Abstract
Radiotherapy for brain metastases has evolved tremendously over the past four decades, allowing for improved intracranial control of disease with reduced neurotoxicity. The main technological advance was provided by volumetric modulated arc therapy (VMAT), a computer-controlled delivery method that has opened the door for single-isocenter multi-metastases stereotactic radiosurgery (SRS) and hippocampal avoidance whole brain radiation therapy (HA-WBRT). Other notable advances have occurred in the combination of immune checkpoint inhibitors (ICI) and radiosurgery. When these two modalities are combined in the proper sequence (within 30 days from each other), it provides promising results in the treatment of intracranial metastases from melanoma. There is emerging evidence of a synergistic interaction between ICI and SRS, providing better intracranial tumor control and lengthening the survival of patients afflicted by this common complication of cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are a common complication of cancer, with incidence ranging from 10 to 30% of all invasive cancers [1, 2]. It has been reported that death from neurological causes can occur in up to 44% of patients with brain metastases [3]. Sequential studies performed over the past 3 decades have also shown that appropriate treatment with radiotherapy can increase time to neurological death, improve functional decline and overall survival for these patients [3,4,5,6].
Current treatment of brain metastases varies depending on several considerations: quantity and volume of intracranial disease, resectability of the lesion(s), age and performance status of the patient, histology, extent of extra-cranial disease, among other factors. These factors eventually dictate the role and the combination of modalities applied (surgery, radiation, and systemic therapy) to achieve the desired clinical benefit.
Technical innovations in radiation therapy
Several Radiation Therapy Oncology Group (RTOG) studies performed in the 1980s established the palliative role and survival benefits of Whole Brain Radiation Therapy (WBRT) [7]. At first, simple opposed-lateral fields through two-dimensional radiation were used. With the advent of computed-tomography (CT) simulation in the 1990s, field-in-field techniques and 3D-conformal radiotherapy facilitated the reduction of hotspots which led to improved dose homogeneity within the brain and fewer side effects (Fig. 1A-B).
A Right lateral ‘beams-eye view’ in a 2D WBRT plan. The (green in online version) arrow represents the horizontal multileaves, which allow the user to reduce dose to critical structures (anterior orbit and oral cavity). B Isodose display of a WBRT planned using 3D-planning tools to deliver a more homogenous dose to the brain. C Dose distribution for HA-WBRT using VMAT, which allowes sparing of specific targets, in this case the hippocampi; the doses received by the avoidance structures are shown on the far right. (Color figure online)
Over time, studies addressing quality of life, established that radiation to the entirety of healthy brain parenchyma contributes to neurocognitive decline. Strategies to mitigate these effects have been evaluated in randomized clinical trials (RCTs); one such study, RTOG 0614, showed modest neurocognitive protection with Memantine, a drug used to treat symptoms of confusion associated with Alzheimer's disease [8]. Furthermore, with the wide adoption of intensity modulated radiation (IMRT), a phase II trial of hippocampal avoidance WBRT (RTOG 0933) was performed. This trial showed that the hippocampi can be spared through the use of IMRT (Fig. 1C) to create hippocampal avoidance WBRT (HA-WBRT), which resulted in similar intracranial control rates but improved cognition compared to historical WBRT trials [9]. HA-WBRT was then compared head-to-head with standard WBRT in NRG CC 001, a phase III trial, with both arms receiving Memantine; the results of this recently published study showed that HA-WBRT with Memantine results in a lower rate of cognitive failure, particularly executive functioning at 4 months, immediate recall and recognition at 6 months, as well as improved speaking, memory, fatigue, and symptom interference with activities of daily living at 6 months [10].
The management of patient with brain metastases can be simplified by breaking down this patient population in three categories: single metastasis, oligo-metastases (2–4 lesions) and multiple metastases (5 lesions or greater). Maximum safe resection has been established as the mainstay of treatment for single brain metastases when the lesion is surgically accessible and the patient has no contraindication to surgery. Three separate phase III trials comparing WBRT alone vs WBRT following resection showed that the addition of resection to WBRT led to improved functional independence and OS compared to WBRT alone but should be limited to well-performing patients [11,12,13]. Resection also provides the benefit of quicker palliation of symptoms compared to radiation.
With the adoption of Stereotactic Radiosurgery (SRS), a specialized form of radiation delivering ablative doses of radiation, the role of WBRT in patients with a single metastasis was re-evaluated; studies have shown that following either surgical resection or primary SRS treatment of a solitary metastasis, addition of WBRT doesn’t necessarily improve overall survival (OS), although WBRT does improve regional intracranial control, and time to neurological death [3, 5, 6].
As such, WBRT is no longer recommended for single metastases that are amenable to surgical resection or treated with SRS, although there is evidence from a RTOG randomized clinical trial of a small clinical benefit in OS for high performance patients (RPA class 1) with a single metastasis when treated with WBRT and an SRS boost [4].
SRS was developed through improvements in the understanding of physics as well as technology that allowed better planning, localization, immobilization, and delivery of high-dose precise radiation. SRS delivers an ablative dose of radiation usually in a single fraction resulting in very high control rates of the treated lesions, through both direct cell-kill, and indirectly through vascular damage and immunologic response [14]. SRS has allowed for a safe and effective option to complement, or in many cases, serve as an alternative to surgery or postoperative WBRT [15]. Randomized clinical trials comparing SRS to surgical resection have not been done, but local recurrence rates after SRS is comparable to surgical resection. For patients with oligometastatic status (2–4 metastases), SRS alone has been shown to be equivalent to WBRT for survival benefit, with the added benefit that it does a better job in preserving neurocognitive functions [16], and there is emerging data to suggest that patients with up to 10 metastases can still benefit from multiple SRS over WBRT [17].
SRS treatment has thus become very popular over the past 20 years, primarily because many brain metastases are small and relatively spherical, and therefore they can be effectively treated with SRS using cones, which are cylindrical collimators of various and pre-defined diameters. However, the utility of cones decreases as the lesion becomes larger or less spherical in shape [18]. For larger and irregular lesions, 3D planning has allowed for more complex plans, particularly using dynamic conformal arc therapy (DCAT), which uses the multi-leaf collimators (MLCs) of the Linac to conform to the shape of the treatment volume at each angle as the gantry rotates around the patient [19]. With the advent of IMRT, reverse treatment planning allowed the user to achieve an even more conformal treatment, by providing a desired target (tumor) dose and avoidance goals; the planning computer, then, individually modulates each beam, allowing the user to choose an optimal plan. The most sophisticated form of IMRT is volumetric modulated arc therapy (VMAT), which like DCAT, involves continuously moving small beams of radiation delivered through multiple revolutions of the gantry; each beam is individually modulated to change the intensity of the beam as the gantry rotates around the head of the patient. The net effect of this modulated beam is a better conformality of the overall radiation dose around the irregular tumor resulting in better sparing of the normal adjacent brain. Thus, VMAT allows for conformal dosing to irregularly shaped target volumes such as non-spherical larger brain tumors, and also allows the delivery HA-WBRT, sparing critical structures of the brain [9, 10, 20].
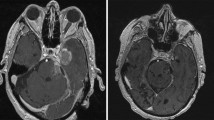
VMAT has also opened the door for single-isocenter treatment of multiple brain metastases in a single radiotherapy session of 30–40 min. Historically, the treatment of each metastasis required a separate isocenter, and subsequently, the repositioning of the patient which in turn required confirmation using image-guidance; thus, treatment of multiple metastases could take hours, resulting in a clinical burden for resource utilization and patient comfort. Through VMAT, multiple lesions can now be treated with a single isocenter in a matter of minutes (Fig. 2) [21]. One minor drawback of VMAT is that there is a higher amount of scatter radiation, but it is minimal and clinically insignificant (Fig. 3).
Planning view of reconstructed head in a thermoplastic SRS mask as part of a VMAT single-isocenter multi-metastases brain plan. Right-sided (orange in online version) and left-sided (blue in online version) arrows represent the 0- and 180-degree arcs of VMAT; other arcs at 30-, 60-, 90, 120-, and 150- degrees, are represented by the thin lines. This patient had metastatic adenocarcinoma of the lung with 9 brain metastases, all treated with a single-isocenter VMAT plan. Each individual lesion was < 2 cm and received 21 Gy in a single fraction. Isodose line figures can be seen in Fig. 3. (Color figure online)
Isodose line distributions (key at far right, color-coded version online) of a single-isocenter multi-metastases VMAT plan, with axial, sagittal, and coronal views. Nine metastases were treated simultaneously, but not all lesions are in view. The outermost (light blue in online version) isodose line represents 525 cGy, the amount of radiation that “spills” in the normal adjacent brain. (Color figure online)
Discussion of the current approaches to treatment of brain metastases with radiation warrants a review of the hypothesis behind cancer spread. Classically, the Halstead hypothesis promoted a continuous, stepwise spread of cancer requiring radical en bloc surgeries. The Fisher hypothesis that followed the Halstead provided an alternative explanation for cancer metastases, that systemic dissemination occurs early on, and therefore treatment should focus on systemic control of microscopic metastases as opposed to local therapy. More recently, the Hellman and Weichselbaum hypothesis proposed the concept of oligometastatic cancer, an intermediate between localized and widely disseminated cancer [22]. A true oligometastatic state occurs when an indolent cancer biology leads to a limited number of macroscopic metastases without extensive background microscopic metastases [23]. Thus, oligometastatic state is a disease concept defined by an operational state in which the patient has a limited number of systemic metastatic tumors for which local ablative therapy such as SRS or Stereotactic Body Radiotherapy (SBRT) could be curative. In this context, treating brain metastases with high dose SRS or SBRT is likely to provide the patient not only with a chance to increase local control (over the low dose radiation approach provided by WBRT) but also potentially give the patient a chance for cure. Although constantly evolving, there already exist some evidence that in select patients, intracranial tumor control can lead to an overall survival benefit following ablative radiation techniques described above [4, 15].
In summary, the current paradigm of treating brain metastases has evolved tremendously from WBRT to HA-WBRT to SRS over the past decade and still depends on numerous factors, but the process is mainly dependent on resectability and number of lesions. Single brain metastases are resected if feasible, followed by an SRS or fractionated SRS (fSRS) boost to the resection cavity. For patients presenting with oligometastatic disease (2–4 lesions), the treatment paradigm is to treat with SRS/fSRS each lesion using VMAT. Finally, patients presenting with 5 or more metastases are commonly treated with WBRT or HA-WBRT and Memantine. In this paradigm, lesions that do not respond completely to WBRT/HA-WBRT (or that continue to grow or recur later) can be boosted with SRS/fSRS (salvage therapy). A recently launched phase III clinical trial, the CCTG CE.7, is currently accruing patients comparing single fraction SRS (doses: 18-22 Gy depending on size) vs. HA-WBRT 30 Gy/10fx for patients with 5–15 brain metastases [24]. The primary objective of this trial is to compare the overall survival as well as the neurocognitive progression-free survival for patients harboring 5–15 lesions.
Synergism between immune checkpoint inhibitors and SRS
Historically, the role of systemic therapy for brain metastases has been limited due to the poor penetration of most chemotherapeutic drugs across the blood–brain barrier (BBB). Recently, however, there has been growing evidence that immune checkpoint inhibitors (ICI) can achieve durable responses even for brain metastases [25]. Although these agents are unable to cross the BBB, due their large molecular size, it is believed that activated tumor-specific T-cells can do so [26]. There is good evidence from numerous retrospective studies that SRS and ICI interact synergistically; the main mechanism of action responsible for such positive outcomes appears to be activation of the immune-cytokine cascade by high dose ablative radiation [14]. More specifically, extensive tumor cell injury and death produced by high dose radiation induces a massive release of tumor-specific antigens, as well as pro-inflammatory and pro-oxidant cytokines. Radiation also promotes antigen presentation through activation and maturation of dendritic cells, as well as an increase in effector T cell traffic to tumor by increasing major histocompatibility complex, adhesion molecules, costimulatory molecules, heat shock proteins, and death receptors expression [27]. The ultimate downstream effect is a tumor-specific heightened immune response [28, 29]. This benefit can be further magnified through the abscopal effect, in which high-dose radiation causes regression not only of the radiation-targeted tumor, but also of distant sites of cancer that were not targeted by radiation, presumably through a systemic immune-mediated response to the radiation [30]. Here, the effects of high-dose radiation-induced immune response are amplified through immunotherapy, which targets and upregulates antigen processing, and generation and trafficking of effector T cells. The proposed synergistic effects of high-dose radiation and immunotherapy have been demonstrated in several recent retrospective studies [31]. In one such study, Skrepnik et al. performed a retrospective review of melanoma brain metastases treated with SRS and ipilimumab and found that such patients had a very long median survival compared to historical controls. Furthermore, they found that there is an optimal window of time for synergy: patients treated with SRS and Ipilimumab within a 30 days window of time had a superior time to CNS progression, and regional brain control (Fig. 4) [32]. Similarly, a more recent meta-analysis by Lehrer et al. of published studies of melanoma brain metastases treated with SRS and ICI, found that concurrent SRS and immunotherapy had a higher 1-year OS, local control, and regional brain control than non-concurrent therapy [33]. While concurrent SRS and immunotherapy appears to be very effective in controlling the disease, there is a growing concern of radionecrosis (RN) given the synergy. Skrepnik et al. found an overall RN rate of 20.7% but a symptomatic RN rate of only 5% that required treatment with Avastin. Similarly, Lehrer et al. found the rate of any RN to be 0–20.7% across all studies, with a combined rate of 5.3%. Multiple prospective trials are ongoing to determine the efficacy [34], optimal fractionation [35], timing [36], and toxicity [37] of combined stereotactic radiation and immunotherapy.
A Overall survival of melanoma brain metastases patients treated with a combination of SRS and ICI leading to high median survival (median OS = 35.8 months). B Time to progression in the CNS by timing of SRS delivery to administration of ICI; delivery up to 30 days from each other leads to a statistically better time to CNS progression (p = 0.02), suggesting synergism between the 2 modalities
Conclusions
The treatment of brain metastases has evolved greatly over the past 30 years and continues to do so. Improved surgical and radiation techniques have increased local and intracranial control, while reducing treatment-related morbidity and mortality. The evidence behind the interplay between radiation, particularly SRS, and immunotherapy is becoming stronger. Furthermore, as systemic therapy continues to improve leading to improved control of extracranial disease, the importance of intracranial control and neurological survival also increases.
References
Nichols EM, Patchell RA, Regine WF, Kowk Y (2013) Palliation of brain and spinal cord metastases. In: Halperin EC, Wazer DE, Perez CA, Brady LW (eds) Perez and brady’s principles and practice of radiation oncology, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1765–1772
Usuki KY, Milano MT, David M, Okunieff P (2015) Metastatic disease: bone, spinal cord, brain, liver, and lung. In: Gunderson Leonard L, Tepper Joel E (eds) Clinical radiation oncology, 4th edn. Elsevier Health Sciences, Philadelphia, pp 443–445
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 280(17):1485–9
Andrews DW, Scott C, Sperduto P, Curran W et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. The Lancet 363(9422):1665–1672
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295(21):2483–2491
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Hassel MB et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29(2):134
Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, Hendrickson FR et al (1980) The palliation of brain metastases final results of the first two studies by the radiation therapy oncology group. Int J Radiat Oncol *Biol*Phys 6(1):1–9. https://doi.org/10.1016/0360-3016(80)90195-9
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Kavadi V et al (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 15(10):1429–1437
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Konski AA et al (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32(34):3810
Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, Grosshans D et al (2020) Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol 38(10):1019–1029
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322(8):494–500
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Brand R et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery. Ann Neurol 33(6):583–590
Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, Levine M et al (1996) A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 78(7):1470–1476
Sperduto PW, Song CW (2016) Radiobiology of stereotactic radiosurgery and stereotactic ablative radiotherapy. In: Khan FM, Gibbons JP, Sperduto PW (eds) Khan’s treatment planning in radiation oncology, 4th edn. Wolters Kluwer, Philadelphia, pp 355–359
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, McGovern S et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18(8):1040–1048
Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M, Chang EL et al (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol*Biol*Phys 91(4):710–717
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Nagano O et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15(4):387–395
Duggan DM, Coffey C (1998) Small photon field dosimetry for stereotactic radiosurgery. Med Dosim 23(3):153–159
Solberg TD, Boedeker KL, Fogg R, Selch MT, DeSalles AA (2001) Dynamic arc radiosurgery field shaping: a comparison with static field conformal and noncoplanar circular arcs. Int J Radiat Oncol*Biol*Phys 49(5):1481–1491
Unkelbach J (2016) Intensity-modulated radiation therapy: photons. In: Khan FM, Gibbons JP, Sperduto PW (eds) Khan’s treatment planning in radiation oncology, 4th edn. Wolters Kluwer, Philadelphia, pp 122–149
Clark GM, Popple RA, Young PE, Fiveash JB (2010) Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol*Biol*Phys 76(1):296–302
Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13(1):8–10
Uppal A, Ferguson MK, Posner MC, Hellman S, Khodarev NN, Weichselbaum RR (2014) Towards a molecular basis of oligometastatic disease: potential role of micro-RNAs. Clin Exp Metas 31(6):735–748
Hu X, Hui Y, Zhenget Y et al (2020) Immune checkpoint inhibitors and survival outcomes in brain metastases: a time series-based meta-analysis. Front Oncol 10:2180. https://doi.org/10.3389/fonc.2020.564382
Wilson EH, Weninger W, Hunter CA (2010) Trafficking of immune cells in the central nervous system. J Clin Investig 120(5):1368–1379
Finkelstein SE, Timmerman R, McBride WH, Schaue D, Hoffe SE, Mantz CA, Wilson GD (2011) The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol 2011:439752. https://doi.org/10.1155/2011/439752
Blank C, Gajewski TF, Mackensen A (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54(4):307–314
Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, Urba WJ et al (2012) Phase 1 study of stereotactic body radiotherapy and interleukin-2–tumor and immunological responses. Sci Transl Med 4(137):13774
Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Sedrak C et al (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366(10):925–931
Okwan-Duodu D, Pollack BP, Lawson D, Khan MK (2015) Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma. Am J Clin Oncol 38(1):119–125
Skrepnik T, Sundararajan S, Cui H, Stea B (2017) Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 6(3):e1283461. https://doi.org/10.1080/2162402X.2017.1283461
Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quiñones-Hinojosa A, Zaorsky NG, Trifiletti DM (2019) Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol 130:104–112
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ho, QA., Stea, B. Innovations in radiotherapy and advances in immunotherapy for the treatment of brain metastases. Clin Exp Metastasis 39, 225–230 (2022). https://doi.org/10.1007/s10585-021-10104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10104-z