Abstract
As a tumor suppressor gene, RAS-association domain family 2 (RASSF2) is inactivated by promoter hypermethylation in different tumor cell lines and primary tumors. However, the role of RASSF2 in esophageal squamous cell carcinoma (ESCC) has remained uninvestigated. The aims of this study were to determine the role and methylation status of RASSF2 in esophageal cancer cell lines, ESCC tissues and white blood cells, and to evaluate the potential prognostic role of RASSF2 in ESCC. In the present study, we found frequent silencing of RASSF2 and up-regulation of the gene by 5-Aza-dC treatment in esophageal cancer cell lines. Aberrant methylation of the CpG sites close to the transcription start site induced silencing of RASSF2 expression and in vitro methylation of RASSF2 led to a significant decrease in luciferase activity. The results were further verified in clinical specimens and aberrant methylation of the CpG sites close to the transcription start site of RASSF2 was found in ESCC tumor tissues and peripheral white blood cells. Furthermore, RASSF2 hypermethylation was associated with lower level of RASSF2 expression. ESCC patients in stage III and IV, with negative expression or hypermethylation of the CpG sites close to the transcription start of RASSF2 demonstrated poor patient survival. Taken together, our results suggest that RASSF2 may function as a tumor suppressor gene that is inactivated through hypermethylation of CpG sites close to the transcription start site in ESCC and its expression or methylation may have prognostic implications for ESCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the eighth most common malignancy and the sixth leading cause of cancer-related death worldwide, the prevalence and death rate of esophageal cancer are continuously increasing and the overall prognosis for esophageal cancer patients is poor [1, 2]. The 5-year survival rate was only 19 % in 2001–2007 in the United States [3]. Esophageal squamous cell carcinoma (ESCC) is the predominant type of esophageal cancer and has a striking geographic distribution in China, especially in some counties bordering Henan, Hebei, and Shanxi Provinces [4]. Smoking tobacco and drinking alcoholic beverages explain nearly 90 % of ESCC cases in the United States and other Western countries [5], but these exposures represent minor factors in high-risk populations in China. There is a strong tendency toward familial aggregation of ESCC in these high-risk areas [4]; however, a large proportion of the etiology in these populations still remains unexplained for the moment.
Ras proteins regulate a broad range of important signaling pathways including MAPK, PI-3K, and Rho GTPases. By interacting with a plenty of regulators and downstream effectors, Ras exhibits a regulatory role in various cellular responses, including cell morphology, cell proliferation, and differentiation, apoptosis, and cell cycle arrest [6]. The RAS-association domain family (RASSF) is defined as a negative effector of RAS and currently consists of ten members, all characterized by the inclusion of an RA-domain (RAS-association domain) at either their C-terminus (RASSF1-6) or N-terminus (RASSF7-10) [7]. The well-characterized members of the RASSF family are RASSF1A and RASSF5A. RASSF1A and RASSF5A have been shown to act as tumor suppressor genes and be frequently epigenetically inactivated in a broad range of tumor cell lines and primary tumors [8, 9]. We also detected epigenetic inactivation of RASSF1A and RASSF5A in ESCC and gastric cardia adenocarcinoma in our previous studies [10–13]. RASSF2 is structurally related to RASSF1A and may also serve as a potential tumor suppressor. RASSF2 binds to K-Ras in a GTP-dependent manner via the effector domain and may serve as a K-Ras-specific effector as it forms an endogenous complex with K-Ras [14]. RASSF2 can interact with other proapoptotic effectors and tumor suppressors, including MST1/2 kinases and PAR-4, thereby regulating the pathways these effectors control [15–17].
Inactivation of RASSF2 by promoter hypermethylation has been shown in different tumor cell lines and primary tumors, including oral squamous cell carcinoma, nasopharyngeal carcinoma, neuroblastoma, colorectal, breast, ovarian, gastric, cervical, thyroid, non-small cell lung cancers, merkel cell carcinoma, and pancreatic ductal adenocarcinoma, indicating the important role of RASSF2 as tumor suppressor gene in carcinogenesis [18–29]. It has been well accepted that DNA methylation changes at the tissue level may play an important role in tumorigenesis. Recently, the investigations on gene-specific methylation in white blood cells (WBC) and cancer risk are rapidly emerging and support the potential for gene-specific methylation measured in WBC as a biomarker of cancer risk [30–33]. To our best knowledge, the roles of RASSF2 on ESCC progression and prognosis have not been investigated and the promoter methylation status of RASSF2 in ESCC tissues and WBC has not been clarified. In the present study, we examined the methylation status and function of RASSF2 in esophageal cancer cell lines, ESCC tumor tissues, and WBC, and further elucidate the role of RASSF2 in the pathogenesis and prognosis of ESCC.
Materials and methods
Cell culture and treatment
The human esophageal cancer cell lines T.Tn, TE1, TE13, and Yes-2 were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % heat-inactivated FBS (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin. Human HEEpiC cells, which are human normal esophageal epithelial cells, were cultured according to the manufacturer’s instructions. Cells were seeded at a low density and incubated for 24 h prior to treatment with DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (5-Aza-dC). All four esophageal cancer Cells and normal esophageal epithelial cells (2 × 105/ml) were treated with 5 μmol/l 5-Aza-dC (Sigma, St Louis, MO, USA) for 72 h and medium containing 5-Aza-dC was changed every 24 h. The dose and timing of 5-Aza-dC was based on similar preliminary study showing optimal reactivation of gene expression [34]. Control cells received no drug treatment. DNA and RNA were isolated from these cells treated or untreated with 5-Aza-dC.
Patients and specimens
All study subjects were ethnically homogeneous Han nationality and residents of Hebei Province and its surrounding regions. Tumor and corresponding adjacent normal tissues were obtained from 118 ESCC cases, which were all inpatients for surgical treatment in the Fourth Affiliated Hospital, Hebei Medical University between the years of 2007 and 2009. Twenty healthy controls were recruited from the cancer-screening program for early detection of upper gastrointestinal tumors in the same area and during the same period. All of the healthy controls were not diagnosed with cancer or precancerous lesions. All subjects were interviewed by professional interviewers for their gender, age, histopathological diagnosis, and upper gastrointestinal cancers (UGIC) family history. The patients included 86 males and 32 females, mean age 58.3 years (ranged from 37 to 76 years). Individuals with at least one first-degree relative or at least two second-degree relatives having esophageal/cardia/gastric cancer were defined as having family history of upper gastrointestinal cancers (UGIC). Five milliliter of venous blood from each subject was drawn in Vacutainer tubes containing EDTA and stored at 4 °C. Tumor and corresponding normal tissues of the patients were divided into two parallel parts, one part were frozen and stored at −80 °C until DNA and RNA was extracted, the other part were formalin-fixed and paraffin-embedded. Histological tumor typing of the cases was carried out on the basis of resected specimens in the department of pathology of the same hospital. Information on clinicopathologic characteristics was available from hospital recordings and pathological diagnosis. Recurrence and survival data were ascertained through the Tumor Registry and Hospital chart review (Supplementary Table 1). All patients were evaluated for recurrent disease by examinations of tumor markers or by diagnostic imaging, including computed tomography, ultrasonography, magnetic resonance imaging, and endoscopy, every 3–6 months. The study was approved by the Ethics Committee of Hebei Cancer Institute and informed consent was obtained from all recruited subjects.
RASSF2 mRNA expression via quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) assay
Total RNA was isolated from 5-Aza-dC treated and untreated cell lines, frozen tumor and corresponding normal tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommended protocol and quantified by UV absorbance at 260–280 nm. Two microgram of total RNA was reverse transcribed to cDNA using the advantage RT-for-PCR kit (Clontech, Palo Alto, CA, USA) with oligo (dT) priming as recommended in the protocol. cDNA from each sample was used as quantitative real-time RT-PCR template and primers for RASSF2 were listed in Supplementary Table 2. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. SYBR Green PCR Master Mix (Life Technology, Foster City, CA, USA) was used as amplification reaction mixture. The PCR reaction was conducted at 95 °C for 5 min and followed by 40 cycles of 95 °C for 30 s and 56 °C for 45 s in the Stepone Plus Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The melting curve analysis was performed to confirm PCR product specificity. The expression levels of target genes were normalized with GAPDH using the 2−ΔΔCT method [35]. All experiments were repeated in triplicate.
RASSF2 protein expression in cancer cell lines via western blot analysis
Proteins were extracted from 5-Aza-dC treated and untreated cell lines by lysing the cells in ice-cold RIPA buffer. Total cell lysates were prepared and 20 μg proteins were separated in 12 % SDS-PAGE gel electrophoresis and analyzed by Western blot analysis with mouse anti-human monoclonal antibody for RASSF2 (1:200 dilution, Santa Cruz Biotechnology, San Diego, CA, USA). To ensure equal loading in all the lanes, the blot was stripped and probed with antibody against housekeeping genes, anti-GAPDH.
Methylation analysis of RASSF2 via bisulfite conversion-specific and methylation-specific polymerase chain reaction (BS-MSP) method
Total DNA was isolated from 5-Aza-dC treated and untreated cells using DNAzol (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s recommendation. Genomic DNA from tumor and corresponding normal sections was isolated from flash frozen tissues using a simplified Proteinase K (Merck, Darmstadt, Germany) digestion method. Five milliliter of venous blood from 118 ESCC cases (before surgery) and 20 healthy controls was drawn in Vacutainer tubes containing EDTA and stored at 4 °C. Genomic DNA was extracted within 1 week after blood collection by using proteinase K digestion method. To examine the DNA methylation patterns, 1 µg of genomic DNA was bisulfite modified using Epitect Fast Bisulfite Conversion Kits (Qiagen, Germany) according to the manufacturer’s instructions. The methylation status of RASSF2 was then determined by bisulfite conversion-specific and methylation-specific polymerase chain reaction method (BS-MSP) as described previously [36]. The MethPrimer program [37] was used to determine the CpG islands of RASSF2 and three regions (region 1: from −443 to −234 bp; region 2: from +65 to +376 bp; region 3: from +720 to +884 bp) of RASSF2 were analyzed. Briefly, BS-MSP consists of two-step PCR amplifications. In the first step of BS-MSP, 100 ng of bisulfite-treated DNA was amplified and a primer set that does not contain any CpG but contains many cytosines of non-CpG sites at the 3′ position was used. Only the sequence that is fully converted by bisulfite is amplified. The second step of BS-MSP used the conventional MSP primer sets that contain many cytosines of CpG sites at the 3′ position specific for methylated and unmethylated sequences. The primers and reaction conditions were listed in Supplementary Table 2. Genomic DNA, methylated in vitro by CpG methyltransferase (Sss I) following the manufacturer’s directions (New England BioLabs, Beverly, MA, USA), was used as a positive control and water blank was used as a negative control. BS-MSP products were analyzed on 2 % agarose gel with ethidium bromide staining, and were determined to have methylation if a visible band was observed in the methylation reaction. Reactions were performed in duplicate with each of the samples.
RASSF2 luciferase constructs
To explore the transcriptional regulation of RASSF2, three promoter reporter plasmids (RASSF2-R1 spanned the −550 to +150 bp; RASSF2-R2 spanned the −350 to +150 bp; RASSF2-R3 spanned the −180 to +150 bp) were constructed. The amplified fragments were inserted into the pGL3-basic vector (Promega, Madison, WI, USA) between the KpnI and HindIII sites. These recombination plasmids were then sequenced for confirmation.
Luciferase assay
1 × 105 T.Tn cells per well were seeded in 24-well dish 24 h before transfection. In all, 200 ng of RASSF2 deletion construct (RASSF2-R1 to R3), pGL3-control vector (positive control) or pGL3-basic vector (negative control) constructs was cotransfected with 10 ng of pRL-TK vector using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). Promoter activities were expressed as the ratio of Firefly luciferase to Renilla luciferase activity and each data point was done in triplicate.
In vitro DNA methylation
Construct pGL3-R3 was in vitro methylated as described previously [38]. 200 ng of mock or SSI-treated vector were transfected in T.Tn cells. After 48 h, luciferase and renilla activity were assayed as described above.
RASSF2 protein expression in ESCC tissues via immunohistochemical staining
RASSF2 protein expression in parallel histopathological sections from paraffin-embedded tumor section and corresponding normal tissues was determined by immunostaining using the avidin–biotin complex immunoperoxidase method. Non-specific binding was blocked by treating the sections with 1.5 % horse normal serum for 10 min, the primary antibody against RASSF2 (1:200 dilution, mouse anti-human monoclonal antibody, sc-376347, Santa Cruz Biotechnology, San Diego, CA, USA) was then applied to sections and incubated at 4 °C overnight. The sections were further incubated with biotinylated secondary antibody and ABC reagent. 0.5 % 3,3′-diaminobenzidine (Sigma, St Louis, MO, USA) was used as the chromagen. For a negative control, the primary antibody was replaced with mouse IgG. Slides with positive staining of RASSF2 were used as positive control.
Immunohistochemical staining was evaluated according to the staining extent and intensity as reported previously [39]. Briefly, the score is the sum of the percentage of positive cells (0, less than 25 % positive cells; 1, 26–50 % positive cells; 2, 51–75 % positive cells, and 3, more than 75 % positive cells) and the staining intensity (0, negative; 1, weak; 2, moderate; 3, strong). Sums between 0 and 2 were scored as negative; sums of 3 and 6 were scored as positive. All of the slides were examined and scored by three independent experienced investigators without knowledge of the patients’ clinical data.
Statistical analysis
Statistical analysis was performed with SPSS19.0 software package (SPSS Company, Chicago, Illinois, USA). The real-time RT-PCR results were expressed as the mean ± S.D. Student’s t test was used to compare the means between different groups. Chi square test was used to analyze the relationship of gene methylation and protein expression between ESCC tissues and corresponding normal tissues. Relationships between variables were tested by Spearman correlation analysis. Survival curves were constructed by using the Kaplan–Meier method and the Log-rank or the Breslow tests were used as needed for the univariate comparison of RASSF2 expression and methylation categories. Cox’s multivariate test applied in a stepwise forward method was used to adjust for potentially confounding variables (e.g., stage and UGIC family history) and to evaluate the role of RASSF2 as independent predictors of patients’ prognosis. All statistical tests were two sided; and P < 0.05 was considered to be statistically significant for all tests.
Results
Frequent silencing of RASSF2 and up-regulation of the gene by 5-Aza-dC treatment in esophageal cancer cell lines
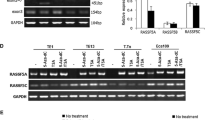
The mRNA and protein expression of RASSF2 was examined in four esophageal cancer cell lines and human normal esophageal epithelial cells to determine the expression status of RASSF2. As shown in Fig. 1a, when we compared the levels of RASSF2 expression in human normal esophageal epithelial cells (HEEpiC) with those in ESCC cell lines (T.Tn, TE1, TE13, and Yes-2), we found that mRNA expression of RASSF2 was remarkably reduced or silenced in four esophageal cancer cell lines. This finding was further confirmed by the results of western blot analysis (Fig. 1b). However, treatment with 5-Aza-dC increased the level of mRNA and protein expression of RASSF2 in the four esohpageal cancer cell lines, which indicated that aberrant methylation may be one of the important mechanisms leading to the inactivation of RASSF2 in esophageal cancer cell lines.
Expression and methylation status of RASSF2 in four human esophageal cancer cell lines. a Relative expression of RASSF2 in four esophageal cancer cell lines and human normal esophageal epithelial cells treated or untreated with 5-Aza-dC. Asterisk compared with untreated cells, P < 0.05. b Protein expression of RASSF2 in treated or untreated esophageal cancer cell lines and human normal esophageal epithelial cells detected by Western-blot method. c Schematic structure of RASSF2 CpG islands. Three CpG islands of RASSF2 are shown and the BS-MSP regions analyzed is indicated. d The methylation status of three regions of RASSF2 detected by BS-MSP analysis in various cancer cell lines with or without 5-Aza-dC treatment. M methylated, U unmethylated. e Luciferase activity of promoter constructs. pGL3-control vector was used as a positive (POS), and empty pGL3-basic vector as a negative control (EV). RASSF2-R3 showed the highest relative luciferase activity. f In vitro methylation of RASSF2-R3 led to a significant decrease in luciferase activity
The aberrant methylation of RASSF2 induces silencing of RASSF2 expression
As shown in Fig. 1c, 3 CpG islands are found to be located in RASSF2 promoter and exon 1. Methylation status of three regions of the CpG islands in RASSF2 was detected according to the distribution of the CpG islands. As shown in Fig. 1d, different degree of hypermethylation of three regions of RASSF2 was respectively observed in four cell lines. In particular, fully methylation of region 2 in T.Tn, TE13, and Yes-2 cell lines was detected. After treatment with 5-Aza-dC, demethylation of three regions of RASSF2 was observed in the four cell lines, and unmethylation of region 2 was especially detected in the four cell lines, indicating that RASSF2 region 2 methylation may have stronger effect in suppressing the expression of RASSF2 in esophageal cancer cells.
In vitro methylation of RASSF2 leads to a significant decrease in luciferase activity
Three constructs (RASSF2-R1 to R3) were designed for functional characterization of the RASSF2 promoter. As shown in Fig. 1e, RASSF2-R3 demonstrated the highest relative luciferase activity which provided a potential explanation for the importance of proximal promoter methylation of RASSF2 on the control of RASSF2 transcription. As a direct evidence for the role of methylation in this region, in vitro methylation of RASSF2-R3 led to an 80 % decrease in luciferase activity (Fig. 1f).
Decreased mRNA and protein expression of RASSF2 in clinical specimens
The mRNA and protein expression of RASSF2 was further detected in 118 ESCC tumor tissues and corresponding normal tissues. As shown in Fig. 2A, mRNA expression of RASSF2 in ESCC tumor tissues was significantly decreased compared to corresponding normal tissues (P < 0.01). When stratified for clinicopathologic characteristics, RASSF2 mRNA expression was associated with TNM stage, pathological differentiation, LN metastasis, distant metastasis or recurrence, and UGIC family history (P < 0.05) (Fig. 2B).
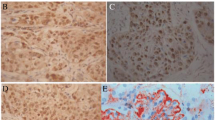
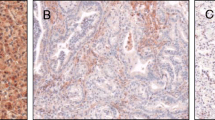
Expression and methylation status of RASSF2 in ESCC tissues. A Relative mRNA expression of RASSF2 in normal tissues and corresponding ESCC tumor tissues. *P < 0.05. B Relative mRNA expression of RASSF2 in different subgroups. *P < 0.05. C Immunohistochemical staining of RASSF2 in ESCC tumor tissues and corresponding normal tissues (SP ×400). a Positive staining of RASSF2 in ESCC tissue; b negative staining of RASSF2 in ESCC tissue; c positive staining of RASSF2 in normal tissue. D The methylation status of three regions of RASSF2 determined by BS-MSP analysis in ESCC tumor tissues. m methylated, u unmethylated. E Relative mRNA expression of RASSF2 in the tumor tissues with and without methylation of the three regions. *P < 0.05
RASSF2 staining was expressed diffusely in the cytoplasm and nucleus, mainly in cytoplasm (Fig. 2C). As shown in Table 1, positive protein expression of RASSF2 in tumor tissues (34.7 %, 41/118) was significantly lower than that in corresponding normal tissues (92.4 %, 109/118) (P < 0.01). According to our scoring method, no tumor tissues showed higher protein expression of RASSF2 than corresponding normal tissues. When stratified for clinicopathologic characteristics, RASSF2 protein expression was associated with TNM stage, pathological differentiation, LN metastasis, distant metastasis or recurrence, and UGIC family history (P < 0.05) (Table 2).
Aberrant methylation of RASSF2 in ESCC tumor tissues and peripheral white blood cells
The methylation analysis was successfully performed in all tissue specimens (Fig. 2D). As shown in Table 1, the methylation frequency of 3 regions of RASSF2 in tumor tissues (38.1 %, 45/118; 52.5 %, 62/118; 35.6 %, 42/118; respectively) was significantly higher than that in corresponding normal tissues (17.8 %, 21/118; 2.5 %, 3/118; 12.7 %, 15/118; respectively) (P < 0.05). When stratified for clinicopathologic characteristics, the methylation status of region 1 and region 3 of RASSF2 was not associated with any clinicopathologic features. However, the methylation status of RASSF2 region 2 was associated with TNM stage, pathological differentiation, LN metastasis, and distant metastasis or recurrence (Table 2).
The methylation status of three regions of RASSF2 was further detected in the peripheral white blood cell DNA of 118 ESCC cases and 20 healthy controls. The methylation frequency of 3 regions of RASSF2 in ESCC WBC was 19.5 % (23/118), 36.4 % (43/118), and 16.9 % (20/118), respectively; while aberrant methylation of 3 regions of RASSF2 was not detected in the WBC of healthy controls. Interestingly, we found that hypermethylation of 3 regions of RASSF2 which occurred in ESCC WBC also occurred in the corresponding tumor tissues. When stratified for clinicopathologic characteristics, the methylation status of region 1 and region 3 of RASSF2 in ESCC WBC was not associated with any clinicopathologic features. However, the methylation status of region 2 of RASSF2 in ESCC WBC was associated with TNM stage, pathological differentiation, and distant metastasis or recurrence (Table 2).
Association between RASSF2 expression and methylation status
RASSF2 mRNA expression in ESCC tumor tissues with positive expression of RASSF2 protein was significantly decreased compared to ESCC tumor tissues with negative protein expression of RASSF2 (P < 0.05). RASSF2 mRNA expression in ESCC tumor tissues where RASSF2 region 2 was methylated was significantly decreased compared to that in ESCC tumor tissues without methylation of this region (P < 0.05), while there was no association between RASSF2 mRNA expression and region 1 and region 3 methylation in ESCC tumor tissues (P > 0.05) (Fig. 2E). The correlation of methylation of three regions of RASSF2 and protein expression was shown in Table 3; a close correlation was observed between RASSF2 region 2 methylation and the loss of protein expression of the gene in ESCC (P < 0.01).
Survival analysis of RASSF2 in ESCC
As shown in Fig. 3a, RASSF2 expression was positively correlated with ESCC patients’ survival. In the RASSF2-positive expression ESCC cases, the 5-year survival rates were significantly longer as opposed to the RASSF2-negative expression ESCC cases (P < 0.05; Log-rank test). As shown in Fig. 3b, RASSF2 region 2 methylation was inversely correlated with ESCC patients’ survival. In the RASSF2-region 2 unmethylated ESCC cases, the 5-year survival rates were significantly longer as opposed to the RASSF2-region 2 methylated ESCC cases (P < 0.05; Log-rank test). ESCC cases with both negative expression and region 2 hypermethylation of RASSF2 showed worse survival rates compared with the ESCC cases with both positive expression and region 2 unmethylation of RASSF2 (Fig. 3c). When TNM stage and RASSF2 expression or region 2 methylation status were combined to analysis, ESCC patients in stage III and IV, with negative expression or region 2 hypermethylation of RASSF2 were most likely to develop metastatic disease and also demonstrated the worse survival (Fig. 3d, e).
Kaplan–Meier univariate survival analysis of RASSF2 expression and methylation in ESCC cases. a Showing a direct correlation between negative RASSF2 expression and poor patient survival. b Showing consistently a direct correlation between RASSF2 methylation and poor patient survival. c ESCC cases with simultaneous negative protein expression and methylation of RASSF2 showing poor patient survival. d ESCC cases in stage III and IV and with negative expression of RASSF2 showing poor patient survival. E. ESCC cases in stage III and IV and with methylation of RASSF2 showing poor patient survival
Cox multivariate analysis was done using RASSF2 expression, methylation, tumor stage, as well as other confounding variables such as patient age, gender and UGIC family history. As shown in Table 4, TNM stages, RASSF2 protein expression, and region 2 methylation were independently associated with ESCC patients’ survival.
Discussion
RASSF2 was first identified by Vos et al. in the year of 2003. RASSF2 shows 28 % identity to that of RASSF1A and 31 % identity to that of RASSF5A. RASSF2 lacks the cysteine-rich domain of RASSF5A and RASSF1A [14]. RASSF2 resides at 20p13; this region has been demonstrated to be frequently lost in human cancers. It has been reported that RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor through inhibiting the growth of tumor cells, impairing tumor xenograft formation in nude mice, and promoting tumor cell apoptosis and cell cycle arrest [14]. Hesson et al. first investigated the CpG island promoter methylation status of RASSF2 in sporadic colorectal cancer, and they found that RASSF2 promoter region CpG island was hypermethylated in a majority of colorectal tumor cell lines (89 %) and in primary colorectal tumors (70 %) [21]. Later after that, inactivation of RASSF2 by promoter hypermethylation has been shown in different tumor cell lines and primary tumors [18–29]. However, the effect of RASSF2 in ESCC has not been previously reported. In the present study, we found frequent silencing of RASSF2 and up-regulation of the gene by 5-Aza-dC treatment in esophageal cancer cell lines; and aberrant methylation of the CpG sites close to the transcription start site induced silencing of RASSF2 expression and in vitro methylation of RASSF2 led to a significant decrease in luciferase activity. The results were further verified in clinical specimens and aberrant methylation of the CpG sites close to the transcription start site of RASSF2 was found in ESCC tumor tissues and peripheral white blood cells. The findings of the correlation between RASSF2 expression, methylation and ESCC patients’ survival further identified the role of RASSF2 as tumor suppressor gene and may be used as a useful marker of ESCC tumor progression and poor prognosis.
The present study showed that mRNA and protein expression of RASSF2 was lost or drastically downregulated in most esophageal cancer cell lines. RASSF2 is a predominantly nuclear protein, however, we found that RASSF2 staining was expressed diffusely in the cytoplasm and nucleus, mainly in cytoplasm. RASSF2 expression was also detected in the cytoplasm and, in some cases, in the nucleus in gastric cancer and squamous cervical cancer [25, 40, 41]. Maruyama et al. analyzed RASSF2 protein motifs and found that RASSF2 carries three putative nuclear localization signals (NLSs) in the N-terminal region adjacent to the RA domain. RASSF2 lacking the NLS was found only in the cytoplasm, indicating its NLS is a key determinant of the intracellular distribution of RASSF2. Deletion of the NLS was also found to lead to induction of apoptosis. Apparently, RASSF2′s proapoptotic activity is mediated by the cytoplasmic protein, and because RASSF2 interacts with Ras, which is localized in the cytoplasm, it would seem reasonable that cytoplasmic RASSF2A possesses the tumor suppressor activity [25].
RASSF2 expression was remarkably restored following treatment with a demethylating agent 5-Aza-dC in esophageal cancer cell lines. We further detected the methylation status of three regions according to the distribution of CpG islands. In esophageal cancer cell lines with lost or drastically downregulated RASSF2 expression such as T.Tn, TE13, and Yes-2, the CpG sites of region 2 was completely methylated. The findings that the hypermethylation of proximal promoter in RASSF2 can efficiently influence transcriptional activity of the gene further verified the importance of the CpG sites close to the transcription start site of RASSF2 in influencing the transcription activity of the gene. In ESCC tumors, methylation in the 5′- (region 1) and 3′- (region 3) furthest regions within the CpG islands was frequently detected in both tumor tissues and corresponding normal tissues, however, methylation near the transcription start sites (region 2) appeared to be mostly cancer-specific. These findings are similar to the investigations of Endoh et al. in gastric cancer [24], indicating that the pattern of spread of methylation, initially at the outskirts of CpG islands and then progressing to regions critical for gene silencing, might be common to various types of methylation-related gene silencing. Cancer-specific hypermethylation near the transcription start site resulting in gene silencing, may be used as a diagnostic marker of malignancy in tissues or other samples, such as peripheral white blood cells, serum or ascites.
In cancer and other diseases, aberrant DNA methylation has been widely observed at the tissue level. Data on whether DNA methylation changes in white blood cells can serve as a useful biomarker for different health outcomes are much more limited, but rapidly emerging. Peripheral blood cell DNA may contain epigenetic information, which is a valuable predictive marker of an individual’s risk of developing cancer [42]. Gene-specific methylation in WBC and cancer risk have mostly focused on selected genes such as BRCA1, IGFII, SFRP1 for breast cancer and colon cancer [30–33]. In the present study, aberrant methylation of three regions of RASSF2 was not detected in the peripheral WBC of healthy controls, however, hymethylation of CpG sites in three regions especially region 2 of RASSF2 was detected in peripheral WBC. Furthermore, the ESCC cases with hypermethylation of region 2 in peripheral WBC all demonstrated hypermethylation of this region in ESCC tumor tissues. These results support the potential for gene-specific methylation measured in peripheral WBC as a biomarker of cancer risk.
Our and others investigations have shown that methylation patterns of tumor suppressor genes may be useful to assess clinical outcomes of carcinomas or response to chemotherapeutic agents [11–13, 43]. A combination of RASSF1A and RASSF2 methylation was found to be significantly associated with poor disease-free survival (DFS) after radiotherapy in oral squamous cell carcinoma [18]. Squamous cervical cancer patients with RASSF2 hypermethylation had shorter survival time, independent of tumor stage [41]. RASSF2 methylation correlated with poor overall survival in Ewing sarcoma and this association was more pronounced in patients under the age of 18 years [44]. In the present study, we showed that RASSF2 expression and methylation was correlated with ESCC patients’ survival. ESCC patients with simultaneous negative protein expression and hypermethylation of RASSF2 demonstrated poor patient survival. Thus, RASSF2 expression and methylation may also have prognostic implications for ESCC patients.
In conclusion, RASSF2 may act as a tumor suppressor gene that is inactivated through hypermethylation of CpG sites close to the transcription start site in ESCC and its expression or methylation may serve as prognostic biomarker for ESCC.
References
Nagaraja V, Eslick GD (2014) Forthcoming prognostic markers for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 5:67–76
Shahbaz Sarwar CM, Luketich JD, Landreneau RJ, Abbas G (2010) Esophageal cancer: an update. Int J Surg 8:417–422
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62:220–241
Guohong Z, Min S, Duenmei W, Songnian H, Min L, Jinsong L, Hongbin L, Feng Z, Dongping T, Heling Y, Zhicai L, Shiyong L, Quansheng G, Xiaoyun L, Yuxia G (2010) Genetic heterogeneity of oesophageal cancer in high-incidence areas of southern and northern China. PLoS One 5:e9668
Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni JF Jr (2003) Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 95:1404–1413
Spandidos DA, Sourvinos G, Tsatsanis C, Zafiropoulos A (2002) Normal ras genes: their onco-suppressor and pro-apoptotic functions. Int J Oncol 21:237–241
Ponting CP, Benjamin DR (1996) A novel family of Ras-binding domains. Trends Biochem Sci 21:422–425
Donninger H, Vos MD, Clark GJ (2007) The RASSF1A tumor suppressor. J Cell Sci 120:3163–3172
Hesson L, Dallol A, Minna JD, Maher ER, Latif F (2003) NORE1A, a homologue of RASSF1A tumour suppressor gene is inactivated in human cancers. Oncogene 22:947–954
Guo W, Dong Z, Chen Z, Yang Z, Wen D, Kuang G, Guo Y, Shan B (2009) Aberrant CpG Island hypermethylation of RASSF1A in gastric cardia adenocarcinoma. Cancer Invest 27:459–465
Guo W, Cui L, Wang C, Guo Y, Shen S, Kuang G, Dong Z (2014) Decreased expression of RASSF1A and up-regulation of RASSF1C is associated with esophageal squamous cell carcinoma. Clin Exp Metastasis 31:521–533
Han L, Dong Z, Wang C, Guo Y, Shen S, Kuang G, Guo W (2014) Decreased expression and aberrant methylation of RASSF5A correlates with malignant progression of gastric cardia adenocarcinoma. Mol Carcinog. doi:10.1002/mc.22245
Guo W, Wang C, Guo Y, Shen S, Guo X, Kuang G, Dong Z (2015) RASSF5A, a candidate tumor suppressor, is epigenetically inactivated in esophageal squamous cell carcinoma. Clin Exp Metastasis 32:83–98
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, Clark GJ (2003) RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem 278:28045–28051
Song H, Oh S, Oh HJ, Lim DS (2010) Role of the tumor suppressor RASSF2 in regulation of MST1 kinase activity. Biochem Biophys Res Commun 391:969–973
Cooper WN, Hesson LB, Matallanas D, Dallol A, von Kriegsheim A, Ward R, Kolch W, Latif F (2009) RASSF2 associates with and stabilizes the proapoptotic kinase MST2. Oncogene 28:2988–2998
Donninger H, Hesson L, Vos M, Beebe K, Gordon L, Sidransky D, Liu JW, Schlegel T, Payne S, Hartmann A, Latif F, Clark GJ (2010) The Ras effector RASSF2 controls the PAR-4 tumor suppressor. Mol Cell Biol 30:2608–2620
Huang KH, Huang SF, Chen IH, Liao CT, Wang HM, Hsieh LL (2009) Methylation of RASSF1A, RASSF2A, and HIN-1 is associated with poor outcome after radiotherapy, but not surgery, in oral squamous cell carcinoma. Clin Cancer Res 15:4174–4180
Zhang Z, Sun D, Van do N, Tang A, Hu L, Huang G (2007) Inactivation of RASSF2A by promoter methylation correlates with lymph node metastasis in nasopharyngeal carcinoma. Int J Cancer 120:32–38
Kiss NB, Kogner P, Johnsen JI, Martinsson T, Larsson C, Geli J (2012) Quantitative global and gene-specific promoter methylation in relation to biological properties of neuroblastomas. BMC Med Genet 13:83
Hesson LB, Wilson R, Morton D, Adams C, Walker M, Maher ER, Latif F (2005) CpG island promoter hypermethylation of a novel Ras-effector gene RASSF2A is an early event in colon carcinogenesis and correlates inversely with K-ras mutations. Oncogene 24:3987–3994
Cooper WN, Dickinson RE, Dallol A, Grigorieva EV, Pavlova TV, Hesson LB, Bieche I, Broggini M, Maher ER, Zabarovsky ER, Clark GJ, Latif F (2008) Epigenetic regulation of the ras effector/tumour suppressor RASSF2 in breast and lung cancer. Oncogene 27:1805–1811
Wu Y, Zhang X, Lin L, Ma XP, Ma YC, Liu PS (2014) Aberrant methylation of RASSF2A in tumors and plasma of patients with epithelial ovarian cancer. Asian Pac J Cancer Prev 15:1171–1176
Endoh M, Tamura G, Honda T, Homma N, Terashima M, Nishizuka S, Motoyama T (2005) RASSF2, a potential tumour suppressor, is silenced by CpG island hypermethylation in gastric cancer. Br J Cancer 93:1395–1399
Maruyama R, Akino K, Toyota M, Suzuki H, Imai T, Ohe-Toyota M, Yamamoto E, Nojima M, Fujikane T, Sasaki Y, Yamashita T, Watanabe Y, Hiratsuka H, Hirata K, Itoh F, Imai K, Shinomura Y, Tokino T (2008) Cytoplasmic RASSF2A is a proapoptotic mediator whose expression is epigenetically silenced in gastric cancer. Carcinogenesis 29:1312–1318
Schagdarsurengin U, Richter AM, Hornung J, Lange C, Steinmann K, Dammann RH (2010) Frequent epigenetic inactivation of RASSF2 in thyroid cancer and functional consequences. Mol Cancer 9:264
Richter AM, Haag T, Walesch S, Herrmann-Trost P, Marsch WC, Kutzner H, Helmbold P, Dammann RH (2013) Aberrant promoter hypermethylation of RASSF family members in merkel cell carcinoma. Cancers (Basel) 5:1566–1576
Park HW, Kang HC, Kim IJ, Jang SG, Kim K, Yoon HJ, Jeong SY, Park JG (2007) Correlation between hypermethylation of the RASSF2A promoter and K-ras/BRAF mutations in microsatellite-stable colorectal cancers. Int J Cancer 120:7–12
Zhao L, Cui Q, Lu Z, Chen J (2012) Aberrant methylation of RASSF2A in human pancreatic ductal adenocarcinoma and its relation to clinicopathologic features. Pancreas 41:206–211
Widschwendter M, Apostolidou S, Raum E, Rothenbacher D, Fiegl H, Menon U, Stegmaier C, Jacobs IJ, Brenner H (2008) Epigenotyping in peripheral blood cell DNA and breast cancer risk: a proof of principle study. PLoS One 3:e2656
Kaaks R, Stattin P, Villar S, Poetsch AR, Dossus L, Nieters A, Riboli E, Palmqvist R, Hallmans G, Plass C, Friesen MD (2009) Insulin-like growth factor-II methylation status in lymphocyte DNA and colon cancer risk in the Northern Sweden Health and Disease cohort. Cancer Res 69:5400–5405
Iwamoto T, Yamamoto N, Taguchi T, Tamaki Y, Noguchi S (2011) BRCA1 promoter methylation in peripheral blood cells is associated with increased risk of breast cancer with BRCA1 promoter methylation. Breast Cancer Res Treat 129:69–77
Wong EM, Southey MC, Fox SB, Brown MA, Dowty JG, Jenkins MA, Giles GG, Hopper JL, Dobrovic A (2011) Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. Cancer Prev Res (Phila) 4:23–33
Kondo Y, Shen L, Issa JP (2003) Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol 23:206–215
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Sasaki M, Anast J, Bassett W, Kawakami T, Sakuragi N, Dahiya R (2003) Bisulfite conversion-specific and methylation-specific PCR: a sensitive technique for accurate evaluation of CpG methylation. Biochem Biophys Res Commun 309:305–309
Li LC, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427–1431
Yu L, Liu C, Vandeusen J, Becknell B, Dai Z, Wu YZ, Raval A, Liu TH, Ding W, Mao C, Liu S, Smith LT, Lee S, Rassenti L, Marcucci G, Byrd J, Caligiuri MA, Plass C (2005) Global assessment of promoter methylation in a mouse model of cancer identifies ID4 as a putative tumor-suppressor gene in human leukemia. Nat Genet 37:265–274
Umemoto M, Yokoyama Y, Sato S, Tsuchida S, Al-Mulla F, Saito Y (2001) Carbonyl reductase as a significant predictor of survival and lymph node metastasis in epithelial ovarian cancer. Br J Cancer 85:1032–1036
Luo D, Ye T, Li TQ, Tang P, Min SD, Zhao GF, Huang H, Chang J, Wang Y, Lv L, Lu ML, Zheng MY (2012) Ectopic expression of RASSF2 and its prognostic role for gastric adenocarcinoma patients. Exp Ther Med 3:391–396
Guerrero-Setas D, Pérez-Janices N, Blanco-Fernandez L, Ojer A, Cambra K, Berdasco M, Esteller M, Maria-Ruiz S, Torrea N, Guarch R (2013) RASSF2 hypermethylation is present and related to shorter survival in squamous cervical cancer. Mod Pathol 26:1111–1122
Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM (2011) DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics 6:828–837
Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, Glöckner S, Piantadosi S, Gabrielson E, Pridham G, Pelosky K, Belinsky SA, Yang SC, Baylin SB, Herman JG (2008) DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med 358:1118–1128
Gharanei S, Brini AT, Vaiyapuri S, Alholle A, Dallol A, Arrigoni E, Kishida T, Hiruma T, Avigad S, Grimer R, Maher ER, Latif F (2013) RASSF2 methylation is a strong prognostic marker in younger age patients with Ewing sarcoma. Epigenetics 8:893–898
Acknowledgments
We thank the patients for taking part in this study. Supported by Grants from the National Natural Science Foundation (No. 81472335).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, W., Dong, Z., Cui, J. et al. Aberrant hypermethylation of RASSF2 in tumors and peripheral blood DNA as a biomarker for malignant progression and poor prognosis of esophageal squamous cell carcinoma. Clin Exp Metastasis 33, 73–85 (2016). https://doi.org/10.1007/s10585-015-9759-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9759-5