Abstract
Bone metastases associated with breast cancer remain a clinical challenge due to their associated morbidity, limited therapeutic intervention and lack of prognostic markers. With a continually evolving understanding of bone biology and its dynamic microenvironment, many potential new targets have been proposed. In this chapter, we discuss the roles of well-established bone markers and how their targeting, in addition to tumour-targeted therapies, might help in the prevention and treatment of bone metastases. There are a vast number of bone markers, of which one of the best-known families is the bone morphogenetic proteins (BMPs). This chapter focuses on their role in breast cancer-associated bone metastases, associated signalling pathways and the possibilities for potential therapeutic intervention. In addition, this chapter provides an update on the role receptor activator of nuclear factor-κB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) play on breast cancer development and their subsequent influence during the homing and establishment of breast cancer-associated bone metastases. Beyond the well-established bone molecules, this chapter also explores the role of other potential factors such as activated leukocyte cell adhesion molecule (ALCAM) and its potential impact on breast cancer cells’ affinity for the bone environment, which implies that ALCAM could be a promising therapeutic target.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
The propensity of breast cancer to metastasise to bone is a well-noted phenomenon. In 1889, through post-mortem study, Paget [1] identified that breast cancer cases were associated with bone metastasis. In spite of this observed occurrence, clinical intervention remains limited and palliative. Breast cancer is the leading cancer in females in the UK and the USA and is a common cause of cancer-related deaths. The most common metastatic site is the bone, and 50–70% of patients develop bone metastases [2]. Though the survival rate of breast cancer patients diagnosed with bone metastases varies greatly in the literature, depending on if it is bone metastases alone or in combination with visceral metastasis (up to 72 months [3]), only 30% of these women are expected to achieve 5-year survival after their bone metastasis diagnosis [4].
The metastatic cascade is not a new concept, and the process is highly inefficient, with only 0.001–0.02% of cancer cells forming metastatic foci [5] of which, our understanding of the biological drivers remains poor. With the success of first-line therapies, breast cancer patients are surviving longer. However, the bone marrow provides a niche for metastasising breast cancer cells, which can be activated many years later. Evidence has shown that tumour cells are detectable in patient bone marrow, but these do not always result in metastatic foci due to a variety of factors including tumour cell dormancy or host response [6, 7]. A better understanding of what reactivates these cancer cells and their interplay, both mechanically and biologically, which occurs between the bone environment and these cells, is fundamental to future identification of patients most at risk of developing bone metastases and development of novel therapeutic interventions.
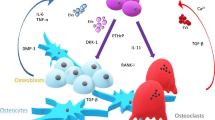
The bone is a dynamic tissue which not only provides structural support and protection but also acts as a reservoir for haematopoietic cells and an elaborate blood supply. As metastatic breast cancer cells colonise the bone environment utilising growth factors, such as transforming growth factor β (TGF-β) and insulin-like growth factors, to stimulate tumour growth and they also feedback into the bone environment through direct cell-to-cell contact and paracrine influence. Tumour cells secrete a range of factors, including interleukins, tumour necrosis factor (TNF)-alpha and parathyroid hormone-related protein (PTHrP), both osteo-inductive factors, which influence the physiological bone environment, enable tumour growth and stimulate osteoclastogenesis [8]. This bidirectional signalling and co-operation are referred to as the ‘vicious cycle’ and involve bone remodelling cells such as osteoclasts and osteoblasts, as well as the recruitment and modulation of other cell types including platelets, immune and nerve cells, which can further facilitate pro-tumorigenic processes, including angiogenesis [9,10,11].
The nature of bone metastases are heterogeneous ranging from bone destructing (osteolytic) to bone forming (osteoblastic), with potential for both cases to also occur at the same time (mixed lesions). Within breast cancer, it is the osteolytic phenotype which is frequently observed. Bisphosphonates, which bind to bone mineral and result in osteoclast apoptosis, and receptor activator of NF-κB ligand (RANKL)-targeted antibodies remain the main standard of care for skeletal-related events (SREs) occurring in breast cancer patients with bone metastases [12]. These interventions currently reduce the morbidities which are associated with bone metastases, including debilitating pain, fractures and hypercalcaemia. However, they do not target the tumorigenic process and only inhibit osteoclast function and osteoclastogenesis. Approximately half of patients continue to develop new bone metastases, and breast cancer patients who develop pathological fracture have a 32% increased risk of death compared to those who do not [13, 14]. Therefore, novel therapies aimed at recently identified targets are required to influence both the metastatic tumour cells and the bone environment.
It is vital to understand the molecular and cellular events involved in bone modelling and remodelling and the effects tumour cells have on the skeleton and vice versa. The key cells, osteoclasts and osteoblasts, from their two distinct lineages, haematopoietic and mesenchymal respectively, were heavily investigated in the 1980s and 1990s to elucidate their roles in bone remodelling, after Epker and Frost (1965) [15] demonstrated an interaction between these crucial cells in the remodelling process. This resulted in the subsequent identification of a trio of key molecules in the 1990s, receptor of activator of NF-κB (RANK), RANKL and osteoprotegerin (OPG), whose interplay is fundamental to the regulation of bone homeostasis [16,17,18]. Since then, these molecules have been under intense investigation in bone-related conditions, including bone metastases.
9.2 OPG, RANK and RANKL in Bone Metastasis Associated with Breast Cancer
RANKL, its receptor RANK and its naturally secreted decoy OPG are all members of the TNF receptor superfamily. Originally linked to bone remodelling and immunity, they have since been extensively studied in a wide range of solid cancers, including breast cancer, particularly focusing on their effects on the bone microenvironment. Furthermore, recent studies in breast cancer have shown that these bone-related molecules could be potential prognostic and therapeutic targets beyond the bone.
9.2.1 RANK/RANKL Signalling in Mammary Gland Development and Carcinogenesis
In the last decade, the RANK/RANKL pathway has come to prominence in breast cancer research beyond the bone environment due to several key observations. RANK and RANKL along with several hormones, including sex hormones, prolactin and PTHrP, have been linked with both normal mammary gland development and lactation, including ductal side branching, alveolar differentiation and lumen formation during pregnancy and carcinogenesis [19,20,21,22]. The interaction of progesterone and RANKL signalling has been particularly relevant given that this can occur in both progesterone-positive and progesterone-negative cells. In progesterone-responsive luminal cells, RANKL is upregulated, which helps stabilise RNA. Furthermore, evidence has shown that through paracrine RANKL signalling on oestrogen and/or progesterone receptor-negative breast cancer cells, proliferation can also be induced [23,24,25,26,27].
Further evidence has shown that RANK loss or overexpression contributes to disrupted mammary gland development and impaired lactation during pregnancy [28]. Furthermore, RANK has been shown to promote proliferation and survival of mammary epithelial cells as well as expansion of mammary stem and luminal progenitor cells [29]. Thus, RANK signalling, through enhanced activation of protein kinase B (PKB/AKT) and extracellular signal-related kinase (ERK) 1/2, causes mammary progenitor populations, which are potentially supported by the paracrine signalling of RANKL, in either its membrane bound or soluble forms, to promote breast tumour formation [30].
Beyond the role that RANK/RANKL signalling plays in mammary gland development, Blake et al. [31] demonstrated that MDA-MB-231 breast cancer cells overexpressing RANK resulted in greater bone colonisation and growth. Furthermore, Casimiro et al. [32] identified that RANK expressing bone-seeking subclones of MDA-MB-231 cells exhibited increased cell migration and invasion through RANKL-mediated c-jun N-terminal kinase (JNK) and ERK 1/2 signalling. We have previously demonstrated that the targeting of RANK expression in breast cancer cells in vitro reduced cell-matrix adhesion, migration and invasion [33]. Whilst Jones et al. [34] reported that mice with RANK deletion, specifically in mammary gland epithelial cells, exhibited decreased cell proliferation under progesterone stimulation compared to the wild-type mice. However, in spite of these observations, no RANK targeting agents currently appear to be in clinical trials for the treatment of primary breast tumours.
Denosumab, which targets osteoclastogenesis by blocking the actions of RANKL, is effective in the treatment of SREs. Literature shows that in vitro cocultured breast cancer cell lines devoid of RANKL could still stimulate RANKL expression in stromal osteoblasts, thus driving osteoclastogenesis and highlighting the relevance in targeting changes which occur in the microenvironment as well as the tumour cells and their related factors [19, 35].
9.2.2 The Pros and Cons of OPG in Breast Cancer
Given that OPG is the natural decoy of RANKL and a negative regulator of bone metabolism, the reversal of its downregulation has been considered for the treatment of breast cancer-associated bone metastasis. Studies have found that basal levels of OPG are expressed in breast cancer cells and tissues. Van Poznak et al. [36] has further demonstrated that in 55% of breast cancer cases studied, OPG expression was detected and correlated with oestrogen and progesterone receptors. However, subsequent functionality tests have suggested a negative correlation between OPG and the oestrogen receptor (ER), whereby activation of the ER results in a decrease in OPG expression. This was demonstrated in the ER-positive cell line MCF-7 when it was treated with 17β-oestradiol, which inhibited OPG at both mRNA and protein levels. The effect was reversed with the addition of the selective ER downregulator Fulvestrant (ICI-182,780) [37]. OPG also has a weak affinity for TNF-related apoptosis inducing ligand (TRAIL), which is believed to aid breast cancer cell survival by evasion of death receptor-induced apoptosis, as has been demonstrated in vitro but not in vivo [38]. It may be less effective in vivo due to evidence suggesting that excessive RANKL can reverse the effect of OPG on TRAIL-induced apoptosis [39, 40]. The identification of OPG in breast cancer and its associated metastasis in vivo appears mixed, potentially due to differing effects between the whole and truncated protein versions of OPG and its response to other factors such as hepatocyte growth factor (HGF) [33, 41,42,43,44]. It seems that outside of the bone microenvironment, OPG might have a tumour-proliferating effect and thus be a driver for metastasis to occur in other parts of the body [45].
Implications of OPG and breast cancer have also been conflicting, potentially due to different breast cancer subtypes [44, 46,47,48,49]. Therefore, OPG as a direct target may not provide the best solution for the treatment of breast cancer-associated bone metastasis. As Croft et al. highlighted in 2013, all clinical trials targeting OPG had been discontinued [50]. Perhaps modulation of OPG as an indirect consequence of other therapeutic intervention could be beneficial. If it is contained to the bone environment, its utilisation for breast cancer-associated bone metastasis treatment may be more advantageous.
9.3 Targeting Aberrant BMP Signalling in Bone Metastasis of Breast Cancer
Bone morphogenetic proteins (BMPs) belong to the TGF-β superfamily which play pivotal roles in embryonic and postnatal development as well as the homeostasis of tissues and organs by coordinating differentiation, proliferation, apoptosis and motility of cells in tissue and organ-specific structures. BMP signalling is relayed through a heteromeric receptor complex comprising two types of serine-threonine kinase transmembrane receptors. Type I receptors mediating BMP signalling include activin receptor-like kinase-1 (ALK-1), BMP receptor type IA (BMPR-IA, also known as ALK-3), BMP receptor type IB (BMPR-IB, or ALK-6), ALK-4, ALK-5 and activin A receptor type I (ActRI). The type II receptors include BMP receptor type II (BMPR-II), activin A receptor type IIA (ActRIIA) and activin A receptor type IIB (ActRIIB). Upon binding of BMP ligands, the type II receptors phosphorylate the glycine-serine (GS) domain of type I receptors, leading to the recruitment of the pathway-restricted Smads (R-Smads, Smads1, 5 and 8) to the complex. With assistance from Smad 4, the R-Smads intracellular signalling complex is translocated into the nucleus, leading to the induction of BMP-responsive genes. Smads 6 and 7 negatively regulate this Smad-dependent signalling. On the other hand, the Smad-independent pathways, such as mitogen-activated protein kinase (MAPK) pathway and the RAS pathway, also relay signals into cells. Thus, these diverse pathways orchestrate cellular responses to BMP ligands [51].
As a group of important regulators for bone formation and turnover, the possible implication of these proteins in bone metastasis of certain solid tumours has been investigated [51]. The attention to BMPs and their role in breast cancer arose nearly a decade ago, with a number of key findings being made in this area.
9.3.1 Aberrant Expression of BMPs in Breast Cancer
Our previous studies have shown that expression of BMPs, including BMP-2, BMP-4, BMP-6, BMP-7, BMP-12, BMP-15 and GDF9a, is reduced in breast cancer. The decreased expression of BMP-2, BMP-7, GDF9a and BMP-15 is associated with poor prognosis [52,53,54], which has also been found in other studies [55, 56]. BMP-7 is reduced in primary tumours with bone metastasis [57]. However, other studies have linked overexpression of BMP-2, BMP-4, BMP-5 and BMP-7 with breast cancer [52, 58,59,60,61]. These contrasting findings suggest that BMPs may play different roles during the development and progression of breast cancer.
Certain BMP receptors have been assessed for their involvement in breast cancer. An increased expression of BMPR-IB has been observed in poorly differentiated tumours, with a higher rate of proliferation and cytogenetic instability. The overexpression of BMPR-IB was also associated with poor prognosis in ER-positive carcinomas [62]. This suggests that the expression of this type I receptor may be associated with the ER status and is regulated by oestrogen. Moreover, our previous study in a breast cancer cohort from the University Hospital of Wales showed that a decreased expression of BMPR-IB was associated with poor prognosis [63]. Differences in the ER status may be a reason for these conflicting findings. Activated Smad1/5/8 and Smad 2 were observed in nuclei of breast cancer cells from both primary tumours and bone metastases. This is supported by findings from an in vivo murine model [64]. TGF-β3 and BMP-2 promoted invasion of MDA-231-D cells, where a blockage of the TGF-β and/or BMP signalling by expression of domain-negative receptors eliminated the TGF-β3- and BMP-2-induced invasion and TGF-β3- and BMP-2-associated bone metastasis. It suggests that BMPs and TGF-ß may synergistically work together to promote the invasion and bone metastasis of breast cancer [64].
9.3.2 Regulatory Aspects of BMP Signalling
The diversity of BMP expression and signalling occurs in malignancies throughout their development and progression, reflecting the temporal and contextual nature of BMP influence. The additional complexity is both the regulatory machinery for BMPs and their interactions with other factors. A number of hormones and growth factors have been indicated within the BMP signalling networks.
9.3.2.1 Crosstalk with Oestrogen Receptor Signalling
Hormone receptor status may have great influence on aberrations in BMP phenotype and signalling with self-adjustment by tumour cells themselves, according to their needs for development and progression at different stages. Indeed, epigenetic regulation of BMPs and BMPRs in breast cancer is associated with ER status [65]. Oestrogen represses the expression of BMPR-IA, BMPR-IB, ActRIIA and ActRIIB but not ActRI and BMPR-II [66]. The expression of BMP-7 has been found to highly correlate with the expression levels of ER, although BMP-7 expression is reduced in response to oestrogen [67, 68] and BMP-2 expression is significantly higher in ER-negative tumours [69].
Oestrogen and BMPs can influence each other’s function through interactions between receptors and downstream signalling [70, 71]. For example, oestrogen interferes with the biological function of BMP-2 by inhibiting the activation of Smad, as a result of biochemical interaction between Smad and ER [70]. Conversely, BMP signalling can affect ER function, as Smad 4 prevents the transcriptional regulation mediated by cytoplasmic ER [71], and BMP-2 inhibits oestradiol-induced proliferation of ER-positive breast cancer cells via upregulation of cyclin kinase inhibitor p21, which in turn inhibits the oestradiol-induced cyclin D1-associated kinase activity [72].
Hypermethylation of BMP-6 and its reduced expression have been observed in ER-negative breast cancer tissues [65]. Methylation of the BMP-6 gene promoter has been detected in ER-negative cell lines, whilst in ER-positive cells, the BMP-6 gene promoter remains demethylated. Studies show overexpression of BMP-6 particularly in ER-positive cell lines and tumour samples [65, 73]. Further in vitro study has demonstrated the interaction of ER with sites on the BMP-6 promoter region [65]. This suggests that ER status is linked with BMP expression at epigenetic level.
9.3.2.2 Crosstalk with Androgen Receptor Signalling
In breast cancer, the androgen receptor (AR) has received increasing attention related to treatment resistance, and its expression has been linked to both good and poor prognosis [74]. In tumours responsive to neoadjuvant endocrine therapy, AR mRNA and protein expression is decreased, whilst this is not seen in treatment-resistant tumours. In a clinical cohort, a high AR/ER ratio was shown as an independent risk for failure of tamoxifen treatment and poor survival. The finding has been corroborated by both in vitro and in vivo studies on breast cancer models, whereby AR overexpression is shown to increase tamoxifen resistance [75]. The underlying mechanisms regarding interactions between BMP signalling and AR are not yet clear in breast cancer. However, it would be a novel area to explore for possible targeted therapy particularly for endocrine treatment-resistant breast cancers.
9.3.2.3 Crosstalk with Growth Factor Signalling
Several other factors and pathways have been indicated in the regulation of BMP expression and function. BMP-4 generally inhibits breast cancer cell growth but enhances proliferation of breast cancer cells induced by fibroblast growth factor (FGF), epidermal growth factor (EGF) and HGF [76]. EGF treatment of breast cancer cells in vitro upregulates BMP-4 signalling, leading to suppression of matrix metalloprotease (MMP) 9. This effect was reduced when treated with BMP-4 antagonists Gremlin or Smad 6 [77]. In addition, BMP-6 in breast cancer cells can be upregulated by EGF and other EGF receptor (EGFR) ligands [55]. Conversely, EGF-, FGF- and HGF-activated MAPK/ERKs phosphorylate a linking region of Smad1/5/8, resulting in reduced nuclear translocation and transcription of BMP target genes [78, 79]. BMPs also exert reciprocal effects, suppressing EGF-induced gene transcription through MAPK/ERK-1 signalling [80]. BMP-9 decreases human epidermal growth factor receptor 2 (HER2) expression, inactivating ERK1/2 and phosphoinositide 3-kinase (PI3K)/AKT signalling pathways and leading to reduced proliferation and metastasis of SK-BR-3 breast cancer cells [81].
There is also interaction between Wnt and BMP signalling. SOSTDC1, a secreted regulator of both pathways, is under-expressed in breast cancer tissue and breast cancer cells. SOSTDC1 increases Wnt3a signalling and decreases BMP-7 signalling whilst eliciting little effect on BMP-2-induced signalling [82].
Nacamuli et al. demonstrated that BMP-3 expression can be controlled by recombinant human FGF in calvarial osteoblasts [83]. Retinoid has been shown to induce expression of BMP-2 in the retinoid-sensitive cell lines [84]. Rapamycin induces BMP-4 and downregulates BMP antagonist Follistatin expression in a prostate cancer cell line (PC3) [85]. Our previous studies showed that HGF upregulated the expression of BMP-7 and BMP receptors in prostate cancer cells. These upregulations were blocked by NK4, an antagonist of HGF [86, 87]. HGF-regulated BMP and BMP signalling may form a part of its contribution to the disease progression and bone metastasis. These studies collectively indicate that BMPs, together with other growth factors, form a collaborative interacting network during the development and progression of cancer, which would be worthy of further study, particularly given how important receptor status has become in breast cancer prognosis and treatments.
9.3.3 BMP Signalling in the Predisposition of Metastasis to Bone and Formation of Osteolytic Lesions
BMPs are the most powerful bone inductive factors which are abundant in bone matrix. In a bone metastatic lesion, BMPs can be synthesised by osteoblasts and stored in bone matrix. In addition, cancer cells can release BMPs and their antagonists to coordinate their functions. Secreted from cancer cells, BMPs contribute to bone lesion by targeting bone cells and in turn enhance aggressiveness of cancer cells. BMPs can also indirectly support the colonisation and development of bone metastasis by promoting tumour-associated angiogenesis, which makes them key factors in the ‘vicious cycle’ of bone metastasis. Both clinical and experimental studies have suggested profound roles for BMPs in the bone metastasis of breast cancer.
9.3.3.1 Profile of BMPs in Bone Dissemination and the Metastatic Bone Microenvironment
Decreased expression of BMP-7 in primary tumours correlates with bone metastases, whilst BMP-7 is capable of inhibiting the growth of breast cancer tumours in bone in vivo [57]. Other studies have shown BMP-7 overexpression in primary tumours is associated with bone metastases [68]. In murine 4T1E/M3 mammary cells, which are highly metastatic to bone, expression of BMP-7, BMPR and phosphorylated Smad1/5/8 are upregulated. These highly invasive features are attenuated when BMP-7 is inhibited [57].
Other studies have found that BMP-induced transcriptional pathways are active in bone metastatic lesions in vivo, and xenograft tumours with dominant negative BMP receptors have fewer bone metastases [64].
BMP-9 suppresses the growth of breast tumour cells in bone, mediated by BMP-9-induced downregulation of connective tissue growth factor (CTGF) [88, 89]. Orthotropic implant of tumours with silk scaffolds coupled with BMP-2 and seeded with bone marrow stromal cells (BMSC), contributed to bone metastasis of breast cancer cells in vivo [90].
Breast cancer cells themselves can display an osteoblast-like phenotype by expressing bone matrix proteins such as bone sialoprotein (BSP), osteopontin (OPN), OPG and osteoblast-specific cadherins [91,92,93]. Breast cancer cells with induced epithelial-to-mesenchymal transition (EMT) exhibited an elevated level of bone-related genes (BRGs) and osteoblast-like features when exposed to BMP-2. Breast cancer cells expressing these BRGs favoured spread and survival in the bone. Interestingly, the cells were also more resistant to chemotherapy [93]. The BMP antagonist Noggin reversed these effects, as did knockdown of runt-related transcription factor 2 (RUNX2), which regulates bone remodelling and osteogenic differentiation [68, 93, 94]. This ‘bone signature’ induced by BMPs may be one of the reasons breast cancer cells home to bone tissue. Once the breast cancer cell is established in the bone, BMPs and their antagonists continue to influence survival of the tumour within the microenvironment [95].
9.3.3.2 Regulation of BMPs in Bone Microenvironment
Local factors such as sexual hormones may play a role in regulation and adaptable expression of BMPs in bone metastases. The selective oestrogen receptor modulator (SERM) raloxifene increases BMP-4 promoter activity in U-2 OS osteoblast-like cells. ER is thought to be indispensable for this effect [96]. In addition, oestradiol enhances BMP-4-induced expression of osteoblastic markers (RUNX2, osterix, osteocalcin) in osteoprogenitor cells [97].
In osteoblasts, BMP-6 reporter activity is increased with antioestrogen treatment and decreased with oestradiol treatment, which provides evidence that ER regulates BMP-6 differentially in the breast and bone. Patients with ER-positive breast tumours are more likely to develop skeletal metastases [73], and this interaction between ER and BMP signalling may be the key influence on skeletal secondary formation in breast cancer.
BMP antagonists also appear to have a significant role in bone metastasis. Conditioned medium (CM) from breast cancer cells (HT-39) resulted in upregulation of BSP mRNA expression in osteoprogenitor cells (MC3T3-E1 cells) and a promotion of their osteoblastic behaviour. This effect was blocked by the addition of BMP antagonist Noggin [98]. High expression levels of Noggin are associated with bone metastases in both cell line/murine models and clinical samples of breast cancer bone metastases [99]. Upregulation of Noggin and another antagonist Follistatin, by zinc finger E-box-binding homeobox 1 (ZEB1) in breast cancer cells, induces differentiation of osteoclasts in vitro, which suggests an osteolytic influence in the bone microenvironment [100].
Another recent study has also demonstrated that lack of Noggin expression in both breast and prostate cancer cells is associated with osteoblastic activities in bone metastases. Overexpression of Noggin in an osteo-inductive prostate cancer cell line (C4-2B) inhibited osteoblastic activities but had little effect on bone resorption and tumour growth [101]. BMPs and their antagonists evidently play a role in coordinating the osteoblastic and osteolytic activities in bone metastatic lesions and thus necessitate further study, particularly in regard to therapeutic potential.
9.3.4 Therapeutic Potential of Targeting BMPs
We require agents that act to prevent or resolve bone metastasis, and in this respect, BMPs/BMP antagonists are largely underexplored. Both clinical and experimental studies suggest profound potential for targeting BMPs in treating breast cancer and bone metastasis. BMPs not only directly affect cancer cells to coordinate their abilities during disease progression and bone metastasis but also indirectly contribute to bone metastasis through regulating tumour-related angiogenesis and the bone microenvironment.
In an in vivo bone tumour model, exposure of tumour-bearing subjects to Noggin, an antagonist of BMPs, reduces the size of bone lesions by a mechanism that involves both osteoblastic and osteolytic processes. The BMP antagonists, Noggin and Follistatin, are also determining factors of the cells response to BMPs. Expression of these antagonists can be regulated by BMPs themselves probably through an autocrine or paracrine feedback loop. A good example is BMP-7, whose endogenous expression is intimately linked to the levels of Noggin and Follistatin in the same cell [102]. These findings collectively indicate the value of BMPs and their antagonists in the management of tumour progression and bone metastasis.
9.3.4.1 BMP Receptor Inhibitors
The BMP type I receptor small molecule inhibitors dorsomorphin and LDN 193189 have been used in several breast cancer studies to abrogate BMP signalling, appearing to reverse stemlike features in breast cancer cells and reduce invasiveness. Their clinical testing is yet to be further developed, and targeting the pathway downstream of the receptors still needs to be explored.
However, clinical trials are already underway for blocking ALK-1. ALK-1 inhibitors block the interaction of BMP-9 and BMP-10 with ALK-1, interrupting the subsequent intracellular signalling pathway. PF-03446962 is an ALK-1-specific neutralising antibody currently being evaluated in Phase II trials for solid tumours as an anti-angiogenic treatment [103]. Dalantercept is a soluble chimeric ALK-1 receptor-like protein (ALK1-Fc), which displays high-affinity binding with BMP-9 and BMP-10, resulting in inhibition of angiogenesis and suppression of tumour growth [104]. Initial studies showed that ALK1-Fc decreased metastasis formation in a breast cancer model [105]. In mice, treatment with ALK1-Fc seemed to remodel tumour vasculature, with increased perfusion and reduced hypoxia. A temporary improvement of tumour perfusion could result in a better delivery and efficacy of chemotherapy. Indeed, pretreatment with ALK1-Fc allowed tumours to be more sensitive to cisplatin, which could repress disease progression [104].
9.3.4.2 BMP/DKK1 Inhibitors
Within the bone environment, Dickkopf 1 (DKK1) is a downstream molecule of BMP signalling that inhibits canonical Wnt signalling and therefore negatively regulates bone mass. Tumour production of DKK1 is thought to contribute to osteolytic bone lesions [14, 106]. A DKK1-neutralising antibody is in clinical trials for multiple myeloma. Bortezomib is a proteasome inhibitor which inhibits osteoclast formation and bone resorption whilst enhancing osteoblastic differentiation and mineralisation in vitro. The detailed mechanisms are unclear but may result from decreased DKK1. The fact that BMP signalling acts upstream makes BMP antagonism and interaction with Wnt signalling a future area of exploration for bone metastases therapeutics [14, 107].
9.3.4.3 mTOR Inhibitors
Another area of therapeutic interest more recently is the PI3K-Akt-mechanistic target of rapamycin (mTOR) pathway – a key mediator of cellular proliferation, apoptosis, migration and angiogenesis, which is commonly activated in breast cancer, conferring resistance to hormonal therapy and trastuzumab. In breast cancer models, BMP-2 induces PI3K in osteoblasts to regulate differentiation. Blocking the PI3K-Akt-mTOR pathway suppresses RANKL and increases OPG secretion by the bone marrow stroma, which reduces osteoclast activity. mTOR inhibitors are part of ongoing trials regarding hormone receptor-positive, treatment-resistant tumours. The relationship of BMPs with this pathway and the apparent involvement of PI3K/mTOR in the bone provide intriguing prospects for the treatment of bone metastasis [108, 109] .
9.4 Activated Leukocyte Cell Adhesion Molecule (ALCAM) in Bone Metastasis
Current projects within our laboratories have highlighted a number of proteins and pathways involved in regulating metastatic characteristics and their potential importance in the development of bone metastasis. One such candidate is activated leukocyte cell adhesion molecule (ALCAM).
9.4.1 Discovery and Characterisation of ALCAM
Bowen et al. first identified and characterised ALCAM in 1995 and subsequently mapped it to chromosome 3q13.1–q13.2 [110]. ALCAM has been reported to be identical to MEMD, a cell adhesion molecule found to be preferentially expressed in metastasising melanoma cell lines compared to non-metastasising lines [111]. ALCAM, also known as CD166, is a member of the immunoglobulin superfamily and is involved in mediating homophilic (ALCAM-ALCAM) and heterophilic (ALCAM-CD6) interactions [110, 111]. Members of this family are characterised by the presence of five NH2 terminals, extracellular immunoglobulin domains comprising two membrane distal variable (V)-type folds and three membrane proximal constant (C2) folds, a transmembrane region and a short cytoplasmic region [112]. The membrane distal domain 1 appears to be important for homophilic binding, whilst the membrane proximal (C2 fold) domain 4 and 5 appear to be important for avidity and ALCAM clustering on the membrane [112, 113]. As with other cell adhesion molecule, ALCAM has been linked with a number of physiological functions but has also been implicated in cancer progression, attracting considerable scientific attention.
9.4.2 Metastatic Potential of ALCAM and Clinical Implications in Breast Cancer
The role played by ALCAM in cancer progression appears to be highly complex. Despite significant research into its expression profile in cancer progression, it still remains unclear as to the precise function or expressional alterations of ALCAM in cancer progression. One factor potentially influencing this complexity is the capacity for ALCAM to exist at a number of cellular and extracellular locations. None the less, there are many contrasting reports highlighting the prognostic potential of ALCAM expression. Such examples, focusing on cellular expression of ALCAM in breast cancer reports, have been summarised in Table 9.1, though similar observations are made within a number of other cancer types as well. Hence, it is apparent from such studies that ALCAM plays a significant, if somewhat unclear, role in breast cancer progression, and with further understanding, it could hold potential as a biomarker or therapeutic strategy. The potential of ALCAM as a prognostic factor is also strengthened due to the capacity of a shed/secreted form being detectable in patient serum. ALCAM can be proteolytically shed into the surrounding extracellular environment by proteases such as A disintegrin and A metalloproteinase 17/tumour necrosis factor-alpha-converting enzyme (ADAM17/TACE) [114]. Unlike cellular ALCAM, a clear trend has emerged within the literature, and elevated serum ALCAM has been detected in breast cancer patients. Serum ALCAM has also been shown to be enhanced in higher-grade breast cancers, and current data indicates it may be a more suitable serum marker than the current established markers, CA15-3 and CEA in breast cancer [115,116,117].
A number of cell-based studies have also explored the functional significance of ALCAM in breast cancer cell lines. ALCAM has been suggested as an important player in programmed cell death and apoptosis. A previous study has identified a protective effect of ALCAM against programmed cell death in breast cancer cells and demonstrated that the overexpression of BCL2 could enhance ALCAM expression and induce apoptosis/autophagy following the silencing of ALCAM. Furthermore, the study highlighted that ALCAM expression might be inhibited by tamoxifen and enhanced by 17-β oestradiol in MCF-7 cells [118]. A further study characterised ALCAM in MDA-MB-231 and MCF-7 breast cancer cell lines and generated knockdown and overexpression models, respectively. The study did not detect any differences in growth rates of such cells, though ALCAM did appear to influence apoptosis. The study also described an enhanced migratory potential of ALCAM-suppressed MDA-MB-231 cells and, in keeping, a reduced level of migration in MCF-7 cells overexpressing ALCAM. However, the invasive potential of MDA-MB-231 knockdown cells was reduced, and no significant impact on invasion was shown in the MCF-7 overexpression line [119]. Another study isolated a human monoclonal antibody, recognising ALCAM (scFv173), which could bind ALCAM on both cancer cell lines and in tumour tissues. It reported that the addition of scFv173 could inhibit the invasive potential of MDA-MB-231 in vitro and reduce tumour development of a colorectal carcinoma cell line (HCT116) in vivo [120].
To further understand the role of ALCAM in breast cancer, a number of studies have explored the potential mechanisms responsible for controlling ALCAM expression. King et al. have reported that DNA methylation of the ALCAM promoter is one such mechanism influencing ALCAM expression and that this may be significant factor in regulating ALCAM expression in tumour tissue. Furthermore, such a loss may inhibit adherence between circulating tumour cells, therefore supporting a role for ALCAM loss in enhancing metastatic potential [121]. Recently, the regulation of ALCAM expression by microRNAs has been reported in breast cancer. ALCAM was found to be one of a panel of genes whose expression was altered following expression of miR-125b. Expression of miR-125b enhanced both ALCAM mRNA and protein levels, which was found to influence MCF-7 growth using a further shRNA study [122]. It has also recently been reported that inhibition of miR-214, overexpression of miR-148b or a combination can inhibit tumour cell crossing of the vessel endothelium through a negative regulation of ALCAM and integrin α5 [123].
9.4.3 Potential Role for ALCAM in Bone Metastasis
Though complex, the literature supports a role for ALCAM in the progression and metastatic dissemination of cancer. To elucidate the potential of ALCAM in influencing the development of bone metastasis, our laboratories further explored ALCAM in a larger, combined breast cancer cohort and examined the association between ALCAM expression and the development of bone metastasis [124]. In keeping with our previous findings, through immunohistochemical analysis, lower ALCAM cytoplasmic expression was noted in breast cancer tissues and in tissue sections from patients who went on to develop skeletal metastasis compared to normal breast tissue. Furthermore, quantitative PCR analysis similarly indicated that significantly lower ALCAM transcript expression was associated with patients with poorer prognostic indicators and that low ALCAM expression was associated with those patients who went on to develop skeletal metastasis. This trend was similarly observed when focusing on ductal carcinoma cases alone [124]. To further explore this potential link, our laboratories examined ALCAM overexpression and knockdown models in MDA-MB-231 and ZR-751 cell lines, respectively, and explored the in vitro impact of culturing such cells in the presence of a bone matrix extract (BME) generated from ground and sonicated femoral heads. Such experiments highlighted a role for ALCAM expression in negatively regulating cell growth and matrix adhesion and migration and underscored a potential relationship between ALCAM expression and the responsiveness of MDA-MB-231 cells to the bone extract, particularly in terms of growth and migratory responses where ALCAM overexpression in the presence of bone matrix extracts brought about greater reductions in such traits [125]. Taken together, these two studies by Davies et al. suggest a potential inhibitory role for ALCAM in the development of bone metastasis. However, an additional study by Hein et al. examining ALCAM immunostaining in a tissue microarray suggested that high ALCAM staining correlated with ER positivity, nodal involvement and the presence of disseminated tumour cells within the bone marrow environment. Furthermore, alteration of ALCAM levels in MDA-MB-231 and MCF-7 cells caused differential expression of a number of molecules including cathepsin D and RUNX2 [119], both of which have implications in bone malignancies and metastasis [126, 127]. Further evidence supportive of a regulator role for ALCAM in bone dissemination was recently reported in a study by Hansen et al. in a prostate cancer model [128]. In their study, Hansen et al. reported that the shedding and detection of tumour-derived ALCAM was significantly elevated in tumour-bearing mice and that reduced ALCAM levels could significantly reduce the incident and metastatic burden of bone metastasis following intracardiac seeding of cells [128].
Given the significant impact of metastatic dissemination and establishment of tumour cells in the bone on patient well-being and ultimately survival, there is a great need to identify and utilise the responsible mechanisms for the development of new therapeutic strategies. ALCAM, a molecule linked to cancer progression and metastasis, though in a somewhat complex fashion, represents an interesting example of one such novel strategy. From early indications, ALCAM is likely to play a role in cancer cell metastasis and development in the bone environment, though additional work is required to further determine the effect of this molecule on this process. Furthermore, the detection of secreted or shed ALCAM in the serum of cancer patients may potentially present a relatively non-invasive biomarker to monitor patients. Therefore, further large-scale study is required to identify and utilise the full potential of ALCAM to monitor cancer dissemination to the bone.
9.5 Concluding Remarks
Targeting bone-associated molecules in the treatment of breast cancer-associated metastases is not a simple or quick fix. With such a rich and diverse environment for molecular targets, opportunities to target bone metastasis are vast. Consideration has been given to treat breast cancer patients in the first-line treatment with denosumab, in the hope of targeting any breast cancer cells which have already become resident in the bone environment. However, such a sledge hammer approach is not sustainable in the long term, as potentially identified targets such as OPG have demonstrated that the anti-tumorigenic benefits it exert on one area of the body may result in detrimental effects elsewhere. Therefore, ongoing efforts are essential for seeking factors which could identify patients at greatest risk of developing bone metastasis or a serum marker which could provide insight into the development of bone metastasis. The answers may not lie in classically identified molecules but in newly identified agents such as miRNAs or emerging regulators such as ALCAM. Complete elucidation of the molecules associated with the development of breast cancer-associated bone metastasis, their interactions and effects on the bone microenvironment is critical to achieve success of developing any future prognostic and therapeutic approach.
References
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8(2):98–101
Yardley DA (2016) Pharmacologic management of bone-related complications and bone metastases in postmenopausal women with hormone receptor-positive breast cancer. Breast Cancer (Dove Med Press) 8:73–82. doi:10.2147/BCTT.S97963
Briasoulis E, Karavasilis V, Kostadima L, Ignatiadis M, Fountzilas G, Pavlidis N (2004) Metastatic breast carcinoma confined to bone: portrait of a clinical entity. Cancer 101(7):1524–1528. doi:10.1002/cncr.20545
Harries M, Taylor A, Holmberg L, Agbaje O, Garmo H, Kabilan S, Purushotham A (2014) Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol 38(4):427–434. doi:10.1016/j.canep.2014.05.005
Fidler IJ (1970) Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 45(4):773–782
Kasimir-Bauer S (2009) Circulating tumor cells as markers for cancer risk assessment and treatment monitoring. Mol Diagn Ther 13(4):209–215. doi:10.2165/11315870-000000000-00000
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70(14):5649–5669. doi:10.1158/0008-5472.CAN-10-1040
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11(6):411–425. doi:10.1038/nrc3055
Guise TA (2013) Breast cancer bone metastases: it's all about the neighborhood. Cell 154(5):957–959. doi:10.1016/j.cell.2013.08.020
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2(8):584–593. doi:10.1038/nrc867
Reddi AH, Roodman D, Freeman C, Mohla S (2003) Mechanisms of tumor metastasis to the bone: challenges and opportunities. J Bone Miner Res 18(2):190–194
Steger GG, Bartsch R (2011) Denosumab for the treatment of bone metastases in breast cancer: evidence and opinion. Ther Adv Med Oncol 3(5):233–243. doi:10.1177/1758834011412656
Roodman GD (2004) Mechanisms of bone metastasis. Discov Med 4(22):144–148
Lipton A, Uzzo R, Amato RJ, Ellis GK, Hakimian B, Roodman GD, Smith MR (2009) The science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumors. J Natl Compr Canc Netw 7(Suppl 7):S1-29:quiz S30
Epker BN, Frost HM (1965) Correlation of bone resorption and formation with the physical behavior of loaded bone. J Dent Res 44:33–41
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K (1997) Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 234(1):137–142
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390(6656):175–179. doi:10.1038/36593
Dougall WC (2012) Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18(2):326–335. doi:10.1158/1078-0432.CCR-10-2507
Karsenty G (1999) The genetic transformation of bone biology. Genes Dev 13(23):3037–3051
Roodman GD (2001) Biology of osteoclast activation in cancer. J Clin Oncol 19(15):3562–3571. doi:10.1200/JCO.2001.19.15.3562
Ross FP (2000) RANKing the importance of measles virus in Paget's disease. J Clin Invest 105(5):555–558. doi:10.1172/JCI9557
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A 107(7):2989–2994. doi:10.1073/pnas.0915148107
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC (2010) RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468(7320):103–107. doi:10.1038/nature09495
Hu H, Wang J, Gupta A, Shidfar A, Branstetter D, Lee O, Ivancic D, Sullivan M, Chatterton RT Jr, Dougall WC, Khan SA (2014) RANKL expression in normal and malignant breast tissue responds to progesterone and is up-regulated during the luteal phase. Breast Cancer Res Treat 146(3):515–523. doi:10.1007/s10549-014-3049-9
Kiesel L, Kohl A (2016) Role of the RANK/RANKL pathway in breast cancer. Maturitas 86:10–16. doi:10.1016/j.maturitas.2016.01.001
Mukherjee A, Soyal SM, Li J, Ying Y, He B, DeMayo FJ, Lydon JP (2010) Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J 24(11):4408–4419. doi:10.1096/fj.10-157982
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50
Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC (2007) RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol 27(4):1442–1454. doi:10.1128/MCB.01298-06
Pellegrini P, Cordero A, Gallego MI, Dougall WC, Munoz P, Pujana MA, Gonzalez-Suarez E (2013) Constitutive activation of RANK disrupts mammary cell fate leading to tumorigenesis. Stem Cells 31(9):1954–1965. doi:10.1002/stem.1454
Blake ML, Tometsko M, Miller R, Jones JC, Dougall WC (2014) RANK expression on breast cancer cells promotes skeletal metastasis. Clin Exp Metastasis 31(2):233–245. doi:10.1007/s10585-013-9624-3
Casimiro S, Mohammad KS, Pires R, Tato-Costa J, Alho I, Teixeira R, Carvalho A, Ribeiro S, Lipton A, Guise TA, Costa L (2013) RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS One 8(5):e63153. doi:10.1371/journal.pone.0063153
Owen S, Sanders AJ, Mason MD, Jiang WG (2016) Importance of osteoprotegrin and receptor activator of nuclear factor kappaB in breast cancer response to hepatocyte growth factor and the bone microenvironment in vitro. Int J Oncol 48(3):919–928. doi:10.3892/ijo.2016.3339
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440(7084):692–696. doi:10.1038/nature04524
Gonzalez-Suarez E, Sanz-Moreno A (2016) RANK as a therapeutic target in cancer. FEBS J 283(11):2018–2033. doi:10.1111/febs.13645
Van Poznak C, Cross SS, Saggese M, Hudis C, Panageas KS, Norton L, Coleman RE, Holen I (2006) Expression of osteoprotegerin (OPG), TNF related apoptosis inducing ligand (TRAIL), and receptor activator of nuclear factor kappaB ligand (RANKL) in human breast tumours. J Clin Pathol 59(1):56–63. doi:10.1136/jcp.2005.026534
Rachner TD, Schoppet M, Niebergall U, Hofbauer LC (2008) 17beta-estradiol inhibits osteoprotegerin production by the estrogen receptor-alpha-positive human breast cancer cell line MCF-7. Biochem Biophys Res Commun 368(3):736–741. doi:10.1016/j.bbrc.2008.01.118
Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, Diamond P, Findlay DM, Zannettino AC, Evdokiou A (2011) Anticancer efficacy of Apo2L/TRAIL is retained in the presence of high and biologically active concentrations of osteoprotegerin in vivo. J Bone Miner Res 26(3):630–643. doi:10.1002/jbmr.244
Holen I, Cross SS, Neville-Webbe HL, Cross NA, Balasubramanian SP, Croucher PI, Evans CA, Lippitt JM, Coleman RE, Eaton CL (2005) Osteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo--a role in tumour cell survival? Breast Cancer Res Treat 92(3):207–215. doi:10.1007/s10549-005-2419-8
Rachner TD, Benad P, Rauner M, Goettsch C, Singh SK, Schoppet M, Hofbauer LC (2009) Osteoprotegerin production by breast cancer cells is suppressed by dexamethasone and confers resistance against TRAIL-induced apoptosis. J Cell Biochem 108(1):106–116. doi:10.1002/jcb.22232
Cody JJ, Rivera AA, Lyons GR, Yang SW, Wang M, Sarver DB, Wang D, Selander KS, Kuo HC, Meleth S, Feng X, Siegal GP, Douglas JT (2010) Arming a replicating adenovirus with osteoprotegerin reduces the tumor burden in a murine model of osteolytic bone metastases of breast cancer. Cancer Gene Ther 17(12):893–905. doi:10.1038/cgt.2010.47
Fisher JL, Thomas-Mudge RJ, Elliott J, Hards DK, Sims NA, Slavin J, Martin TJ, Gillespie MT (2006) Osteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeutically. Cancer Res 66(7):3620–3628. doi:10.1158/0008-5472.CAN-05-3119
Fradet A, Sorel H, Bouazza L, Goehrig D, Depalle B, Bellahcene A, Castronovo V, Follet H, Descotes F, Aubin JE, Clezardin P, Bonnelye E (2011) Dual function of ERRalpha in breast cancer and bone metastasis formation: implication of VEGF and osteoprotegerin. Cancer Res 71(17):5728–5738. doi:10.1158/0008-5472.CAN-11-1431
Weichhaus M, Segaran P, Renaud A, Geerts D, Connelly L (2014) Osteoprotegerin expression in triple-negative breast cancer cells promotes metastasis. Cancer Med 3(5):1112–1125. doi:10.1002/cam4.277
Zinonos I, Luo KW, Labrinidis A, Liapis V, Hay S, Panagopoulos V, Denichilo M, Ko CH, Yue GG, Lau CB, Ingman W, Ponomarev V, Atkins GJ, Findlay DM, Zannettino AC, Evdokiou A (2014) Pharmacologic inhibition of bone resorption prevents cancer-induced osteolysis but enhances soft tissue metastasis in a mouse model of osteolytic breast cancer. Int J Oncol 45(2):532–540. doi:10.3892/ijo.2014.2468
Owen S, Ye L, Sanders AJ, Mason MD, Jiang WG (2013) Expression profile of receptor activator of nuclear-kappaB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancer. Anticancer Res 33(1):199–206
Park HS, Lee A, Chae BJ, Bae JS, Song BJ, Jung SS (2014) Expression of receptor activator of nuclear factor kappa-B as a poor prognostic marker in breast cancer. J Surg Oncol 110(7):807–812. doi:10.1002/jso.23737
Sanger N, Ruckhaberle E, Bianchini G, Heinrich T, Milde-Langosch K, Muller V, Rody A, Solomayer EF, Fehm T, Holtrich U, Becker S, Karn T (2014) OPG and PgR show similar cohort specific effects as prognostic factors in ER positive breast cancer. Mol Oncol 8(7):1196–1207. doi:10.1016/j.molonc.2014.04.003
Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A, Ortega C, Porta C, Galluzzo S, Armento G, La Verde N, Caroti C, Treilleux I, Ruggiero A, Perrone G, Addeo R, Clezardin P, Muda AO, Tonini G (2011) Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One 6(4):e19234. doi:10.1371/journal.pone.0019234
Croft M, Benedict CA, Ware CF (2013) Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 12(2):147–168. doi:10.1038/nrd3930
Ye L, Lewis-Russell JM, Kyanaston HG, Jiang WG (2007) Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol 22(10):1129–1147
Davies SR, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG (2008) Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol 7(4):327–338
Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG (2007) The role of growth differentiation factor-9 (GDF-9) and its analog, GDF-9b/BMP-15, in human breast cancer. Ann Surg Oncol 14(7):2159–2166. doi:10.1245/s10434-007-9397-5
Li J, Ye L, Parr C, Douglas-Jones A, Kyanaston HG, Mansel RE, Jiang WG (2009) The aberrant expression of bone morphogenetic protein 12 (BMP-12) in human breast cancer and its potential prognostic value. Gene Ther Mol Biol 13:186–193
Clement JH, Sanger J, Hoffken K (1999) Expression of bone morphogenetic protein 6 in normal mammary tissue and breast cancer cell lines and its regulation by epidermal growth factor. Int J Cancer 80(2):250–256
Reinholz MM, Iturria SJ, Ingle JN, Roche PC (2002) Differential gene expression of TGF-beta family members and osteopontin in breast tumor tissue: analysis by real-time quantitative PCR. Breast Cancer Res Treat 74(3):255–269
Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clezardin P, Papapoulos SE, Cecchini MG, Lowik CW, van der Pluijm G (2007) Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res 67(18):8742–8751. doi:10.1158/0008-5472.CAN-06-2490
Bobinac D, Maric I, Zoricic S, Spanjol J, Dordevic G, Mustac E, Fuckar Z (2005) Expression of bone morphogenetic proteins in human metastatic prostate and breast cancer. Croat Med J 46(3):389–396
Raida M, Clement JH, Ameri K, Han C, Leek RD, Harris AL (2005) Expression of bone morphogenetic protein 2 in breast cancer cells inhibits hypoxic cell death. Int J Oncol 26(6):1465–1470
Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjarvi T, Kallioniemi A (2006) Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer 45(4):411–419
Alarmo EL, Kuukasjarvi T, Karhu R, Kallioniemi A (2007) A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat 103(2):239–246. doi:10.1007/s10549-006-9362-1
Helms MW, Packeisen J, August C, Schittek B, Boecker W, Brandt BH, Buerger H (2005) First evidence supporting a potential role for the BMP/SMAD pathway in the progression of oestrogen receptor-positive breast cancer. J Pathol 206(3):366–376
Bokobza S, Ye L, Kynaston H, Mansel RE, Jiang WG (2009) Reduced expression of BMPR-IB correlates with poor prognosis and increased proliferation of breast cancer cells. Cancer Genom Proteom 6(2): 101–108
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27(49):6322–6333. doi:10.1038/onc.2008.232
Zhang M, Wang Q, Yuan W, Yang S, Wang X, Yan JD, Du J, Yin J, Gao SY, Sun BC, Zhu TH (2007) Epigenetic regulation of bone morphogenetic protein-6 gene expression in breast cancer cells. J Steroid Biochem Mol Biol 105(1–5):91–97. doi:10.1016/j.jsbmb.2007.01.002
Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, Ogura T, Makino H, Doihara H (2008) Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol 199(3):445–455. doi:10.1677/JOE-08-0226
Schwalbe M, Sanger J, Eggers R, Naumann A, Schmidt A, Hoffken K, Clement JH (2003) Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol 23(1):89–95
Alarmo EL, Kallioniemi A (2010) Bone morphogenetic proteins in breast cancer: dual role in tumourigenesis? Endocr Relat Cancer 17(2):R123–R139. doi:10.1677/ERC-09-0273
Julien S, Ivetic A, Grigoriadis A, QiZe D, Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, Tutt A, Taylor-Papadimitriou J, Pinder SE, Burchell JM (2011) Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res 71(24):7683–7693. doi:10.1158/0008-5472.CAN-11-1139
Yamamoto T, Saatcioglu F, Matsuda T (2002) Cross-talk between bone morphogenic proteins and estrogen receptor signaling. Endocrinology 143(7):2635–2642. doi:10.1210/endo.143.7.8877
Wu L, Wu Y, Gathings B, Wan M, Li X, Grizzle W, Liu Z, Lu C, Mao Z, Cao X (2003) Smad4 as a transcription corepressor for estrogen receptor alpha. J Biol Chem 278(17):15192–15200. doi:10.1074/jbc.M212332200
Ghosh-Choudhury N, Ghosh-Choudhury G, Celeste A, Ghosh PM, Moyer M, Abboud SL, Kreisberg J (2000) Bone morphogenetic protein-2 induces cyclin kinase inhibitor p21 and hypophosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cells. Biochim Biophys Acta 1497(2):186–196
Ong DB, Colley SM, Norman MR, Kitazawa S, Tobias JH (2004) Transcriptional regulation of a BMP-6 promoter by estrogen receptor alpha. J Bone Miner Res 19(3):447–454. doi:10.1359/JBMR.0301249
Feng J, Li L, Zhang N, Liu J, Zhang L, Gao H, Wang G, Li Y, Zhang Y, Li X, Liu D, Lu J, Huang B (2016) Androgen and AR contribute to breast cancer development and metastasis: an insight of mechanisms. Oncogene. doi:10.1038/onc.2016.432
Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D'Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gomez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16(1):R7. doi:10.1186/bcr3599
Montesano R, Sarkozi R, Schramek H (2008) Bone morphogenetic protein-4 strongly potentiates growth factor-induced proliferation of mammary epithelial cells. Biochem Biophys Res Commun 374(1):164–168. doi:10.1016/j.bbrc.2008.07.007
Laulan NB, St-Pierre Y (2015) Bone morphogenetic protein 4 (BMP-4) and epidermal growth factor (EGF) inhibit metalloproteinase-9 (MMP-9) expression in cancer cells. Oncoscience 2(3):309–316. Doi:10.18632/oncoscience.144
Kretzschmar M, Doody J, Massague J (1997) Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature 389(6651):618–622. doi:10.1038/39348
Guo X, Wang XF (2009) Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res 19(1):71–88. doi:10.1038/cr.2008.302
Ghosh Choudhury G, Jin DC, Kim Y, Celeste A, Ghosh-Choudhury N, Abboud HE (1999) Bone morphogenetic protein-2 inhibits MAPK-dependent elk-1 transactivation and DNA synthesis induced by EGF in mesangial cells. Biochem Biophys Res Commun 258(2):490–496
Ren W, Liu Y, Wan S, Fei C, Wang W, Chen Y, Zhang Z, Wang T, Wang J, Zhou L, Weng Y, He T, Zhang Y (2014) BMP9 inhibits proliferation and metastasis of HER2-positive SK-BR-3 breast cancer cells through ERK1/2 and PI3K/AKT pathways. PLoS One 9(5):e96816. doi:10.1371/journal.pone.0096816
Clausen KA, Blish KR, Birse CE, Triplette MA, Kute TE, Russell GB, D'Agostino RB Jr, Miller LD, Torti FM, Torti SV (2011) SOSTDC1 differentially modulates Smad and beta-catenin activation and is down-regulated in breast cancer. Breast Cancer Res Treat 129(3):737–746. doi:10.1007/s10549-010-1261-9
Nacamuli RP, Fong KD, Lenton KA, Song HM, Fang TD, Salim A, Longaker MT (2005) Expression and possible mechanisms of regulation of BMP3 in rat cranial sutures. Plast Reconstr Surg 116(5):1353–1362
Hallahan AR, Pritchard JI, Chandraratna RA, Ellenbogen RG, Geyer JR, Overland RP, Strand AD, Tapscott SJ, Olson JM (2003) BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med 9(8):1033–1038
van der Poel HG, Hanrahan C, Zhong H, Simons JW (2003) Rapamycin induces Smad activity in prostate cancer cell lines. Urol Res 30(6):380–386
Ye L, Lewis-Russell JM, Sanders AJ, Kynaston H, Jiang WG (2008) HGF/SF up-regulates the expression of bone morphogenetic protein 7 in prostate cancer cells. Urol Oncol 26(2):190–197. doi:10.1016/j.urolonc.2007.03.027
Ye L, Lewis-Russell JM, Davies G, Sanders AJ, Kynaston H, Jiang WG (2007) Hepatocyte growth factor up-regulates the expression of the bone morphogenetic protein (BMP) receptors, BMPR-IB and BMPR-II, in human prostate cancer cells. Int J Oncol 30(2):521–529
Ren W, Sun X, Wang K, Feng H, Liu Y, Fei C, Wan S, Wang W, Luo J, Shi Q, Tang M, Zuo G, Weng Y, He T, Zhang Y (2014) BMP9 inhibits the bone metastasis of breast cancer cells by downregulating CCN2 (connective tissue growth factor, CTGF) expression. Mol Biol Rep 41(3):1373–1383. doi:10.1007/s11033-013-2982-8
Wang K, Feng H, Ren W, Sun X, Luo J, Tang M, Zhou L, Weng Y, He TC, Zhang Y (2011) BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J Cancer Res Clin Oncol 137(11):1687–1696. doi:10.1007/s00432-011-1047-4
Moreau JE, Anderson K, Mauney JR, Nguyen T, Kaplan DL, Rosenblatt M (2007) Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res 67 (21):10304–10308. doi:10.1158/0008-5472.CAN-07-2483
Kapoor P, Suva LJ, Welch DR, Donahue HJ (2008) Osteoprotegerin and the bone homing and colonization potential of breast cancer cells. J Cell Biochem 103(1):30–41. doi:10.1002/jcb.21382
Ibrahim T, Leong I, Sanchez-Sweatman O, Khokha R, Sodek J, Tenenbaum HC, Ganss B, Cheifetz S (2000) Expression of bone sialoprotein and osteopontin in breast cancer bone metastases. Clin Exp Metastasis 18(3):253–260
Tan CC, Li GX, Tan LD, Du X, Li XQ, He R, Wang QS, Feng YM (2016) Breast cancer cells obtain an osteomimetic feature via epithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction. Oncotarget. doi:10.18632/oncotarget.12939
Carreira AC, Alves GG, Zambuzzi WF, Sogayar MC, Granjeiro JM (2014) Bone morphogenetic proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys 561:64–73. doi:10.1016/j.abb.2014.07.011
Rucci N, Teti A (2010) Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2:907–915
van den Wijngaard A, Mulder WR, Dijkema R, Boersma CJ, Mosselman S, van Zoelen EJ, Olijve W (2000) Antiestrogens specifically up-regulate bone morphogenetic protein-4 promoter activity in human osteoblastic cells. Mol Endocrinol 14(5):623–633
Matsumoto Y, Otsuka F, Takano-Narazaki M, Katsuyama T, Nakamura E, Tsukamoto N, Inagaki K, Sada KE, Makino H (2013) Estrogen facilitates osteoblast differentiation by upregulating bone morphogenetic protein-4 signaling. Steroids 78(5):513–520. doi:10.1016/j.steroids.2013.02.011
Bunyaratavej P, Hullinger TG, Somerman MJ (2000) Bone morphogenetic proteins secreted by breast cancer cells upregulate bone sialoprotein expression in preosteoblast cells. Exp Cell Res 260(2):324–333. doi:10.1006/excr.2000.5019
Tarragona M, Pavlovic M, Arnal-Estape A, Urosevic J, Morales M, Guiu M, Planet E, Gonzalez-Suarez E, Gomis RR (2012) Identification of NOG as a specific breast cancer bone metastasis-supporting gene. J Biol Chem 287(25):21346–21355. doi:10.1074/jbc.M112.355834
Mock K, Preca BT, Brummer T, Brabletz S, Stemmler MP, Brabletz T (2015) The EMT-activator ZEB1 induces bone metastasis associated genes including BMP-inhibitors. Oncotarget
Schwaninger R, Rentsch CA, Wetterwald A, van der Horst G, van Bezooijen RL, van der Pluijm G, Lowik CW, Ackermann K, Pyerin W, Hamdy FC, Thalmann GN, Cecchini MG (2007) Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am J Pathol 170 (1):160–175. doi:10.2353/ajpath.2007.051276
Ye L, Lewis-Russell JM, Kynaston H, Jiang WG (2007) Endogenous BMP-7 controls the motility of prostate cancer cells through regulation of BMP antagonists. J Urol 178(3) Pt 1: 1086–1091
Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM (2015) Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther 9:4479–4499. doi:10.2147/DDDT.S86621
Hawinkels LJ, de Vinuesa AG, Paauwe M, Kruithof-de Julio M, Wiercinska E, Pardali E, Mezzanotte L, Keereweer S, Braumuller TM, Heijkants RC, Jonkers J, Lowik CW, Goumans MJ, ten Hagen TL, ten Dijke P (2016) Activin receptor-like kinase 1 ligand trap reduces microvascular density and improves chemotherapy efficiency to various solid tumors. Clin Cancer Res 22(1):96–106. doi:10.1158/1078-0432.CCR-15-0743
Cunha SI, Pietras K (2011) ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood 117(26):6999–7006. doi:10.1182/blood-2011-01-330142
Chen G, Deng C, Li YP (2012) TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8(2):272–288. doi:10.7150/ijbs.2929
Suvannasankha A, Chirgwin JM (2014) Role of bone-anabolic agents in the treatment of breast cancer bone metastases. Breast Cancer Res 16(6):484. doi:10.1186/s13058-014-0484-9
Zhang L, Ye Y, Long X, Xiao P, Ren X, Yu J (2016) BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget 7(47):78206–78218. doi:10.18632/oncotarget.12151
Royce ME, Osman D (2015) Everolimus in the treatment of metastatic breast cancer. Breast Cancer (Auckl) 9:73–79. doi:10.4137/BCBCR.S29268
Bowen MA, Patel DD, Li X, Modrell B, Malacko AR, Wang WC, Marquardt H, Neubauer M, Pesando JM, Francke U et al (1995) Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med 181(6):2213–2220
Degen WG, van Kempen LC, Gijzen EG, van Groningen JJ, van Kooyk Y, Bloemers HP, Swart GW (1998) MEMD, a new cell adhesion molecule in metastasizing human melanoma cell lines, is identical to ALCAM (activated leukocyte cell adhesion molecule). Am J Pathol 152(3):805–813
Swart GW (2002) Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol 81(6):313–321. doi:10.1078/0171-9335-00256
van Kempen LC, Nelissen JM, Degen WG, Torensma R, Weidle UH, Bloemers HP, Figdor CG, Swart GW (2001) Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)-ALCAM interaction. J Biol Chem 276(28):25783–25790. doi:10.1074/jbc.M011272200
Micciche F, Da Riva L, Fabbi M, Pilotti S, Mondellini P, Ferrini S, Canevari S, Pierotti MA, Bongarzone I (2011) Activated leukocyte cell adhesion molecule expression and shedding in thyroid tumors. PLoS One 6(2):e17141. doi:10.1371/journal.pone.0017141
Kulasingam V, Zheng Y, Soosaipillai A, Leon AE, Gion M, Diamandis EP (2009) Activated leukocyte cell adhesion molecule: a novel biomarker for breast cancer. Int J Cancer 125(1):9–14. doi:10.1002/ijc.24292
Witzel I, Schroder C, Muller V, Zander H, Tachezy M, Ihnen M, Janicke F, Milde-Langosch K (2012) Detection of activated leukocyte cell adhesion molecule in the serum of breast cancer patients and implications for prognosis. Oncology 82(6):305–312. doi:10.1159/000337222
Al-Shehri FS, Abd El Azeem EM (2015) Activated leukocyte cell adhesion molecule (ALCAM) in Saudi breast cancer patients as prognostic and predictive indicator. Breast Cancer (Auckl) 9:81–86. doi:10.4137/BCBCR.S25563
Jezierska A, Matysiak W, Motyl T (2006) ALCAM/CD166 protects breast cancer cells against apoptosis and autophagy. Med Sci Monit 12(8):BR263–BR273
Hein S, Muller V, Kohler N, Wikman H, Krenkel S, Streichert T, Schweizer M, Riethdorf S, Assmann V, Ihnen M, Beck K, Issa R, Janicke F, Pantel K, Milde-Langosch K (2011) Biologic role of activated leukocyte cell adhesion molecule overexpression in breast cancer cell lines and clinical tumor tissue. Breast Cancer Res Treat 129(2):347–360. doi:10.1007/s10549-010-1219-y
Wiiger MT, Gehrken HB, Fodstad O, Maelandsmo GM, Andersson Y (2010) A novel human recombinant single-chain antibody targeting CD166/ALCAM inhibits cancer cell invasion in vitro and in vivo tumour growth. Cancer Immunol Immunother 59(11):1665–1674. doi:10.1007/s00262-010-0892-3
King JA, Tan F, Mbeunkui F, Chambers Z, Cantrell S, Chen H, Alvarez D, Shevde LA, Ofori-Acquah SF (2010) Mechanisms of transcriptional regulation and prognostic significance of activated leukocyte cell adhesion molecule in cancer. Mol Cancer 9:266. doi:10.1186/1476-4598-9-266
Akman HB, Selcuklu SD, Donoghue MT, Akhavantabasi S, Sapmaz A, Spillane C, Yakicier MC, Erson-Bensan AE (2015) ALCAM is indirectly modulated by miR-125b in MCF7 cells. Tumour Biol 36(5):3511–3520. doi:10.1007/s13277-014-2987-5
Orso F, Quirico L, Virga F, Penna E, Dettori D, Cimino D, Coppo R, Grassi E, Elia AR, Brusa D, Deaglio S, Brizzi MF, Stadler MB, Provero P, Caselle M, Taverna D (2016) miR-214 and miR-148b targeting inhibits dissemination of melanoma and breast cancer. Cancer Res 76(17):5151–5162. doi:10.1158/0008-5472.CAN-15-1322
Davies SR, Dent C, Watkins G, King JA, Mokbel K, Jiang WG (2008) Expression of the cell to cell adhesion molecule, ALCAM, in breast cancer patients and the potential link with skeletal metastasis. Oncol Rep 19(2):555–561
Davies S, Jiang WG (2010) ALCAM, activated leukocyte cell adhesion molecule, influences the aggressive nature of breast cancer cells, a potential connection to bone metastasis. Anticancer Res 30(4):1163–1168
Gemoll T, Epping F, Heinrich L, Fritzsche B, Roblick UJ, Szymczak S, Hartwig S, Depping R, Bruch HP, Thorns C, Lehr S, Paech A, Habermann JK (2015) Increased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignancies. Oncotarget 6 (18):16517–16526. doi:10.18632/oncotarget.4140
Vishal M, Swetha R, Thejaswini G, Arumugam B, Selvamurugan N (2017) Role of Runx2 in breast cancer-mediated bone metastasis. Int J Biol Macromol 99:608–614. doi:10.1016/j.ijbiomac.2017.03.021
Hansen AG, Arnold SA, Jiang M, Palmer TD, Ketova T, Merkel A, Pickup M, Samaras S, Shyr Y, Moses HL, Hayward SW, Sterling JA, Zijlstra A (2014) ALCAM/CD166 is a TGF-beta-responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res 74(5):1404–1415. doi:10.1158/0008-5472.CAN-13-1296
King JA, Ofori-Acquah SF, Stevens T, Al-Mehdi AB, Fodstad O, Jiang WG (2004) Activated leukocyte cell adhesion molecule in breast cancer: prognostic indicator. Breast Cancer Res 6(5):R478–R487. doi:10.1186/bcr815
Burkhardt M, Mayordomo E, Winzer KJ, Fritzsche F, Gansukh T, Pahl S, Weichert W, Denkert C, Guski H, Dietel M, Kristiansen G (2006) Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. J Clin Pathol 59(4):403–409. doi:10.1136/jcp.2005.028209
Jezierska A, Olszewski WP, Pietruszkiewicz J, Olszewski W, Matysiak W, Motyl T (2006) Activated leukocyte cell adhesion molecule (ALCAM) is associated with suppression of breast cancer cells invasion. Med Sci Monit 12(7):BR245–BR256
Ihnen M, Muller V, Wirtz RM, Schroder C, Krenkel S, Witzel I, Lisboa BW, Janicke F, Milde-Langosch K (2008) Predictive impact of activated leukocyte cell adhesion molecule (ALCAM/CD166) in breast cancer. Breast Cancer Res Treat 112(3):419–427. doi:10.1007/s10549-007-9879-y
Ihnen M, Kohler N, Kersten JF, Milde-Langosch K, Beck K, Holler S, Muller V, Witzel I, Janicke F, Kilic E (2010) Expression levels of activated leukocyte cell adhesion molecule (ALCAM/CD166) in primary breast carcinoma and distant breast cancer metastases. Dis Markers 28(2):71–78. doi:10.3233/DMA-2010-0685
Ihnen M, Wirtz RM, Kalogeras KT, Milde-Langosch K, Schmidt M, Witzel I, Eleftheraki AG, Papadimitriou C, Janicke F, Briassoulis E, Pectasides D, Rody A, Fountzilas G, Muller V (2010) Combination of osteopontin and activated leukocyte cell adhesion molecule as potent prognostic discriminators in HER2- and ER-negative breast cancer. Br J Cancer 103(7):1048–1056. doi:10.1038/sj.bjc.6605840
Zhou P, Du LF, Lv GQ, Yu XM, Gu YL, Li JP, Zhang C (2011) Functional polymorphisms in CD166/ALCAM gene associated with increased risk for breast cancer in a Chinese population. Breast Cancer Res Treat 128(2):527–534. doi:10.1007/s10549-011-1365-x
Ihnen M, Kilic E, Kohler N, Loning T, Witzel I, Hagel C, Holler S, Kersten JF, Muller V, Janicke F, Milde-Langosch K (2011) Protein expression analysis of ALCAM and CEACAM6 in breast cancer metastases reveals significantly increased ALCAM expression in metastases of the skin. J Clin Pathol 64(2):146–152. doi:10.1136/jcp.2010.082602
Piao D, Jiang T, Liu G, Wang B, Xu J, Zhu A (2012) Clinical implications of activated leukocyte cell adhesion molecule expression in breast cancer. Mol Biol Rep 39(1):661–668. doi:10.1007/s11033-011-0783-5
Varadi V, Bevier M, Grzybowska E, Johansson R, Enquist-Olsson K, Henriksson R, Butkiewicz D, Pamula-Pilat J, Tecza K, Hemminki K, Lenner P, Forsti A (2012) Genetic variation in ALCAM and other chromosomal instability genes in breast cancer survival. Breast Cancer Res Treat 131(1):311–319. doi:10.1007/s10549-011-1765-y
Tan F, Mosunjac M, Adams AL, Adade B, Taye O, Hu Y, Rizzo M, Ofori-Acquah SF (2014) Enhanced down-regulation of ALCAM/CD166 in African-American breast cancer. BMC Cancer 14:715. doi:10.1186/1471-2407-14-715
Burandt E, Bari Noubar T, Lebeau A, Minner S, Burdelski C, Janicke F, Muller V, Terracciano L, Simon R, Sauter G, Wilczak W, Lebok P (2014) Loss of ALCAM expression is linked to adverse phenotype and poor prognosis in breast cancer: a TMA-based immunohistochemical study on 2,197 breast cancer patients. Oncol Rep 32(6):2628–2634. doi:10.3892/or.2014.3523
Chen MJ, Cheng YM, Chen CC, Chen YC, Shen CJ (2017) MiR-148a and miR-152 reduce tamoxifen resistance in ER+ breast cancer via downregulating ALCAM. Biochem Biophys Res Commun 483(2):840–846. doi:10.1016/j.bbrc.2017.01.012
Acknowledgements
Authors would like to thank Cancer Research Wales and the Life Sciences Research Network Wales for supporting their work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Owen, S., Zabkiewicz, C., Ye, L., Sanders, A.J., Gong, C., Jiang, W.G. (2017). Key Factors in Breast Cancer Dissemination and Establishment at the Bone: Past, Present and Future Perspectives. In: Song, E., Hu, H. (eds) Translational Research in Breast Cancer. Advances in Experimental Medicine and Biology, vol 1026. Springer, Singapore. https://doi.org/10.1007/978-981-10-6020-5_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-6020-5_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6019-9
Online ISBN: 978-981-10-6020-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)