Abstract
A participant in key developmental processes, the adhesion glycoprotein CD44 is also expressed in several types of malignancies and can promote metastasis. In addition, the expression of CD44 isoforms in different types of cancer such as prostate and breast cancers may facilitate bone metastases by enhancing tumorigenicity, osteomimicry, cell migration, homing to bone, and anchorage within the bone specialized domains. Moreover, there is evidence that the CD44-ICD fragments in breast cancer cells may promote the cells’ osteolytic nature. Yet the mechanisms by which CD44 and its downstream effectors promote the establishment of these cells within the bone are not fully elucidated. In this review, we summarize the current data on the roles played by CD44 in cancer progression and bone metastasis and the possible effects of its interaction with the different components of the bone marrow milieu.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adhesion molecule CD44 is expressed on several types of cells, including hematopoietic and mesenchymal stem cells, lymphocytes, and epithelial cells [1, 2]. CD44 isoforms, which are similarly expressed on many types of cancer cells, can facilitate tumor cell migration, invasion, proliferation, and survival [3]. Prostate and breast cancer cells commonly express CD44 and are known for their high predilection to metastasize to bone, which occurs partly in a CD44- dependent manner [4, 5]. Bone is a frequent site of metastases that are characterized in most cases by poor prognoses and for which, to date, efficient treatment is lacking [6]. According to several investigations, CD44 may confer on these cancer cells an advantage: during the bone metastasis process, CD44 promotes their specific migration and homing to, and their subsequent anchorage within, the bone specialized domains, and it fuels the vicious cycle of bone metastasis through a variety of signaling cascades [5, 7, 8]. Some of the aforementioned processes are facilitated by the acquisition of stemness properties during the epithelial to mesenchymal transition (EMT). After this transition, the genetic profile expressed by the cancer cells may resemble that of bone microenvironment cells, thus conferring on the cancer cells a phenotype that promotes their survival in bone. Termed osteomimicry, this phenomenon has been observed mainly in prostate and breast cancers and the results of some studies indicate that CD44 may play a role in osteomimicry [9]. Moreover, CD44 expression is considered a marker for cancer stem cells (CSCs), a phenotype that confers on the cells additional advantages during their interaction with the endosteal microenvironment [10].
In this article, we discuss the complexity of CD44’s functions, its possible effects on the multi-step, bone metastasis process, and its possible role in the fate decisions of the invading cells.
Structure and function
CD44 is a family of transmembrane glycoproteins that are involved in cellular communication and adhesion between cells and the extracellular matrix (ECM) [11] and that contribute to cell division and migration, lymphocyte homing and activation, and haematopoiesis signaling [12, 13]. As such, CD44 is expressed in several healthy tissues and cell types, including bone marrow endothelial cells, connective tissue and on embryonic stem cells [2, 5]. In humans, the CD44 coding gene, located on the short arm of chromosome 11, contains 50 kb of DNA that comprises 20 exons, 12 of which undergo splicing processes [13].
The primary domains of CD44 comprise its extracellular, transmembrane, and intracellular/cytoplasmic domains [14] (Fig. 1). The extracellular domain interacts with the external microenvironment, and it is roughly divided into two main regions, one that is constant and another that is variable. The constant region is generated from exons 1–5, which is common to all CD44 isoforms, while exons 6–15 generate the variable region. The variant exons v1–v10 that are derived from exons 6–15 are either completely absent as in the standard isoform (CD44s), or they are present in different combinations that lead to the creation of variant isoforms (CD44v) [15, 16] (Fig. 1). The transmembrane domain of CD44 participates in signal transduction by cofactors and adaptor proteins and directs lymphocyte homing [17]. The intracellular domain (CD44-ICD) has short-tail and long-tail configurations that can be detached and transmitted to the nucleus, where it mediates transcription [18]. Lastly, the cytoplasmatic domain can interact with cytoskeletal linker proteins such as those from the ezrin/radixin/moesin (ERM) family and ankyrin, which regulate cell migration and cell shape as well as protein resorting in the plasma membrane [19, 20].
After its translation, CD44 can also be modified by N- and O- linked glycosylation and glycosaminoglycanation, which are carried out via the addition of chondroitin sulfate [21, 22]. Likewise, several CD44v isoforms undergo post-translational modifications, for instance, the heparan-sulfate (HS) site in CD44v3. These modifications, such as at the binding sites for growth factors, are essential to support CD44 function and abilities [23]. Indeed, each isoform possesses a specific role in cancer progression, and may have a distinct influence on its malignancy. For example, CD44v6 supports metastasis in colon cancer and tumorigenicity and chemoresistance in prostate cancer [3].
CD44 ligands, signaling pathways and function
Evidence is mounting that CD44 is a signaling hub that regulates multiple types of cell surface receptors [24]. A surface molecule, CD44 can interact with a range of ECM components such as laminin, fibronectin, collagen, osteopontin, and hyaluronan (HA), its main ligand [25,26,27]. As an extracellular and cell-surface-associated matrix, HA is a high-molecular-weight, linear glycosaminoglycan composed of repeating disaccharides of glucuronic acid and N-acetylglucosamine [28]. The interaction of HA with the binding domain of CD44 (part of its extracellular domain) induces conformational changes that allow adaptor proteins or cytoskeletal elements to bind to intracellular domains. That latter binding event, in turn, activates signaling pathways that lead to cell proliferation, adhesion, migration, and invasion [3].
Embryonic development
In early embryos, cells produce large amounts of HA as a pericellular coating and as a filler of the intercellular space to prevent cells from packing too closely together [29]. In addition, HA has an expanded random coil state that leads to an entanglement structure that surrounds embryos in the medium [30, 31]. The activity of HA via the CD44 receptor is known to facilitate migration and to preserve cellular activities by creating hydrated pathways for the passage of migrating cells and hydrated pericellular matrices to facilitate cell rounding during mitosis [32]. However, these pivotal and finely tuned processes, which are observed during morphogenesis and tissue homeostasis, may also be recruited during the development of various malignancies [33].
Cancer progression
The mechanisms by which CD44 receptors facilitate tumor cell migration, proliferation, and survival have been researched in depth. CD44-HA interaction is considered an integral part of tumor progression and metastasis [34], and the binding capacity of CD44 to HA may be activation dependent [35]. Cells of healthy tissues express low levels of CD44, but not necessarily in its activated form, whereas solid tumors, in contrast, overexpress mainly the activated form of CD44 [36].
CD44 can also interact with matrix metalloproteinase 9 (MMP9), an important protease that plays vital roles in many biological processes, such as in ECM degradation and remodeling [37]. Associated with cancer pathology, MMP9 is known to be involved in cancer invasion, metastasis, and angiogenesis [38]. Specifically, according to Gupta et al., PC3 cell migration and invasion were reduced by disrupting CD44/MMP9 interactions on the cell surface [39].
Several CD44 isoforms exhibit a binding site for cytokines and chemokines, among them osteopontin (OPN). A non-collagenous extracellular sialylated glycoprotein that is incorporated in the bone matrix, OPN is considered to be a key component of osteoclast attachment to bone during resorption [40]. Moreover, OPN is able to interact with both the standard and variant forms of CD44, and some variant isoforms can bind to OPN independently of the arginine-glycine-aspartic acid (RGD) sequences located in the N-terminal domain of CD44 [18]. According to several studies, OPN interacts mainly with CD44v6 and/or v7 [27, 41, 42]. Elevated OPN expression levels correlate with tumor invasion, progression and metastasis in various types of cancer [43]. Furthermore, OPN and CD44 were reported to interact with ERM protein family members to alter cytoskeletal dynamics, cell adhesion, and motility through the cortical actin filaments [44,45,46]. It has also been shown by Desai et al. that the OPN/αvβ3 signaling pathway promotes CD44 expression on prostate cancer cells, CD44/MMP-9 interaction, MMP-9 activation and secretion, and cell migration [47].
CD44 can also mediate signaling by interacting with specific ligands, which can induce proteolytic cleavage of the extracellular domain by MMPs, such as disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and membrane type 1 matrix metalloprotease (MT1-MMP)[48, 49]. This process can lead to an additional cleavage of the intracellular domain by presenilin-1/γ secretase that results in three cleavage products, i.e., the extracellular domain (ECD) fragment, the CD44β-like peptide or transmembrane domain (TMD), and the CD44 intracellular domain (ICD) fragment. After the cleavage, the ICD fragment can be translocated to the nucleus where it functions as a transcription factor and activates genes that promote metastases and cancer cell survival [50, 51]. Cho et al. showed that CD44-ICD-overexpressed breast cancer cells displayed strong tumorigenicity and greater metastatic potential than control cells and that the nuclear localization of CD44-ICD is important for the transcriptional activation of the stemness factors [52]. Interestingly, efficient cancer cell migration on the HA matrix was observed to be dependent on cleavage of the CD44 ectodomain [53].
Bone metastasis
Bone is a frequent site of metastases that, in most cases, are characterized by poor survival. To date, efficient treatment for cancer that has spread to the bones is lacking, but bone metastases are often amenable to treatment, which extends patient life expectancy [54]. This medical condition is typically associated with severe pain, impaired mobility, pathologic fractures, spinal cord compression, bone marrow aplasia and hypercalcemia [55]. Bone metastases can be classified as osteolytic, osteosclerotic or mixed when elements of both types of lesions are present simultaneously [56]. Osteolytic lesions which entail digestion of the bone that eventually leads to its destruction, are induced mostly by breast cancer, multiple myeloma, renal cell carcinoma, melanoma, non-small cell lung cancer, non-Hodgkin lymphoma and thyroid cancer [57, 58]. Several theories have been proposed to explain the cause of this destructive process. However, it is widely accepted that resorption is a result of the ability of the tumor cells to induce osteoclastogenesis, which is the enhanced differentiation and subsequent activation of bone resorbing cells [59]. Osteosclerotic/osteogenic lesions, in contrast, are characterized by excessive osteoblast activation that is triggered by tumor cells and that results in the formation of new bone of poor quality. Moreover, excessive osteoclast activation usually precedes, and likely triggers, osteosclerotic metastases, whose lesions are triggered mainly by prostate cancer, carcinoid tumors, small cell lung cancer, Hodgkin lymphoma and medulloblastomas [58, 60].
The development of bone metastases involves multiple steps that begin with the establishment of a premetastatic-niche in distant organs and that are followed by penetration of the bloodstream by disseminating tumor cells (DTCs), subsequent DTC evasion of the immune system, and finally, DTC homing to and colonization of the bone [61, 62]. The process of homing to the bone is mediated by chemokines such as CXCL12 (SDF-1), which interacts with its receptor CXCR4 [63, 64]. CXCL12 is secreted by osteoblast and endothelial cells from the bone marrow and found to regulate hematopoietic stem cell (HSC) homing and retention [65]. This homing process is harnessed by invading DTCs that upon their arrival to the bone, may either proliferate immediately or enter a dormant state that may last decades [66, 67]. Dormant cells can be reactivated by a set of cues to create an overt lesion that may acquire a more aggressive metastatic phenotype via mechanisms that are not yet fully understood [62]. Such reactivated cells may then interact with the microenvironment in what has been described as the “vicious cycle”.
Driven by various signals, the vicious cycle of bone metastasis is characterized by (a) secretion by tumor cells of different molecules that may induce the proliferation and differentiation of bone-lining osteoblasts. These include parathyroid hormone related protein (PTHrP), traditionally identified as a mediator of this process, and TNFα, MMP1 and others [68]. Additional factors that participate in the initiation of osteolytic and osteogenic lesions comprise Wnt ligands, bone morphogenetic proteins (BMPs), endothelins, TGF-\(\beta\) and interleukins (ILs) such as IL-1, IL-6 etc. (b) In response to the increase in metastasis-derived signals, the bone-lining osteoblasts produce receptor activator of nuclear factor kappa-Β ligand (RANKL) that, in turn, stimulates osteoclast differentiation and activity. Taken together, these events can lead to high osteoclast activity and, therefore, to osteolytic bone destruction, the latter of which releases factors that had been integrated within the bone matrix, such as TGFβ, IGF1 and PDGF. The increased presence of these osteoclast-generated factors encourages metastasis growth and expansion by enhancing tumor cell survival and growth and by promoting more bone destruction, thus completing the vicious cycle of reactivated cancer cell interaction with the microenvironment [69,70,71].
The nature of these lesions is markedly affected by the unique microenvironment of the bone marrow, which constitutes the home of several stem cells and a “factory” for blood cells. As such, this microenvironment possesses unique properties that enable the regulation of this complex biological system, e.g., high Ca2+ flux and low O2 pressure, especially in the vicinity of the endosteal niche [72]. The bone microenvironment is also enriched with a variety of extracellular matrix components including collagen, fibronectin, OPN, and HA [73]. The prominent presence of these ECM components, especially HA and OPN, in the bone microenvironment may hint at a connection between CD44 and bone that applies not only to blood cells and stem cells, but also to cancer cells that are associated with CD44, such as breast and prostate cancer cells.
CD44 in prostate and breast cancer bone metastasis
Breast and prostate tumor cells exhibit a strong tendency to home to bone based on their ability to arrest on, adhere to, and extravasate across the bone marrow endothelium to the underlying bone matrix [4]. CD44 plays a dual role in the adhesion and affinity of these tumor cells to the bone marrow. First, according to Draffin et al., activated CD44 is expressed on the bone marrow endothelium, and it was shown to be linked with HA retained by CD44 on the surface of breast and prostate tumor cells [5]. Second, CD44 also co-operates in CXCR4\CXCL12 signaling, which is the most established axis related to DTC homing to the bone. CD44-binding of high-molecular weight HA was shown to enhance CXCR4 activation by CXCL12, while low molecular weight HA completely abrogated CXCL12-induced CXCR4 signaling. Moreover, CD44 and CXCR4 were found in a CXCL12-dependent complex regulated by HA [74].
Several investigations have shown that CD44 expression in cancer cells helps promote bone metastasis. Hiraga et al. showed that CD44 expression in breast cancer cells promotes osteoclast formation and activation that led to osteolytic bone metastasis. Furthermore, the inhibition of HA synthesis was found to decrease tumor sphere formation and osteoclast-like cell differentiation in vitro, and it suppressed bone metastasis formation with a reduced number of osteoclasts [75]. These results suggest that CD44 expression in cancer cells may promote bone metastases by enhancing cell migration, homing to the bone, invasion and HA production.
The role of CD44 in bone metastasis, a proposed integrative model. (A) HIF signaling in the hypoxic environment of the solid tumor functions in concert with other factors, leading to an increase in CD44 expression. (B) The pericellular HA coating on prostate and breast cancer cells linked with active CD44 on the bone marrow endothelium enhances the ability of these tumor cells to specifically adhere to and invade bone. (C) Invading prostate and breast cancer cells may express a gene profile similar to that of the osteoblasts. These osteomimetic cells integrate within the endosteal niche mainly by interacting with osteoblasts, giving the cells a long-term survival advantage in the bone microenvironment. (D) CD44 overexpressing tumor cells adhere to the endosteal niche via the interaction between CD44 and the high levels of HA present in this region. Ca2+ influx induces ADAM10 activation that leads to cleavage of the CD44 ectodomain followed by the cleavage of the CD44-ICD, which further activates target genes. CD44 also activates Smad5 via phosphorylation, which results in the secretion of RANKL in PC3 to induce osteoclastogenesis. (E) As a result of osteoclast activity, TGF-\(\beta\) is released from the bone matrix and interacts with its receptor (TGFR) on tumor cells, which was also found to induce CD44 cleavage. The target genes MMP9 and Cathepsin K can then be expressed in the breast cancer cells, which also promotes bone resorption and the release of additional TGF-\(\beta\), thus propelling the “vicious cycle”. The hypoxic environment of the endosteal niche may further trigger tumor cell CD44 overexpression, and the switching to CD44 variants that enable independent RGD interaction with OPN
Beyond investigations of the role played by CD44 expression in bone metastasis, several studies focused on a possible mechanism that takes place after the invading cells become anchored within the bone marrow microenvironment. Sottnik and Theodorescu proposed that the OPN-CD44-TIAM1-Rac1 axis in tumor cells is exploited while OPN is secreted by osteoblasts in response to inflammatory mediators [76]. Hill et al. focused on the involvement of CD44 in breast cancer cells in the “vicious cycle” by examining two of its downstream targets, MMP9 and Cathepsin K, the latter of which is a unique and potent collagenase that is expressed primarily in osteoclasts. Both proteases enable bone resorption and the release of factors (i.e., TGF-b, IGF, FGF, PDGF and BMPs) that together lead to increased PTHrP secretion by tumor cells and the corresponding release of stimulating growth factors, thus driving the “vicious cycle” [8]. Interestingly, it was shown by Kuo et al. that in MDA-MB-435s breast cancer cells, TGF-\(\beta\) induces MT1-MMP expression, which can lead to proteolytic cleavage not only of CD44‘s extracellular domain but also of its intracellular domain [77,78,79,80]. The findings of Kuo et al. may indeed be relevant to the “vicious cycle” in bone metastasis, wherein the CD44-ICD fragment can be further translocated into the nucleus and function as a transcription factor, as mentioned earlier, and activate MMP9 [18]. This process, therefore, may promote bone resorption and the release of additional TGF-\(\beta\) as described above (Fig. 2).
Of note, the ectodomain cleavage may be triggered by multiple stimulants, including extracellular Ca2+ influx, while high Ca2+ flux is one of the defining characteristics of the endosteal niche [80, 81]. In this case, the cleavage triggered by the Ca2+ influx was that executed by ADAM10, which was recently found to be involved in the cell migration of breast cancer cells [80, 82]. Therefore, it is possible that the high calcium flux in the bone induces enhanced CD44 cleavage that leads to the mediation of target gene transcription (e.g., MMP9, OPN, etc.), and thus, calcium flux plays a causative role in bone metastasis (Fig. 2) [18].
As mentioned earlier, despite being a highly vascularized tissue, bone is a particularly hypoxic environment, especially in the endosteum [83]. Under hypoxic conditions, hypoxia-inducible factor (HIF) signaling is activated, and it affects several cellular processes, including glycolysis, angiogenesis, drug resistance, and several steps within the metastatic cascade [84]. Moreover, large, solid tumors, such as breast tumors, are known to create a hypoxic microenvironment that presumably affects their aggressive phenotype [85]. The results of Krishnamachary et al. suggest that HIF-1\(\alpha\) regulates CD44 by increasing CD44 expression and the percentage of CD44 positive cells that express variant exons v6 and v7/8 in breast cancer cells in a hypoxic environment [86]. This mechanism may be one of the factors involved in the high expression levels of CD44 in breast cancer cells, especially in aggressive cell lines (Fig. 2). Moreover, in the case of bone metastasis, these findings could be due to the hypoxia inducing upregulation of CD44 expression in the bone or to the hypoxic environment that attracts CD44-expressing cells to the bone. The latter assumption is warranted, since hypoxia leads to increased amounts of CXCR4 and its ligand SDF-1 (CXCL12), which mediate cell homing to the bone marrow, wherein CD44 plays a major role as an adhesion molecule [86].
The upregulation of CD44 due to hypoxia may affect the interplay between CD44 and OPN. This interaction may promote tumor progression in the bone microenvironment by triggering vessel regeneration and by recruiting immune cells and bone marrow stromal cells [87]. Unlike CD44 standard, variants of the molecule interact with \(\beta\)1-containing integrins to permit cells to bind to osteopontin independently of RGD, thereby stimulating cell motility and chemotaxis [88]. In the case of hypoxia that drives the increased expression of the standard and variant forms of CD44 in the bone microenvironment, hypoxia might also enhance the effects of the interaction between OPN and CD44, in the process promoting bone metastases. For instance, according to Nemoto et al., OPN-deficient mice have reduced numbers of metastases to bone. These results indicate that OPN may play a role in the adhesion and migration of the tumor cells to bone [89]. We can therefore speculate that the hypoxia-facilitated up regulation of CD44 in a primary tumor may contribute to this process.
As mentioned here, bone marrow, particularly that in the endosteal niche, is enriched with hyaluronic acid [90]. HSCs synthesize and express HA, and that expression correlates with HSC adhesion to the endosteal niche. Therefore, CD44 overexpressing cancer cells that invade the bone marrow can exploit similar capacities, and combining their action with that of other adhesion molecules allows them to firmly anchor within the endosteal microenvironment [91]. This tight interaction together with additional cues, such as high calcium flux, specific ECM components and growth factors, can further navigate these cells toward more stem-like properties. Similar to the case of HSCs, this unique microenvironment may direct the invading cells either into a dormant state or, given the proper signals, toward reactivation [92]. Considering the growing evidence that CD44 is a pillar signaling hub, one can speculate that CD44 may also have an effect on the quiescence of the invading cells, in part via their influence over cell stemness state. CD44 is a well-known marker for cancer stem cells (CSCs), mainly for breast cancer cells, which are identified by their characteristically high CD44 and low CD24 expression levels and high aldehyde dehydrogenase (ALDH) activity [3, 93]. Several studies have shown that slow cycling CSCs, known to have high tumorigenicity and self-renewal capacity, constitute a key component of tumor heterogeneity and may be responsible for metastatic dormancy [94, 95]. Furthermore, CD44-dependent localization of MMP9 was suggested to lead to TGF-\(\beta\) activation that, in turn, hampered proliferation of the dormant cancer cells (DCCs) within the bone niches [96].
Within the unique physical and biochemical landscapes of the endosteal niche, some of the causative signals are tightly regulated to drive mesenchymal stem cells (MSCs) toward differentiation along the osteoblast lineage. The mature osteoblasts precipitate the mineral constituents of the bone and they play a role in the formation of the niche microenvironment that regulates HSC production and fate decisions [97]. DTCs that preferentially metastasize to the bone can initially integrate in this microenvironment and later orchestrate the endosteal niche to suit their specific needs. This process is partially facilitated by the ability of the invading cells to express a genetic profile similar to that of the cells of the bone microenvironment, thereby acquiring a favorable phenotype for survival in the bone. This phenomenon, termed osteomimicry, has been observed mainly in prostate and breast cancers [9]. Most researchers refer to an osteoblast-like phenotype, in which these cells produce bone matrix proteins (i.e., OCN, OPN, BSP) that are regulated by Runx2 [98,99,100]. In addition to its role in metastasis, Runx2 is a well-known master transcription factor that is essential for osteoblast differentiation and that is involved in the transcription of many osteoblast and bone-formation-related factors [101]. In prostate and breast cancer cells, the CD44-ICD fragment can interact with Runx2 in the nucleus and activate genes such as MMP9 and OPN, which may promote the osteolytic nature of breast cancer bone metastasis. Moreover, CD44-ICD may also play a role in Runx2 expression, but further research is needed to resolve this issue definitively [102].
An early investigation related to osteomimetic behavior through CD44 and Runx2 showed that CD44 and integrin αvβ3 signaling regulate RANKL expression in human-derived PC3 prostate cancer cells isolated from bone metastases by phosphorylating Smad5 and Runx2. These results suggest a role for CD44 in the promotion of osteoblastic behavior for PC3 cells [103]. Recently, Fontanella et al. suggested that CD44v8-10 activates the tafazzin (TAZ) and Wnt/TAZ signaling pathways, which would confer on the CD44 variant an important role in the osteomimetic process of PC3 cells [104]. The importance of this seminal study notwithstanding, although it shed light on a mechanism in which CD44 regulates osteomimicry in PC3 cells, the authors’ conclusions are based on the analysis of 2D culture models, which may skew the results, particularly those related to hierarchical, three-dimensional microenvironments such as the bone and the bone-marrow cavity [105].This suggested mechanism, therefore, requires further investigation by using 3D models that more closely recapitulate the natural microenvironment and/or in vivo models.
Interestingly, according to Kim et al., CD44 may be a viable marker for the osteogenic differentiation of MSCs. Several researchers have reported that as the differentiation process progresses from MSCs to osteocytes, CD44 expression levels rise [106,107,108]. In a study of this issue in our laboratory, we showed that CD44 expression in 4T1 breast cancer cells cultured on a 3D model increased over time (Fig. 3). It was also shown by Cox et al. that 4T1 cells treated with osteogenic condition media were capable of osteomimicry when cultured on a 2D or 3D scaffold [109]. Taken together with the conclusions of Kim et al., these results may indicate that, as in the process of osteoblastic differentiation wherein CD44 expression increases, a similar correlation may exist for cancer cells that acquire an osteoblastic nature (Fig. 2). However, further research is needed of the interplay between CD44 upregulation over time and the possible trans-differentiation of breast cancer cells toward an osteoblastic phenotype.
Within the hosting microenvironment of the bone marrow, the osteomimetic phenotype may be advantageous, primarily as yet another mechanism that supports the invading cells’ immune evasion capacity. According to Huang et al., the upregulation of the bone-specific proteins in the primary tumor allows the cancer cells to escape immune surveillance and successfully colonize the bone [110, 111]. Hence, it can be assumed that this favorable phenotype that is acquired by the osteomimetic cancer cell enables them to “fall off the radar” of the immune system also after bone colonization (Fig. 2). The favorable phenotype acquisition together with other CD44-dependent mechanisms that affect DCCs may support their long-term dormancy, and thereby their preferential survival within the hostile bone microenvironment.
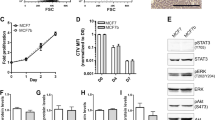
4T1 breast cancer cells cultured on a 3D model that is made of porous coralline skeletal material to mimic the endosteal microenvironment. (A-B) SEM images of 4T1 cells on the scaffold. (A) Pores of the coralline skeletal material are indicated with orange arrows and the cells are indicated with yellow arrows. Bar = 100 \(\mu m\) (B) Seeded cell adherence to and interactions with the scaffold can be seen. The cells are indicated with yellow arrows. Bar = 10 \(\mu m\) (C) Immunofluorescence staining of CD44 for the BCCs on the 3D scaffold, an extended depth of field (EDF) image. Bar = 100 \(\mu m\) (D) Enlargement of the indicated section of image C, blue represents nuclei and red CD44 staining. (E) CD44 protein expression of 4T1 cells over a timeline. \(5*{10}^{3}\) cells were seeded on the 3D model and cultured over 3, 4 or 14 days. CD44 proteins were immuno-stained according to the immunofluorescence protocol of Cell Signaling Technology Inc. The fluorescent labeled samples were observed using a fluorescent microscope (Eclipse-Ti, Nikon) connected to an LED-based excitation system (CoolLED pE, Life Sciences & Analytical, UK), and imaged with a 10x/0.3 NA objective on a digital camera (DS-Qi1Mc, Nikon). Since the surface of the 3D model is not optically flat, Z-stacks of images were acquired and later flattened using EDF to obtain a focused image. Images were segmented using machine-learning based image recognition, as implemented in Ilastik, a freely available platform (https://www.ilastik.org). NucBlueTM and CD44 channels were separately segmented, after which a dilated mask was created for each channel. The obtained mask images were applied to the original images and the total fluorescence was quantified. To correct for the possible variation in fluorescence intensity due to variation of cell axial position (owing to the 3D nature of the substrate), the fluorescence intensity of the protein was corrected by dividing it by the fluorescence intensity of the nuclei (assumed to be of uniform across cells). Data are represented as mean ± SEM. n > 10. Statistical significance between data points was determined using pairwise post-ANOVA comparison (performed by Tukey HSD), **P < 0.01.
Conclusions and perspective
CD44 has long been known to play a key role in a variety of normal and pathological processes, and in recent years, its role in cancer progression and metastasis was widely investigated and established. In the case of bone metastasis, however, the current experimental data is somewhat limited and not fully elucidated. Nevertheless, as discussed here, evidence is mounting that CD44 may facilitate bone metastasis by enhancing tumorigenicity, osteomimicry, cell migration, homing to and anchorage within the bone, and presumably the dormant state. Stemming from the insights presented herein, we suggest that CD44 may confer on the invading cells the features they need to hijack the endosteal niche “in their favor”. Depending on a variety of signals from the microenvironment, these interactions with the endosteal niche may direct the fate of invading cells in two main possible directions:
-
(1)
Toward quiescence, in which case CD44 is a double-edged sword because on the one hand, it supports the stem cell-like phenotype, which may enable the cells to enter dormancy, and on the other hand, it is potentially able to engage in osteomimicry, representing a sort of differentiated state of the cancer cells.
-
(2)
Alternatively, in the process of reactivation, CD44 may play a role through enhanced tumorigenic properties via the expression of proteolytic factors and by inducing osteoclastogenesis, thus propelling the vicious cycle.
Furthermore, the upregulation of CD44 over time that emerged in our results may be correlated with the process of breast cancer cell trans-differentiation, and therefore, this finding could support the growing evidence of CD44’s role in osteomimicry. More research is needed, however, to untangle the intricacies of the relations between CD44 upregulation over time (including how CD44’s role in cell stemness state and EMT may also be involved) and its role in breast and prostate cancer cell trans-differentiation toward an osteoblastic phenotype.
Data Availability
Not applicable.
References
Isacke CM, Yarwood H (2002) The hyaluronan receptor, CD44. Int J Biochem Cell Biol 34:718–721
Naor D, Sionov RV, Ish-Shalom D (1997) CD44: structure, function and association with the malignant process. Adv Cancer Res 71:241–319
Chen C, Zhao S, Karnad A, Freeman JW (2018) The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11:1–23
Draffin JE, Hill A, Johnston PG, Waugh DJ (2003) CD44 expression on prostate cancer cells correlates with adhesion to bone marrow endothelial cells. Clin Cancer Res 9:6181S
Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ (2004) CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res 64:5702–5711
Adjei IM, Temples MN, Brown SB, Sharma B (2018) Targeted nanomedicine to treat bone metastasis. Pharmaceutics 10:205
Hiraga T, Ito S, Nakamura H (2013) Cancer stem–like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and Hyaluronan Production. Cancer Res 73:4112–4122
Hill A, McFarlane S, Johnston PG, Waugh DJ (2006) The emerging role of CD44 in regulating skeletal micrometastasis. Cancer Lett 237:1–9
Rucci N, Teti A (2018) Osteomimicry: how the seed grows in the soil. Calcif Tissue Int 102:131–140
G van der Pluijm (2011) Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone 48:37–43
Ouhtit A, Rizeq B, Saleh HA, Rahman MM, Zayed H (2018) Novel CD44-downstream signaling pathways mediating breast tumor invasion. Int J Biol Sci 14:1782
Jackson DG, Buckley J, Bell JI (1992) Multiple variants of the human lymphocyte homing receptor CD44 generated by insertions at a single site in the extracellular domain. J Biol Chem 267:4732–4739
NS Basakran (2015) CD44 as a potential diagnostic tumor marker. Saudi Med J 36:273
KA Iczkowski (2011) Cell adhesion molecule CD44: its functional roles in prostate cancer. Am J translational Res 3:1
Sacks JD, Barbolina MV (2015) Expression and function of CD44 in epithelial ovarian carcinoma. Biomolecules 5:3051–3066
Orian-Rousseau V (2015) CD44 acts as a signaling platform controlling tumor progression and metastasis. Front Immunol 6:154
Williams K, Motiani K, Giridhar PV, Kasper S (2013) CD44 integrates signaling in normal stem cell, cancer stem cell and (pre) metastatic niches. Exp Biol Med 238:324–338
Senbanjo LT, Chellaiah MA (2017) CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front cell Dev biology 5:18
Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126:391–401
Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S et al (1998) Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol 140:885–895
Greenfield B, Wang W, Marquardt H, Piepkorn M, Wolff EA, Aruffo A et al (1999) Characterization of the heparan sulfate and chondroitin sulfate assembly sites in CD44. J Biol Chem 274:2511–2517
Gasbarri A, Del Prete F, Girnita L, Martegani MP, Natali PG, Bartolazzi A (2003) CD44s adhesive function spontaneous and PMA-inducible CD44 cleavage are regulated at post-translational level in cells of melanocytic lineage. Melanoma Res 13:325–337
Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B et al (1995) CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol 128:687–698
Orian-Rousseau V (2015) CD44 acts as a signaling platform controlling tumor progression and metastasis. Front Immunol 6:154
Jalkanen S, Jalkanen M (1992) Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol 116:817–825
Toyama-Sorimachi N, Miyasaka M (1994) A novel ligand for CD44 is sulfated proteoglycan. Int Immunol 6:655–660
Weber GF, Ashkar S, Glimcher MJ, Cantor H (1996) Receptor-ligand interaction between CD44 and osteopontin (Eta-1), Science. 271:509–512
Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10:1558–1570
Borland G, Ross JA, Guy K (1998) Forms and functions of CD44. Immunology 93:139
Astachov L, Vago R, Aviv M, Nevo Z (2011) Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front Bioscience-Landmark 16:261–276
Toyokawa K, Harayama H, Miyake M (2005) Exogenous hyaluronic acid enhances porcine parthenogenetic embryo development in vitro possibly mediated by CD44, Theriogenology. 64:378–392
BP Toole (1998) Hyaluronan in morphogenesis and tissue remodeling. Glycoforum 2:A9
Kosaki R, Watanabe K, Yamaguchi Y (1999) Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 59:1141–1145
Alaniz L, Cabrera PV, Blanco G, Ernst G, Rimoldi G, Alvarez E et al (2002) Interaction of CD44 with different forms of hyaluronic acid. Its role in adhesion and migration of tumor cells. Cell Communication & Adhesion 9:117–130
Guo Q, Yang C, Gao F (2021) The state of CD44 activation in cancer progression and therapeutic targeting,The FEBS Journal.
Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR et al (2011) Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J 278:1429–1443
Huang H (2018) Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors 18:3249
John A, Tuszynski G (2001) The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 7:14–23
Gupta A, Cao W, Sadashivaiah K, Chen W, Schneider A, Chellaiah MA (2013) Promising noninvasive cellular phenotype in prostate cancer cells knockdown of matrix metalloproteinase 9, The Scientific World Journal. (2013)
Luukkonen J, Hilli M, Nakamura M, Ritamo I, Valmu L, Kauppinen K et al (2019) Osteoclasts secrete osteopontin into resorption lacunae during bone resorption. Histochem Cell Biol 151:475–487
Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S et al (2000) Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860–864
Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K et al (1999) CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res 59:219–226
Wai PY, Kuo PC (2008) Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev 27:103–118
Smith LL, Giachelli CM (1998) Structural requirements for α9β1-mediated adhesion and migration to thrombin-cleaved osteopontin. Exp Cell Res 242:351–360
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS (2001) Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
Zohar R, Cheifetz S, McCulloch CA, Sodek J (1998) Analysis of intracellular osteopontin as a marker of osteoblastic cell differentiation and mesenchymal cell migration. Eur J Oral Sci 106:401–407
Desai B, Rogers MJ, Chellaiah MA (2007) Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer 6:1–16
Cheng Y, Lin L, Li X, Lu A, Hou C, Wu Q et al (2021) ADAM10 is involved in the oncogenic process and chemo-resistance of triple-negative breast cancer via regulating Notch1 signaling pathway, CD44 and PrPc. Cancer Cell Int 21:1–15
Marrero-Diaz R, Bravo‐Cordero JJ, Megías D, García MA, Bartolomé RA, Teixido J et al (2009) Polarized MT1‐MMP‐CD44 interaction and CD44 cleavage during cell retraction reveal an essential role for MT1‐MMP in CD44‐mediated invasion. Cell Motil Cytoskeleton 66:48–61
Thorne RF, Legg JW, Isacke CM (2004) The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci 117:373–380
Senbanjo LT, Chellaiah MA (2017) CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front cell Dev biology 5:18
Cho Y, Lee H, Kang H, Kim H, Kim S, Chun K (2015) Cleaved CD44 intracellular domain supports activation of stemness factors and promotes tumorigenesis of breast cancer. Oncotarget 6:8709
Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG et al (2015) The role of CD44 in epithelial-mesenchymal transition and cancer development. OncoTargets and therapy 8:3783–3792
Adjei IM, Temples MN, Brown SB, Sharma B (2018) Targeted nanomedicine to treat bone metastasis. Pharmaceutics 10:205
Brook N, Brook E, Dharmarajan A, Dass CR, Chan A (2018) Breast cancer bone metastases: pathogenesis and therapeutic targets. Int J Biochem Cell Biol 96:63–78
Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F et al (2021) Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev 101:797–855
Chen Y, Sosnoski DM, Mastro AM (2010) Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res 12:1–11
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L et al (2017) Bone metastases: an overview, oncology reviews.11
Dai R, Liu M, Xiang X, Xi Z, Xu H (2022) Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis. J Experimental Clin Cancer Res 41:1–12
Maurizi A, Rucci N (2018) The osteoclast in bone metastasis: player and target, Cancers. 10 218
Wang H, Pan J, Barsky L, Jacob JC, Zheng Y, Gao C et al (2021) Characteristics of pre-metastatic niche: the landscape of molecular and cellular pathways. Mol Biomed 2:1–32
Barsky L, Cohen-Erez I, Bado I, Zhang XH, Vago R (2022) Review old bone, new tricks,Clin.Exp.Metastasis.1–16
Wu M, Li C, Yiang G, Cheng Y, Tsai AP, Hou Y et al (2018) Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem 46:1423–1438
Shao H, Varamini P (2022) Breast Cancer bone metastasis: a narrative review of emerging targeted drug Delivery Systems, cells. 11:388
Hsu YS, Greenbaum A, Schuettpelz LG, Christopher M, Borgerding JN, Day RB et al (2012) CXCL12 production by early mesenchymal progenitors is required for hematopoietic stem cell maintenance. Blood 120:510
Mishra A, Shiozawa Y, Pienta KJ, Taichman RS (2011) Homing of cancer cells to the bone. Cancer Microenvironment 4:221–235
Sandiford OA, Donnelly RJ, El-Far MH, Burgmeyer LM, Sinha G, Pamarthi SH et al (2021) Mesenchymal stem cell–secreted Extracellular vesicles Instruct Stepwise dedifferentiation of breast Cancer cells into Dormancy at the bone marrow Perivascular RegionBone Marrow Perivascular Niche in breast Cancer dormancy. Cancer Res 81:1567–1582
Virk MS, Lieberman JR (2007) Tumor metastasis to bone. Arthritis Res therapy 9:1–10
Russo S, Scotto di F, Carlo F, Gianfrancesco (2022) The osteoclast traces the Route to Bone Tumors and Metastases, Frontiers in Cell and Developmental Biology.788
CC Lynch (2011) Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone 48:44–53
Sharma G, Pothuraju R, Kanchan RK, Batra SK, Siddiqui JA (2022) Chemokines network in bone metastasis: vital regulators of seeding and soiling,
Miftakhova R, Hedblom A, Semenas J, Robinson B, Simoulis A, Malm J et al (2016) Cyclin A1 and P450 aromatase promote metastatic homing and growth of stem-like prostate Cancer cells in the bone MarrowStem-like prostate Cancer cells in bone marrow metastases. Cancer Res 76:2453–2464
Zanetti C, Krause DS (2020) Caught in the net”: the extracellular matrix of the bone marrow in normal hematopoiesis and leukemia. Exp Hematol 89:13–25
Fuchs K, Hippe A, Schmaus A, Homey B, Sleeman JP, Orian-Rousseau V (2013) Opposing effects of high-and low-molecular weight hyaluronan on CXCL12-induced CXCR4 signaling depend on CD44, cell death & disease. 4:e819
Hiraga T, Ito S, Nakamura H (2013) Cancer stem–like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and Hyaluronan ProductionCancer stem-like cell marker CD44 promotes bone metastases. Cancer Res 73:4112–4122
Sottnik JL, Theodorescu D (2016) CD44: a metastasis driver and therapeutic target. Oncoscience 3:320
Murakami D, Okamoto I, Nagano O, Kawano Y, Tomita T, Iwatsubo T et al (2003) Presenilin-dependent γ-secretase activity mediates the intramembranous cleavage of CD44. Oncogene 22:1511–1516
Thorne RF, Legg JW, Isacke CM (2004) The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci 117:373–380
Kuo Y, Su C, Liu C, Chen T, Chen C, Wang H (2009) Transforming growth factor-β induces CD44 cleavage that promotes migration of MDA‐MB‐435s cells through the up‐regulation of membrane type 1‐matrix metalloproteinase. Int J Cancer 124:2568–2576
Nagano O, Saya H (2004) Mechanism and biological significance of CD44 cleavage, Cancer science. 95:930–935
Hashimoto R, Katoh Y, Miyamoto Y, Itoh S, Daida H, Nakazato Y et al (2015) Increased extracellular and intracellular Ca2 lead to adipocyte accumulation in bone marrow stromal cells by different mechanisms. Biochem Biophys Res Commun 457:647–652
Fanali C, Lucchetti D, Farina M, Corbi M, Cufino V, Cittadini A et al (2014) Cancer stem cells in colorectal cancer from pathogenesis to therapy: controversies and perspectives. World J gastroenterology: WJG 20:923
Das R, Jahr H, van Osch GJ, Farrell E (2010) The role of hypoxia in bone marrow–derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B: Reviews 16:159–168
Rankin EB, Giaccia AJ (2016) Hypoxic control of metastasis. Science 352:175–180
Yamamoto Y, Ibusuki M, Okumura Y, Kawasoe T, Kai K, Iyama K et al (2008) Hypoxia-inducible factor 1α is closely linked to an aggressive phenotype in breast cancer. Breast Cancer Res Treat 110:465–475
Krishnamachary B, Penet M, Nimmagadda S, Mironchik Y, Raman V, Solaiyappan M et al (2012) Hypoxia regulates CD44 and its variant isoforms through HIF-1α in triple negative breast cancer,
Pang X, Gong K, Zhang X, Wu S, Cui Y, Qian B (2019) Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol Res 144:235–244
Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K et al (1999) CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res 59:219–226
Nemoto H, Rittling SR, Yoshitake H, Furuya K, Amagasa T, Tsuji K et al (2001) Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res 16:652–659
R Stern (2009) Association between cancer and “acid mucopolysaccharides”: an old concept comes of age, finally, Hyaluronan in cancer biology.3–16
Khaldoyanidi SK, Goncharova V, Mueller B, Schraufstatter IU (2014) Hyaluronan in the healthy and malignant hematopoietic microenvironment. Adv Cancer Res 123:149–189
Byrne NM, Summers MA, McDonald MM (2019) Tumor cell dormancy and reactivation in bone: skeletal biology and therapeutic opportunities. JBMR plus 3:e10125
Alvarez-Elizondo MB, Weihs D (2022) Breast cancer stem cells: mechanobiology reveals highly invasive cancer cell subpopulations. Cell Mol Life Sci 79:1–9
Fukuoka M, Ichikawa Y, Osako T, Fujita T, Baba S, Takeuchi K et al (2022) The ELEANOR noncoding RNA expression contributes to cancer dormancy and predicts late recurrence of estrogen receptor-positive breast cancer. Cancer Sci 113:2336
De Angelis ML, Francescangeli F, Zeuner A (2019) Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers 11:1569
Damen MP, van Rheenen J, Scheele CL (2021) Targeting dormant tumor cells to prevent cancer recurrence. FEBS J 288:6286–6303
Galán-Díez M, Kousteni S (2017) The osteoblastic niche in hematopoiesis and hematological myeloid malignancies. Curr Mol biology Rep 3:53–62
Curatolo C, Ludovico GM, Correale M, Pagliarulo A, Abbate I, Marucco EC et al (1992) Advanced prostate cancer follow-up with prostate-specific antigen, prostatic acid phosphatase, osteocalcin and bone isoenzyme of alkaline phosphatase. Eur Urol 21:105–107
Koeneman KS, Yeung F, Chung LW (1999) Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 39:246–261
Bellahcene A, Bachelier R, Detry C, Lidereau R, Clezardin P, Castronovo V (2007) Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res Treat 101:135–148
Komori T (2009) Regulation of osteoblast differentiation by Runx2, Osteoimmunology. Springer, pp 43–49
Senbanjo LT, AlJohani H, Majumdar S, Chellaiah MA (2019) Characterization of CD44 intracellular domain interaction with RUNX2 in PC3 human prostate cancer cells. Cell Communication and Signaling 17:1–13
Gupta A, Cao W, Chellaiah MA (2012) Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB ligand signaling axis, molecular cancer. 11:1–17
Fontanella RA, Sideri S, Stefano CD, Catizone A, Agostino SD, Angelini DF et al (2021) CD44v8-10 is a marker for malignant traits and a potential driver of bone metastasis in a subpopulation of prostate cancer cells. Cancer Biology & Medicine 18:788
Levinger I, Ventura Y, Vago R (2014) Life is three dimensional—as in vitro Cancer cultures should be. Adv Cancer Res 121:383–414
Phuong DTK, Yoon TR, Kim HK, Lee ES (2015) AB139. The role of CD44 in the osteoblastic differentiation from mesenchymal stem cells,Annals of Translational Medicine.3
Jamal HH, Aubin JE (1996) CD44 expression in fetal rat bone: in Vivoandin VitroAnalysis. Exp Cell Res 223:467–477
Kim HK, Kim JH, Abbas AA, Yoon TR (2009) Alendronate enhances osteogenic differentiation of bone marrow stromal cells: a preliminary study. Clin Orthop Relat Research® 467:3121–3128
Cox RF, Jenkinson A, Pohl K, O’Brien FJ, Morgan MP (2012) Osteomimicry of mammary adenocarcinoma cells in vitro; increased expression of bone matrix proteins and proliferation within a 3D collagen environment. PLoS ONE 7:e41679
Huang W, Odero-Marah V, Chung LW (2005) Biologic and therapeutic implications of Osteomimicry and epithelial-mesenchymal transition in prostate Cancer, bone metastasis. Springer, pp 75–86
Ban J, Fock V, Aryee DN, Kovar H (2021) Mechanisms, diagnosis and treatment of bone metastases. Cells 10:2944
Acknowledgements
The authors thank Mr. Patrick Martin for valuable comments on the manuscript. The figures in this review were created with BioRender.com and parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the writing of this manuscript and they have given their approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zer, N.S., Ben-Ghedalia-Peled, N., Gheber, L.A. et al. CD44 in Bone Metastasis Development: A Key Player in the Fate Decisions of the Invading Cells?. Clin Exp Metastasis 40, 125–135 (2023). https://doi.org/10.1007/s10585-023-10203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10203-z