist, and Zeb1/2 transcription factors, favoring promigratory properties. In the first step, the invading tumor cells become dis-cohesive (“loosening up” of tumor cells) as morphologically evidenced by single cell infiltration or nests of tumor cells at the leading edge of the invasive BC (Fig. 4). This is followed by degradation of the extracellular matrix, attachment to “remodeled” extracellular matrix components, and migration facilitating the invasion of tumor cells. The basement membrane and interstitial connective tissue degradation is achieved by autocrine or paracrine secretions, proteolytic enzymes such as matrix metalloproteinase, cathepsin D, and urokinase plasminogen activator. The final step of invasion is the mechanical migration of cancer cells by locomotion through basement membranes and areas of proteolysis by the contractile actin cytoskeleton.
Once in the bloodstream, most circulating tumor cells die but surviving cells home to and colonize specific sites, such as bone or internal viscera (most commonly lung, liver, or brain), showing organ tropism. The invasion process at the primary site in the breast is reversed in the end organ. The underlying molecular mechanism is thought to be through the expression of adhesion or chemokine receptors, whose ligands are expressed by endothelial cells at the metastatic site [122].
Tumor phenotypic plasticity and disrupted differentiation [107]: During primary site invasion and metastases and at the metastatic sites, BCs change their molecular programs and transform dynamically, adapting to fluctuating environmental conditions and therapeutic pressures. Tumor plasticity plays a critical role in tumor progression, metastases, and therapeutic resistance and is driven by a complex interplay between genetic, epigenetic, and environmental factors within the BC cell and TME.
Plasticity is thought to be achieved through genetic heterogeneity, clonal evolution, EMT, and cancer stem cells [123]. A tumor may consist of multiple distinct clones, each with genetic alterations that can confer selective advantages in response to environmental pressures. During clonal evolution, genetic alterations are selectively acquired through stepwise acquisition, leading to aggressive subclones. During the process of EMT, invasive BC cells change morphology to spindled mesenchymal phenotype. These de-differentiated cells gain more migration and invasion capability, typically at the leading edge of the tumor, while at the center of the tumor mass, the cells re-differentiate, become smaller, and often go into senescence mode. This might help them to escape from host immunity or therapeutic pressures. Thus, BC cells are not fixed entities but a dynamic population of cells that can undergo genetic and epigenetic changes in response to TME signals such as hypoxia, nutrient deprivation, and immune surveillance. Cancer stem cells are a subpopulation of cancer cells possessing stem cell-like properties, including self-renewal and differentiation capacity, and may be responsible for tumor initiation, maintenance, and recurrence.
There still is much to know and learn about the tumor progression biology and plasticity that molds the tumor at different time points. TME plays a significant role in tumor progression in BC. I leave you with an example to support the above statement and ponder. A well-differentiated invasive tubular carcinoma of the breast has the best long-term prognosis [124] while a well-differentiated pancreatic adenocarcinoma with tumor cells forming well-formed tubules/ducts and “similar” dense desmoplastic stroma has dismal outcomes.
Can we cure Stage IV BC?
Alejandro Ríos-Hoyo, Lajos Pusztai
Clinical presentations of metastatic BC and its molecular underpinnings
Metastatic BC can present either as recurrence of a stage I-III disease, or as de novo stage IV BC with distant metastases at the time of diagnosis. Recurrent metastatic disease that becomes apparent years after the initial diagnosis arises from clinically undetectable micro-metastases that were already present at the time of diagnosis and have survived the systemic therapies (such as chemotherapy, immunotherapy, and targeted therapy) that a patient received as part of her initial treatment. A fundamental clinical difference between recurrent metastatic cancers and de novo stage IV disease is exposure to prior therapies. Recurrent metastatic cancers are composed of cells that survived or descended from survivors of prior treatments, whereas de novo stage IV cancers are treatment naïve. Systemic therapies administered before or after surgery for stage I-III cancers have become increasingly more effective at eradicating micro-metastatic disease. Consequently, recurrence rates for stage I-III BCs are steadily decreasing and the proportion of de novo stage IV cancers among metastatic cancers is increasing. Historically, about 20% of newly diagnosed metastatic BCs were de novo stage IV disease, and 80% were recurrences. The contemporary ratio is closer to 40–60% [125].
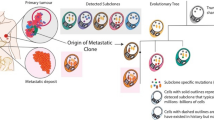
Molecular analysis of metastatic lesions reveals clinically important observations. Data from phylogenetic analysis of DNA sequences of paired primary tumors and metastases reveals 3 distinct paths to metastases formation [126,127,128]. Metastatic dissemination may occur early from a common ancestor of both the primary tumor and metastatic lesions which may explain why some small primary tumors already have micro-metastases at diagnosis. Metastases may also arise from a small subpopulation of cells within the primary breast tumor, which might explain why tumors left untreated for months to years will eventually metastasize. Thirdly, metastatic lesions can also give rise to new metastases, which contributes to the relentless spread of metastatic disease to new organ sites. All 3 types of dissemination can be observed in the same patient. Molecular analysis of BC tissues obtained at different time points during the course of the disease show that genomic features of cancer change over time. Metastatic lesions acquire new genomic alterations spontaneously or due to selective pressure from therapies (Table 2) [126,127,128,129,130,131,132,133,134,135]. Comparison of primary tumors and metastatic lesions in the same patient reveal that synchronous metastases, as in stage IV disease or regional LN metastases at the time of diagnosis, have more genomic similarities to the primary tumors, presumably due to shorter divergence time, than asynchronous metastases that arise after years of dormancy as in recurrent metastatic disease [126,127,128, 130, 136]. This observation suggests that synchronous metastases may have similar treatment sensitivities to the primary tumor. This hypothesis is consistent with clinical observations. When stage II-III BC patients with synchronous LN metastases receive preoperative chemotherapy, the response rates in the breast and LNs are similar. High rates of complete pathologic response (CPR) of the primary BC are accompanied by similarly high rates of CPR from biopsy proven positive LNs [137, 138].
Oligometastatic cancer
The extent of metastatic disease at the time of diagnosis can vary from a single or few metastatic lesions to extensive metastatic disease with many lesions in multiple organs. Oligometastasis refers to the subset of metastatic cancers (either recurrent or de novo stage IV) as low volume metastatic disease involving up to 5 lesions in different organs [139]. Approximately 40-60% of newly diagnosed metastatic BCs are oligometastatic [140]. Multiple studies in many different cancer types show patients with oligometastatic disease have longer survival than patients who present with extensive metastatic disease [140]. This is likely due to a combination of more indolent biology (i.e., slower growth rate or maybe more limited metastatic ability), lead time bias (i.e., being diagnosed at an earlier time point during the course of the disease), and different treatment approaches towards oligometastatic cancers. It is often possible to radiate or surgically resect all metastatic lesions in oligometastatic disease. One could hypothesize that surgical resection and radio-ablation of all detectable cancer will reduce the rate of metastases-to-metastases spread. Eliminating billions of cells also reduces cancer cell heterogeneity that could delay or even prevent development of drug resistance. Because of these considerations, some oligometastatic cancers are treated with multi-modality therapy involving surgery, radiation, and systemic chemotherapy, endocrine therapy, and immunotherapy, whereas non-oligometastatic cancers usually receive systemic therapies only. Several small nonrandomized clinical studies and retrospective database analyses document 20-year disease free survival in up to 26% of patients who received systemic therapy combined with local treatment that eliminated all macroscopic metastatic lesions [141,142,143,144]. Unfortunately, results from non-randomized studies and retrospective database analyses are susceptible to selection bias. Patients selected for more aggressive multimodality therapy are likely to be younger, healthier, and have overall better prognosis to start with and therefore, these studies cannot prove that the multimodality therapy made patients live longer. Randomized clinical trials yield conflicting results regarding the importance of ablative therapy to metastatic sites in addition to standard of care systemic therapies. The SABR-COMET trial accrued both recurrent and de novo stage IV metastatic disease of various cancer types (including 18 BC patients) and showed improved 5-year overall survival in the arm that received radio-ablation to all known metastatic sites plus standard of care systemic therapy compared to systemic therapy alone [145]. In contrast, another randomized clinical trial, NRG-BR002, that included recurrent or de novo stage IV oligometastatic BC patients showed no improvement in survival in the radio-ablation arm [146]. An important limitation of this trial is that 80% of patients had recurrent metastatic disease. It is uncertain to what extent these results can be extrapolated to de novo stage IV disease.
De novo, stage IV oligometastatic BCs, a unique subset of metastatic cancers
With current treatment strategies, both recurrent and de novo stage IV BCs remain incurable and almost all patients succumb to their disease. This is in sharp contrast with our ability to eradicate micro-metastatic disease that is present in many stage II-III BCs with multi-drug systemic adjuvant therapies leading to cure for the majority of these patients. Oligometastatic de novo stage IV BCs are a unique subset of metastatic cancers that share several important features with stage II-III cancers. These include lack of exposure to prior systemic therapies, technical feasibility of resecting or ablating all detectable cancer, and genomic similarities between the primary tumor and synchronous metastases in regional LNs or at distant organ sites. This raises the question if the difference in outcome, high rates of cure in one, no-cure in the other, are driven by differences in the treatment approach. Despite the unique clinical and molecular features of de novo stage IV oligometastatic BCs, current United States oncology practice guidelines do not make specific treatment recommendations for this disease subset [147]. Several clinical trials evaluated whether surgical excision of the primary BC in stage IV patients improves survival or not; these trials included both oligometastatic and non-oligometastatic cancers and radio-ablation of distant metastatic lesions was not required. Systemic therapies also varied and were given with palliative intent (i.e., single agent therapies rather than multi-drug treatment regimens that are administered with curative intent in stage I-III BCs). The majority of these trials demonstrated no benefit from resection of the primary BC [148,149,150], which dampened enthusiasm for aggressive multimodality therapy of de novo stage IV BCs. Unfortunately, no trial addressed whether complete eradication of all detectable disease (i.e., primary tumor and distant metastatic sites), coupled with the most aggressive molecular subtype-appropriate multidrug systemic therapy, identical to that deployed against stage III BC, could improve survival in de novo stage IV oligometastatic BC. How to optimally treat de novo stage IV cancers is an increasingly relevant clinical question due to the growing absolute number and proportion of these patients among metastatic cancers. New clinical trials are being planned for this unique patient population to test if the same treatment strategy that reduced recurrence rates and improved survival in locally advanced stage III BC might also cure at least some oligometastatic de novo stage IV BCs. Until 2003, supraclavicular LN involvement at presentation was considered stage IV metastatic disease by the clinical staging system and these patients were considered incurable and often received systemic therapies only with palliative intent. However, clinical data indicate that these groups of patients when treated with combined modality therapy had long-term survival similar to stage III BCs [149, 150], eventually leading to reclassification to stage IIIC disease, and today they all receive multimodality therapy with curative intent. Might we someday consider de novo oligometastatic BC stage IIID disease treating aggressively with intent to cure?
References
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A et al (2022) Breast cancer statistics, 2022. CA Cancer J Clin 72:524–541. https://doi.org/10.3322/caac.21754
Leonard-Murali S, Nathanson SD, Springer K, Baker P, Susick L (2023) Early breast cancer survival of black and white american women with equal diagnostic and therapeutic management. Eur J Surg Oncol 49:583–588. https://doi.org/10.1016/j.ejso.2022.11.101
Dieterich LC, Tacconi C, Ducoli L, Detmar M (2022) Lymphatic vessels in cancer. Physiol Rev 102:1837–1879. https://doi.org/10.1152/physrev.00039.2021
Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14:159–172. https://doi.org/10.1038/nrc3677
Dieterich LC, Detmar M (2016) Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev 99:148–160. https://doi.org/10.1016/j.addr.2015.12.011
Chen JM, Luo B, Ma R, Luo XX, Chen YS, Li Y (2021) Lymphatic endothelial markers and tumor lymphangiogenesis assessment in human breast cancer. Diagnostics (Basel) 12:4. https://doi.org/10.3390/diagnostics12010004
Agarwal B, Saxena R, Morimiya A, Mehrotra S, Badve S (2005) Lymphangiogenesis does not occur in breast cancer. Am J Surg Pathol 29:1449–1455. https://doi.org/10.1097/01.pas.0000174269.99459.9d
van der Schaft DW, Pauwels P, Hulsmans S, Zimmermann M, van de Poll-Franse LV, Griffioen AW (2007) Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer Lett 254:128–136. https://doi.org/10.1016/j.canlet.2007.03.001
Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG (2003) Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol 200:195–206. https://doi.org/10.1002/path.1343
Van der Auwera I, Colpaert C, Van Marck E, Vermeulen P, Dirix L (2006) Lymphangiogenesis in breast cancer. Am J Surg Pathol 30:1055–1056 author reply 1056–1057. https://doi.org/10.1097/00000478-200608000-00021
Van der Auwera I, Van den Eynden GG, Colpaert CG, Van Laere SJ, van Dam P, Van Marck EA, Dirix LY et al (2005) Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res 11:7637–7642. https://doi.org/10.1158/1078-0432.CCR-05-1142
Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB et al (2004) Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res 10:7965–7971. https://doi.org/10.1158/1078-0432.CCR-04-0063
Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, Jindal S et al (2014) Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest 124:3901–3912. https://doi.org/10.1172/JCI73777
Niemiec J, Adamczyk A, Ambicka A, Mucha-Malecka A, Wysocki W, Mitus J, Rys J (2012) Lymphangiogenesis assessed using three methods is related to tumour grade, breast cancer subtype and expression of basal marker. Pol J Pathol 63:165–171. https://doi.org/10.5114/pjp.2012.31500
Niemiec JA, Adamczyk A, Ambicka A, Mucha-Malecka A, W MW, Rys J (2014) Triple-negative, basal marker-expressing, and high-grade breast carcinomas are characterized by high lymphatic vessel density and the expression of podoplanin in stromal fibroblasts. Appl Immunohistochem Mol Morphol 22:10–16. https://doi.org/10.1097/PAI.0b013e318286030d
Ma Q, Dieterich LC, Ikenberg K, Bachmann SB, Mangana J, Proulx ST, Amann VC et al (2018) Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci Adv 4:eaat4758. https://doi.org/10.1126/sciadv.aat4758
Van den Eynden GG, Van der Auwera I, Van Laere SJ, Huygelen V, Colpaert CG, van Dam P, Dirix LY et al (2006) Induction of lymphangiogenesis in and around axillary lymph node metastases of patients with breast cancer. Br J Cancer 95:1362–1366. https://doi.org/10.1038/sj.bjc.6603443
Westhoff CC, Muller SK, Jank P, Kalder M, Moll R (2023) Nodal lymphangiogenesis and immunophenotypic variations of sinus endothelium in sentinel and non-sentinel lymph nodes of invasive breast carcinoma. PLoS ONE 18:e0280936. https://doi.org/10.1371/journal.pone.0280936
Commerford CD, Dieterich LC, He Y, Hell T, Montoya-Zegarra JA, Noerrelykke SF, Russo E et al (2018) Mechanisms of tumor-induced lymphovascular niche formation in draining lymph nodes. Cell Rep 25:3554–3563e3554. https://doi.org/10.1016/j.celrep.2018.12.002
Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M (2007) VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109:1010–1017. https://doi.org/10.1182/blood-2006-05-021758
Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS (2014) Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun 5:4715. https://doi.org/10.1038/ncomms5715
Olmeda D, Cerezo-Wallis D, Riveiro-Falkenbach E, Pennacchi PC, Contreras-Alcalde M, Ibarz N, Cifdaloz M et al (2017) Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 546:676–680. https://doi.org/10.1038/nature22977
Garcia-Silva S, Benito-Martin A, Nogues L, Hernandez-Barranco A, Mazariegos MS, Santos V, Hergueta-Redondo M et al (2021) Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat Cancer 2:1387–1405. https://doi.org/10.1038/s43018-021-00272-y
Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M (2005) VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 201:1089–1099. https://doi.org/10.1084/jem.20041896
Leary N, Walser S, He Y, Cousin N, Pereira P, Gallo A, Collado-Diaz V et al (2022) Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. J Extracell Vesicles 11:e12197. https://doi.org/10.1002/jev2.12197
Nathanson SD, Kwon D, Kapke A, Alford SH, Chitale D (2009) The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann Surg Oncol 16:3396–3405. https://doi.org/10.1245/s10434-009-0659-2
Nathanson S D, Leonard-Murali S, Burmeister C, Susick L, Baker P (2020) Clinicopathological evaluation of the potential anatomic pathways of systemic metastasis from primary breast cancer suggests an orderly spread through the regional lymph nodes. Ann Surg Oncol 27:4810–4818. https://doi.org/10.1245/s10434-020-08904-w
Zhang S, Zhang D, Gong M, Wen L, Liao C, Zou L (2017) High lymphatic vessel density and presence of lymphovascular invasion both predict poor prognosis in breast cancer. BMC Cancer 17:335. https://doi.org/10.1186/s12885-017-3338-x
Chen Y, Yan J, Yuan Z, Yu S, Yang C, Wang Z, Zheng Q (2013) A meta-analysis of the relationship between lymphatic microvessel density and clinicopathological parameters in breast cancer. Bull Cancer 100:1–10. https://doi.org/10.1684/bdc.2013.1719
Wang J, Guo Y, Wang B, Bi J, Li K, Liang X, Chu H et al (2012) Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: a systematic review and meta-analysis of the literature. Mol Biol Rep 39:11153–11165. https://doi.org/10.1007/s11033-012-2024-y
Liang B, Li Y (2014) Prognostic significance of VEGF-C expression in patients with breast cancer: a meta-analysis. Iran J Public Health 43:128–135
Zhang Z, Luo G, Tang H, Cheng C, Wang P (2016) Prognostic significance of high VEGF-C expression for patients with breast cancer: an update meta analysis. PLoS ONE 11:e0165725. https://doi.org/10.1371/journal.pone.0165725
Wang F, Li S, Zhao Y, Yang K, Chen M, Niu H, Yang J et al (2016) Predictive role of the overexpression for CXCR4, C-Met, and VEGF-C among breast cancer patients: a meta-analysis. Breast 28:45–53. https://doi.org/10.1016/j.breast.2016.04.016
Gao S, Ma JJ, Lu C (2014) Prognostic significance of VEGF-C immunohistochemical expression in breast cancer: a meta-analysis. Tumour Biol 35:1523–1529. https://doi.org/10.1007/s13277-013-1211-3
Fujimoto N, Dieterich LC (2021) Mechanisms and clinical significance of tumor lymphatic invasion. Cells 10:2585. https://doi.org/10.3390/cells10102585
Mohammed SI, Torres-Luquis O, Walls E, Lloyd F (2019) Lymph-circulating tumor cells show distinct properties to blood-circulating tumor cells and are efficient metastatic precursors. Mol Oncol 13:1400–1418. https://doi.org/10.1002/1878-0261.12494
Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S et al (2001) Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 20:672–682. https://doi.org/10.1093/emboj/20.4.672
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L et al (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7:192–198. https://doi.org/10.1038/84643
Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G et al (2011) Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest 121:2000–2012. https://doi.org/10.1172/JCI44751
Teichgraeber DC, Guirguis MS, Whitman GJ (2021) Breast cancer staging: updates in the AJCC Cancer Staging Manual, 8th Edition, and current challenges for radiologists, from the AJR Special Series on Cancer Staging. AJR Am J Roentgenol 217:278–290. https://doi.org/10.2214/ajr.20.25223
Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG et al (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 7:186–191. https://doi.org/10.1038/84635
Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS et al (2015) The evolutionary history of lethal metastatic prostate cancer. Nature 520:353–357. https://doi.org/10.1038/nature14347
Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin SM et al (2018) Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359:1403–1407. https://doi.org/10.1126/science.aal3622
Nathanson SD, Detmar M, Padera TP, Yates LR, Welch DR, Beadnell TC, Scheid AD et al (2022) Mechanisms of breast cancer metastasis. Clin Exp Metastasis 39:117–137. https://doi.org/10.1007/s10585-021-10090-2
Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago-Horvath Z et al (2018) Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359:1408–1411. https://doi.org/10.1126/science.aal3662
Hong MK, Macintyre G, Wedge DC, Van Loo P, Patel K, Lunke S, Alexandrov LB et al (2015) Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun 6:6605. https://doi.org/10.1038/ncomms7605
Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T et al (2017) Origins of lymphatic and distant metastases in human colorectal cancer. Science 357:55–60. https://doi.org/10.1126/science.aai8515
Zhang C, Zhang L, Xu T, Xue R, Yu L, Zhu Y, Wu Y et al (2020) Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat Commun 11:1993. https://doi.org/10.1038/s41467-020-15886-6
Pretti MAM, Bernardes SS, da Cruz JGV, Boroni M, Possik PA (2020) Extracellular vesicle-mediated crosstalk between melanoma and the immune system: impact on tumor progression and therapy response. J Leukoc Biol 108:1101–1115. https://doi.org/10.1002/jlb.3mr0320-644r
Hood JL, San RS, Wickline SA (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 71:3792–3801. https://doi.org/10.1158/0008-5472.CAN-10-4455
Srinivasan S, Vannberg FO, Dixon JB (2016) Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep 6:24436. https://doi.org/10.1038/srep24436
Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D et al (2016) SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352:242–246. https://doi.org/10.1126/science.aaf1328
Sun B, Zhou Y, Fang Y, Li Z, Gu X, Xiang J (2019) Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int J Cancer 145:1648–1659. https://doi.org/10.1002/ijc.32196
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396:1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL et al (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499:214–218. https://doi.org/10.1038/nature12213
Miller LD, Chou JA, Black MA, Print C, Chifman J, Alistar A, Putti T et al (2016) Immunogenic subtypes of breast cancer delineated by gene classifiers of immune responsiveness. Cancer Immunol Res 4:600–610. https://doi.org/10.1158/2326-6066.CIR-15-0149
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Bassez A, Vos H, Van Dyck L, Floris G, Arijs I, Desmedt C, Boeckx B et al (2021) A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med 27:820–832. https://doi.org/10.1038/s41591-021-01323-8
Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, van Nimwegen M et al (2020) The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell 38:685–700e688. https://doi.org/10.1016/j.ccell.2020.09.001
Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu Y, Xiong D et al (2022) The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell 185:4049–4066e4025. https://doi.org/10.1016/j.cell.2022.09.020
O’Melia MJ, Manspeaker MP, Thomas SN (2021) Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer Immunol Immunother 70:2179–2195. https://doi.org/10.1007/s00262-020-02792-5
Prokhnevska N, Cardenas MA, Valanparambil RM, Sobierajska E, Barwick BG, Jansen C, Reyes Moon A et al (2023) CD8(+) T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56:107–124e105. https://doi.org/10.1016/j.immuni.2022.12.002
Francis DM, Manspeaker MP, Schudel A, Sestito LF, O’Melia MJ, Kissick HT, Pollack BP et al (2020) Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci Transl Med 12:eaay3575. https://doi.org/10.1126/scitranslmed.aay3575
Jalkanen S, Salmi M (2020) Lymphatic endothelial cells of the lymph node. Nat Rev Immunol 20:566–578. https://doi.org/10.1038/s41577-020-0281-x
Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG et al (2010) Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med 207:681–688. https://doi.org/10.1084/jem.20092465
Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F et al (2014) Steady-state antigen scavenging, cross-presentation, and CD8 + T cell priming: a new role for lymphatic endothelial cells. J Immunol 192:5002–5011. https://doi.org/10.4049/jimmunol.1302492
Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A et al (2012) VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 1:191–199. https://doi.org/10.1016/j.celrep.2012.01.005
Cohen JN, Tewalt EF, Rouhani SJ, Buonomo EL, Bruce AN, Xu X, Bekiranov S et al (2014) Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PLoS ONE 9:e87740. https://doi.org/10.1371/journal.pone.0087740
Fujimoto N, He Y, D’Addio M, Tacconi C, Detmar M, Dieterich LC (2020) Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes. PLoS Biol 18:e3000704. https://doi.org/10.1371/journal.pbio.3000704
Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR et al (2012) Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120:4772–4782. https://doi.org/10.1182/blood-2012-04-427013
Dieterich LC, Ikenberg K, Cetintas T, Kapaklikaya K, Hutmacher C, Detmar M (2017) Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-cell activation. Front Immunol 8:66. https://doi.org/10.3389/fimmu.2017.00066
Sibler E, He Y, Ducoli L, Rihs V, Sidler P, Puig-Moreno C, Frey J et al (2022) Immunomodulatory responses of subcapsular sinus floor lymphatic endothelial cells in tumor-draining lymph nodes. Cancers (Basel) 14:3602. https://doi.org/10.3390/cancers14153602
Takeda A, Hollmen M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lonnberg T et al (2019) Single-cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity 51:561–572e565. https://doi.org/10.1016/j.immuni.2019.06.027
Cousin N, Cap S, Dihr M, Tacconi C, Detmar M, Dieterich LC (2021) Lymphatic PD-L1 expression restricts tumor-specific CD8 + T cell responses. Cancer Res 81:4133–4144. https://doi.org/10.1158/0008-5472.CAN-21-0633
Lane RS, Femel J, Breazeale AP, Loo CP, Thibault G, Kaempf A, Mori M et al (2018) IFNgamma-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J Exp Med 215:3057–3074. https://doi.org/10.1084/jem.20180654
Gkountidi AO, Garnier L, Dubrot J, Angelillo J, Harle G, Brighouse D, Wrobel LJ et al (2021) MHC class II antigen presentation by lymphatic endothelial cells in tumors promotes intratumoral regulatory T cell-suppressive functions. Cancer Immunol Res 9:748–764. https://doi.org/10.1158/2326-6066.CIR-20-0784
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664. https://doi.org/10.1056/NEJMra030831
Ell B, Kang Y (2012) SnapShot: bone metastasis. Cell 151:690–690e691. https://doi.org/10.1016/j.cell.2012.10.005
Yoneda T, Sasaki A, Dunstan C, Williams PJ, Bauss F, De Clerck YA, Mundy GR (1997) Inhibition of osteolytic bone metastasis of breast cancer by combined treatment with the bisphosphonate ibandronate and tissue inhibitor of the matrix metalloproteinase-2. J Clin Invest 99:2509–2517. https://doi.org/10.1172/JCI119435
Suzman DL, Boikos SA, Carducci MA (2014) Bone-targeting agents in prostate cancer. Cancer Metastasis Rev 33:619–628. https://doi.org/10.1007/s10555-013-9480-2
Satcher RL, Zhang XH (2022) Evolving cancer-niche interactions and therapeutic targets during bone metastasis. Nat Rev Cancer 22:85–101. https://doi.org/10.1038/s41568-021-00406-5
Campbell JP, Merkel AR, Masood-Campbell SK, Elefteriou F, Sterling JA (2012) Models of bone metastasis. J Vis Exp:e4260. https://doi.org/10.3791/4260
Corey E, Quinn JE, Bladou F, Brown LG, Roudier MP, Brown JM, Buhler KR et al (2002) Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. Prostate 52:20–33. https://doi.org/10.1002/pros.10091
Yu C, Wang H, Muscarella A, Goldstein A, Zeng HC, Bae Y, Lee BH et al (2016) Intra-iliac artery injection for efficient and selective modeling of microscopic bone metastasis. J Vis Exp e53982. https://doi.org/10.3791/53982
Wang H, Tian L, Goldstein A, Liu J, Lo HC, Sheng K, Welte T et al (2017) Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat Commun 8:15045. https://doi.org/10.1038/ncomms15045
Wu YH, Gugala Z, Barry MM, Shen Y, Dasgupta S, Wang H (2022) Optimization and characterization of a bone culture model to study prostate cancer bone metastasis. Mol Cancer Ther 21:1360–1368. https://doi.org/10.1158/1535-7163.MCT-21-0684
Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK et al (2016) Early dissemination seeds metastasis in breast cancer. Nature 540:552–558. https://doi.org/10.1038/nature20785
Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH et al (2006) Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12:5615–5621. https://doi.org/10.1158/1078-0432.CCR-06-0169
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D et al (2013) The PMN regulates breast tumour dormancy. Nat Cell Biol 15:807–817. https://doi.org/10.1038/ncb2767
Ghajar CM (2015) Metastasis prevention by targeting the dormant niche. Nat Rev Cancer 15:238–247. https://doi.org/10.1038/nrc3910
Zhang W, Xu Z, Hao X, He T, Li J, Shen Y, Liu K et al (2023) Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2 + cells. Cancer Discov 13:474–495. https://doi.org/10.1158/2159-8290.CD-22-0220
Sivaraj KK, Adams RH (2016) Blood vessel formation and function in bone. Development 143:2706–2715. https://doi.org/10.1242/dev.136861
Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, Olivere L et al (2016) Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med 8:340ra373. https://doi.org/10.1126/scitranslmed.aad4059
Muscarella AM, Dai W, Mitchell PG, Zhang W, Wang H, Jia L, Stossi F et al (2020) Unique cellular protrusions mediate breast cancer cell migration by tethering to osteogenic cells. NPJ Breast Cancer 6:42. https://doi.org/10.1038/s41523-020-00183-8
Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H et al (2015) The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27:193–210. https://doi.org/10.1016/j.ccell.2014.11.017
Wang H, Tian L, Liu J, Goldstein A, Bado I, Zhang W, Arenkiel BR et al (2018) The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34:823–839e827. https://doi.org/10.1016/j.ccell.2018.10.002
Bado IL, Zhang W, Hu J, Xu Z, Wang H, Sarkar P, Li L et al (2021) The bone microenvironment increases phenotypic plasticity of ER(+) breast cancer cells. Dev Cell 56:1100–1117e1109. https://doi.org/10.1016/j.devcel.2021.03.008
Zhang W, Bado IL, Hu J, Wan YW, Wu L, Wang H, Gao Y et al (2021) The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184:2471–2486e2420. https://doi.org/10.1016/j.cell.2021.03.011
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249. https://doi.org/10.1158/1078-0432.CCR-06-0931. s
Tian Z, Wu L, Yu C, Chen Y, Xu Z, Bado I, Loredo A et al (2021) Harnessing the power of antibodies to fight bone metastasis. Sci Adv 7:eabf2051. https://doi.org/10.1126/sciadv.abf2051
Yuan X, Qian N, Ling S, Li Y, Sun W, Li J, Du R et al (2021) Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 11:1429–1445. https://doi.org/10.7150/thno.45351
Chen Z, Orlowski RZ, Wang M, Kwak L, McCarty N (2014) Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood 123:2204–2208. https://doi.org/10.1182/blood-2013-07-517136
Zhang XH, Giuliano M, Trivedi MV, Schiff R, Osborne CK (2013) Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 19:6389–6397. https://doi.org/10.1158/1078-0432.CCR-13-0838
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Rakha EA, Tse GM, Quinn CM (2023) An update on the pathological classification of breast cancer. Histopathology 82:5–16. https://doi.org/10.1111/his.14786
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12:31–46. https://doi.org/10.1158/2159-8290.CD-21-1059
Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, Minisini AM et al (2014) Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist 19:608–615. https://doi.org/10.1634/theoncologist.2014-0002
Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, Blamey RW et al (2008) Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol 26:3153–3158. https://doi.org/10.1200/JCO.2007.15.5986
Cserni G, Quinn CM, Foschini MP, Bianchi S, Callagy G, Chmielik E, Decker T et al (2021) Triple-negative breast cancer histological subtypes with a favourable prognosis. Cancers (Basel) 13:5694. https://doi.org/10.3390/cancers13225694
WHO Classification of Tumours Editorial Board (2019) WHO classification of tumours: breast tumours, 5th edn. World Health Organization, Lyon, France
Rakha EA, Ellis IO (2010) Lobular breast carcinoma and its variants. Semin Diagn Pathol 27:49–61. https://doi.org/10.1053/j.semdp.2009.12.009
Giuliano AE, Edge SB, Hortobagyi GN (2018) Eighth Edition of the AJCC Cancer staging Manual: breast Cancer. Ann Surg Oncol 25:1783–1785. https://doi.org/10.1245/s10434-018-6486-6
Jang N, Choi JE, Kang SH, Bae YK (2019) Validation of the pathological prognostic staging system proposed in the revised eighth edition of the AJCC staging manual in different molecular subtypes of breast cancer. Virchows Arch 474:193–200. https://doi.org/10.1007/s00428-018-2495-x
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752. https://doi.org/10.1038/35021093
Masood S (2016) Breast cancer subtypes: morphologic and biologic characterization. Womens Health (Lond) 12:103–119. https://doi.org/10.2217/whe.15.99
Kumar V, Abbas AK, Aster JC (2022) Robbins & Cotran pathologic basis of disease, 10th edn. Elsevier, Philadelphia, PA
Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87:159–170. https://doi.org/10.1016/s0092-8674(00)81333-1
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669. https://doi.org/10.1158/0008-5472.CAN-10-1040
Akhtar M, Haider A, Rashid S, Al-Nabet A (2019) Paget’s seed and soil theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol 26:69–74. https://doi.org/10.1097/PAP.0000000000000219
Langley RR, Fidler IJ (2007) Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev 28:297–321. https://doi.org/10.1210/er.2006-0027
Riggi N, Aguet M, Stamenkovic I (2018) Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annu Rev Pathol 13:117–140. https://doi.org/10.1146/annurev-pathol-020117-044127
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273. https://doi.org/10.1038/nrc2620
Javid SH, Smith BL, Mayer E, Bellon J, Murphy CD, Lipsitz S, Golshan M (2009) Tubular carcinoma of the breast: results of a large contemporary series. Am J Surg 197:674–677. https://doi.org/10.1016/j.amjsurg.2008.05.005
Malmgren J, Hurlbert M, Atwood M, Kaplan HG (2019) Examination of a paradox: recurrent metastatic breast cancer incidence decline without improved distant disease survival: 1990–2011. Breast Cancer Res Treat 174:505–514. https://doi.org/10.1007/s10549-018-05090-y
Brown D, Smeets D, Szekely B, Larsimont D, Szasz AM, Adnet PY, Rothe F et al (2017) Phylogenetic analysis of metastatic progression in breast cancer using somatic mutations and copy number aberrations. Nat Commun 8:14944. https://doi.org/10.1038/ncomms14944
Fimereli D, Venet D, Rediti M, Boeckx B, Maetens M, Majjaj S, Rouas G et al (2022) Timing evolution of lobular breast cancer through phylogenetic analysis. EBioMedicine 82:104169. https://doi.org/10.1016/j.ebiom.2022.104169
Garcia-Recio S, Hinoue T, Wheeler GL, Kelly BJ, Garrido-Castro AC, Pascual T, De Cubas AA et al (2023) Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nat Cancer 4:128–147. https://doi.org/10.1038/s43018-022-00491-x
Siegel MB, He X, Hoadley KA, Hoyle A, Pearce JB, Garrett AL, Kumar S et al (2018) Integrated RNA and DNA sequencing reveals early drivers of metastatic breast cancer. J Clin Invest 128:1371–1383. https://doi.org/10.1172/JCI96153
Yates LR, Knappskog S, Wedge D, Farmery JHR, Gonzalez S, Martincorena I, Alexandrov LB et al (2017) Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32:169–184e167. https://doi.org/10.1016/j.ccell.2017.07.005
Ng CKY, Bidard FC, Piscuoglio S, Geyer FC, Lim RS, de Bruijn I, Shen R et al (2017) Genetic heterogeneity in therapy-naive synchronous primary breast cancers and their metastases. Clin Cancer Res 23:4402–4415. https://doi.org/10.1158/1078-0432.CCR-16-3115
Ullah I, Karthik GM, Alkodsi A, Kjallquist U, Stalhammar G, Lovrot J, Martinez NF et al (2018) Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J Clin Invest 128:1355–1370. https://doi.org/10.1172/JCI96149
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM et al (2015) Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5:1164–1177. https://doi.org/10.1158/2159-8290.CD-15-0369
Cejalvo JM, Martinez de Duenas E, Galvan P, Garcia-Recio S, Burgues Gasion O, Pare L, Antolin S et al (2017) Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res 77:2213–2221. https://doi.org/10.1158/0008-5472.CAN-16-2717
Aftimos P, Oliveira M, Irrthum A, Fumagalli D, Sotiriou C, Gal-Yam EN, Robson ME et al (2021) Genomic and transcriptomic analyses of breast cancer primaries and matched metastases in AURORA, the breast International Group (BIG) molecular screening initiative. Cancer Discov 11:2796–2811. https://doi.org/10.1158/2159-8290.CD-20-1647
Birkbak NJ, McGranahan N (2020) Cancer genome evolutionary trajectories in metastasis. Cancer Cell 37:8–19. https://doi.org/10.1016/j.ccell.2019.12.004
Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML, Group E (2021) Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg 156:e210891. https://doi.org/10.1001/jamasurg.2021.0891
Samiei S, van Nijnatten TJA, de Munck L, Keymeulen K, Simons JM, Kooreman LFS, Siesling S et al (2020) Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 271:574–580. https://doi.org/10.1097/SLA.0000000000003126
Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, Dingemans AC et al (2020) Characterisation and classification of oligometastatic disease: a european Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 21:e18–e28. https://doi.org/10.1016/S1470-2045(19)30718-1
Lacaze JL, Aziza R, Chira C, De Maio E, Izar F, Jouve E, Massabeau C et al (2021) Diagnosis, biology and epidemiology of oligometastatic breast cancer. Breast 59:144–156. https://doi.org/10.1016/j.breast.2021.06.010
Lehrer EJ, Singh R, Wang M, Chinchilli VM, Trifiletti DM, Ost P, Siva S et al (2021) Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: a systematic review and meta-analysis. JAMA Oncol 7:92–106. https://doi.org/10.1001/jamaoncol.2020.6146
Milano MT, Katz AW, Zhang H, Huggins CF, Aujla KS, Okunieff P (2019) Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: some patients survive longer than a decade. Radiother Oncol 131:45–51. https://doi.org/10.1016/j.radonc.2018.11.022
Hanrahan EO, Broglio KR, Buzdar AU, Theriault RL, Valero V, Cristofanilli M, Yin G et al (2005) Combined-modality treatment for isolated recurrences of breast carcinoma: update on 30 years of experience at the University of Texas M.D. Anderson Cancer Center and assessment of prognostic factors. Cancer 104:1158–1171. https://doi.org/10.1002/cncr.21305
Abbott DE, Brouquet A, Mittendorf EA, Andreou A, Meric-Bernstam F, Valero V, Green MC et al (2012) Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery 151:710–716. https://doi.org/10.1016/j.surg.2011.12.017
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L et al (2020) Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 38:2830–2838. https://doi.org/10.1200/JCO.20.00818
Chmura SJ, Winter KA, Woodward WA, Borges VF, Salama JK, Al-Hallaq HA, Matuszak M et al (2022) NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol 40:1007–1007. https://doi.org/10.1200/JCO.2022.40.16_suppl.1007
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B et al (2022) Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20:691–722. https://doi.org/10.6004/jnccn.2022.0030
Soran A, Ozbas S, Kelsey SF, Gulluoglu BM (2009) Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (protocol MF07-01): a study of turkish federation of the National Societies for breast Diseases. Breast J 15:399–403. https://doi.org/10.1111/j.1524-4741.2009.00744.x
Fitzal F, Bjelic-Radisic V, Knauer M, Steger G, Hubalek M, Balic M, Singer C et al (2019) Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE trial. Ann Surg 269:1163–1169. https://doi.org/10.1097/SLA.0000000000002771
Khan SA, Zhao F, Goldstein LJ, Cella D, Basik M, Golshan M, Julian TB et al (2022) Early local therapy for the primary site in de novo stage IV breast cancer: results of a randomized clinical trial (EA2108). J Clin Oncol 40:978–987. https://doi.org/10.1200/JCO.21.02006
Funding
SDN supported by the Nathanson/Rands Breast Cancer Research Chair, Detroit, MI. LD supported by a Heisenberg Fellowship (DI 2861/1–1) awarded by Deutsche Forschungsgemeinschaft (DFG). L.P receives funding from the Breast Cancer Research Foundation (BCRF-22-133) and is a Susan G. Komen Scholar funded through a Leadership Award (SAC220225). A.R-H supported by a grant from the Spanish Society of Medical Oncology. XHZ supported by NIH grants R01CA221946; R01CA183878; R01CA251950; U01CA253553; DoD BC201371P1.
Author information
Authors and Affiliations
Contributions
S. David Nathanson conceived the idea for this review article. All the co-authors performed their own literature searches and concept analysis and drafted and critically revised their own work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Other acknowledgements
The authors wish to acknowledge the crucial contributions to this manuscript of Stephanie Stebens MLIS, AHIP, Sladen Librarian at HFH.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nathanson, S.D., Dieterich, L.C., Zhang, X.HF. et al. Associations amongst genes, molecules, cells, and organs in breast cancer metastasis. Clin Exp Metastasis 41, 417–437 (2024). https://doi.org/10.1007/s10585-023-10230-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10230-w