Abstract
Gekkotan lizards are a highly specious (∼1600 described species) clade of squamate lizards with nearly cosmopolitan distribution in warmer areas. The clade is primarily nocturnal and forms an ecologically dominant part of the world nocturnal herpetofauna. However, molecular cytogenetic methods to study the evolution of karyotypes have not been widely applied in geckos. Our aim here was to uncover the extent of chromosomal rearrangements across the whole group Gekkota and to search for putative synapomorphies supporting the newly proposed phylogenetic relationships within this clade. We applied cross-species chromosome painting with the recently derived whole-chromosomal probes from the gekkonid species Gekko japonicus to members of the major gekkotan lineages. We included members of the families Diplodactylidae, Carphodactylidae, Pygopodidae, Eublepharidae, Phyllodactylidae and Gekkonidae. Our study demonstrates relatively high chromosome conservatism across the ancient group of gekkotan lizards. We documented that many changes in chromosomal shape across geckos can be attributed to intrachromosomal rearrangements. The documented rearrangements are not totally in agreement with the recently newly erected family Phyllodactylidae. The results also pointed to homoplasy, particularly in the reuse of chromosome breakpoints, in the evolution of gecko karyotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reconstruction of major chromosomal rearrangements can be a source of reliable synapomorphies necessary to resolve phylogenetic relationships within a clade. This approach has proved to be useful in supporting relationships between mammalian orders not recognized by classical taxonomic approaches (e.g. monophyly of Afrotheria) or in resolving phylogenetic relationships within particular mammalian lineages (reviewed e.g. in Graphodatsky et al. 2011). Mammals in general possess extraordinary high rates of interchromosomal rearrangements (Ferguson-Smith and Trifonov 2007). Recently, high levels of chromosomal conservation were revealed by whole genome sequencing (Hillier et al. 2004; Warren et al. 2010; Alföldi et al. 2011), physical gene mapping (e.g. Matsubara et al. 2006, 2014a; Srikulnath et al. 2009, 2014; Uno et al. 2012), qPCR mapping to sex chromosomes (Gamble et al. 2014; Rovatsos et al. 2014a, b, c) and comparative painting (e.g. Pokorná et al. 2011, 2012; Kasai et al. 2012) in sauropsids, the clade encompassing squamate reptiles, tuataras, turtles, crocodiles and birds. Therefore, reconstruction of the ancestral karyotype could be easier for sauropsids than it was for mammals (Ferguson-Smith and Trifonov 2007; Deakin and Ezaz 2014). However, lack of data in many important lineages prevents a robust reconstruction of ancestral karyotypes and detection of cytogenetic synapomorphies of particular lineages.
Here, we focus on comparative chromosome painting in one of the major lineages of squamate reptiles, geckos (Gekkota), in order to contribute to the reconstruction of the gekkotan ancestral karyotype and to search for chromosomal signatures of phylogenetic relationships within this group. Gekkotan lizards are a highly specious clade of squamate lizards (approximately 1600 described species; Uetz and Hošek 2014) with nearly cosmopolitan distribution in warmer areas. The clade is primarily nocturnal and forms an ecologically dominant part of the world nocturnal herpetofauna. The monophyly of this group is well supported by morphological and sequence data (Estes et al. 1988; Townsend et al. 2004; Pyron et al. 2013). Molecular studies place geckos as a sister group to the clade Unidentata encompassing all other squamates with the exception of dibamids (Townsend et al. 2004; Pyron et al. 2013). Alternatively, Gekkota and dibamids were suggested to form a single clade basal to all other squamate lineages (Wiens et al. 2012). These topologies are in contrast to classical morphological phylogenetic trees, which placed Gekkota into the clade Scleroglossa together with all the other lineages of squamates apart from the sister clade Iguania (iguanas, agamids, chameleons) (Estes et al. 1988). The putative ancestral karyotype of geckos (2n = 38) includes probably only acrocentric chromosomes (Gorman 1973; Pokorná et al. 2010), and it is therefore largely different from the putative ancestral karyotypes of Unidentata consisting of 12 metacentric chromosomes and 24 microchromosomes (Gorman 1973; Oguiura et al. 2010; Johnson Pokorná et al. 2014). It was reported previously that in geckos, the genome organization is in general rather conservative, and inversions were suggested as a primary source of the rearrangements (King 1987a, b, 1990; King and Mengdon 1990).
The phylogenetic relationships within Gekkota were recently considerably revised in comparison to a classical understanding of the phylogeny and taxonomy of the group (cf. Kluge 1983; Grismer 1988). Phylogeny based on sequence data places the clade of the families Diplodactylidae, Carphodactylidae and the legless Pygopodidae as a sister group to all remaining Gekkota (Han et al. 2004; Gamble et al. 2008a; Pyron et al. 2013). Traditionally, eyelid geckos (family Eublepharidae) were assigned as sister to all other gekkotan lineages (Grismer 1988), but in the recent molecular analyses, they are placed as sister to the geckos with hard, highly calcified eggshells (Gamble et al. 2012; Wiens et al. 2012; Pyron et al. 2013). The split of the clade of the hard-shelled geckos, classically mostly assigned as the family Gekkonidae, into three families was recently suggested. Within this group, Gamble et al. (2008b) redefined the family Sphaerodactylidae originally established by Underwood (1954), now covering some Old World and New World genera, as sister to the newly erected trans-Atlantic family Phyllodactylidae and the newly understood, restricted family Gekkonidae (Gamble et al. 2008a, 2011, 2012).
Our aim here was to uncover the extent of interchromosomal rearrangements across the whole group Gekkota and to search for putative synapomorphies supporting the newly proposed phylogenetic relationships within this clade using a molecular-cytogenetic approach. Specifically, we applied chromosome painting with the recently derived whole-chromosomal probes from the gekkonid species Gekko japonicus (Trifonov et al. 2011) to members of the major gekkotan lineages (families Diplodactylidae, Carphodactylidae, Pygopodidae, Eublepharidae, Phyllodactylidae and Gekkonidae sensu Gamble et al. 2008a, 2011, 2012).
Material and methods
Species studied and metaphase chromosome preparation
We prepared metaphase chromosomes from one specimen of six species of the gekkotan lizards from five gekkotan families (taxonomy according to Gamble et al. 2012): Oedura monilis (Diplodactylidae, female), Lialis burtonis (Pygopodidae, male), Coleonyx variegatus (Eublepharidae, female), Tarentola annularis (Phyllodactylidae, female), Paroedura bastardi (Gekkonidae, female) and Agamura persica (Gekkonidae, female). To our knowledge, the karyotype of A. persica has not been published (cf. Chromorep database by Olmo and Signorino; http://ginux.univpm.it/scienze/chromorep). Moreover, molecular cytogenetic data on chromosome syntenies with chromosomes of G. japonicus (GJA) are available for six species from the family Gekkonidae, three from the genus Hemidactylus (Hemidactylus turcicus, Hemidactylus platyurus, Hemidactylus frenatus) and three from the genus Gekko (Gekko gecko, Gekko vittatus and Gekko badenii, assigned there by its junior synonym as Gekko ulikovskii; Trifonov et al. 2011), and we included them in our analyses of karyotype evolution within Gekkota.
All individuals used in this study were captive bred animals maintained in the laboratory breeding rooms at the Faculty of Science, Charles University in Prague, Czech Republic (accreditation No. 13060/2014-MZE-17214), and originated from the pet trade. Metaphase chromosome spreads were prepared from whole blood cell cultures following protocols described in Pokorná et al. (2010) with slight modifications.
Fluorescence in situ hybridization and signal detection
The chromosome paints of GJA were prepared from chromosomes sorted with a dual laser cell sorter (Mo-Flo, Dako) at the Cambridge Resource Centre for Comparative Genomics, Department of Veterinary Medicine, University of Cambridge, Cambridge, UK. Sorted chromosomes were used as templates for DNA amplification by degenerate oligonucleotide-primed PCR (DOP-PCR). Primary DOP-PCR product was used as a template in a secondary DOP-PCR to incorporate biotin-16-dUTP (Roche). The probe preparation and flow karyotype of GJA are described in Trifonov et al. (2011). To prevent misidentifications of chromosome syntenies in phylogenetically distant lineages, we used only those probes that have been assigned to only a single chromosomal pair in GJA.
Fluorescence in situ hybridization (FISH) was performed using the protocol described in Yang et al. (1995) and Rens et al. (1999; 2006) with several modifications. Briefly, slides were dehydrated through ethanol series, aged for 1 h at 65 °C and denatured in 70 % formamide/0.6× SSC at 70 °C for 1 up to 4 min (time depending on species and metaphase preparation) and dehydrated again. Eight microlitres of biotinylated secondary PCR product was precipitated in ethanol and resuspended in 11 μl of hybridization buffer [40 % deionized formamide (v/v), 10 % dextran sulphate, 2× SSC, 0.05 M phosphate buffer, pH 7.3]. This mixture was denatured for 10 min at 75 °C, preannealed at 37 °C for 30 min and applied to each slide. Hybridization was carried out at 37 °C for three nights. Posthybridization washes were performed in 40 % formamide/1.8× SSC twice for 5 min each, followed by 2× SSC twice for 5 min each and 4× SSC with 0.05 % Tween-20 (4xT) once for 4 min. The washes were performed at 42 °C. Probe detection was carried out using 200 μl of diluted (1:500) Cy3-streptavidin antibody (Amersham) per slide at 37 °C for 30 min. After detection, slides were washed in 4xT three times for 3 min each at 42 °C and mounted in Vectashield Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI; VECTOR Laboratories).
Microscopy and data analyses
Images were captured using the Leica DMRXA microscope equipped with CCD camera (Photometrics Sensys). Leica CW4000 FISH software (Leica Microsystems) was used to capture grey-scale images and to superimpose the source images into colours to visualize the FISH results. Chromosome analyses were made from images that were processed with a 9 × 9 high-pass spatial filter, displayed in contrast-adjusted reversed greyscale images and classified using Leica CW4000 Karyo software (Leica Microsystems). The final composition of the images was performed in CorelDraw X5 software (Corel Corporation).
Results
Karyotype description
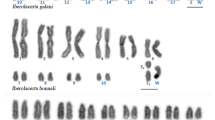
The karyotype of A. persica consists of 2n = 42 chromosomes. There are seven metacentric or submetacentric pairs in the karyotype; all the others are acrocentric or subtelocentric (Fig. 1). We had only one individual of this species available for the cytogenetic examination, which might not be adequate for the description of the karyotype for the whole species, as our specimen could be atypical. However, the genus Agamura is closely related to the genus Cyrtopodion (Červenka et al. 2008; Bauer et al. 2013), where the same number of chromosomes (2n = 42) was found in all five species studied so far (Manilo 1996), and this chromosome number thus seems to be stable within this clade. The karyotypes in O. monilis 2n = 38 (Diplodactylidae), C. variegatus 2n = 32 (Eublepharidae), T. annularis 2n = 42 (Phyllodactylidae) and P. bastardi 2n = 34 (Gekkonidae) correspond to the original descriptions (Yaseen et al. 1995; Pokorná et al. 2010, 2011; Aprea et al. 2013; Koubová et al. 2014). The number of chromosomes in the male of L. burtonis (Pygopodidae) 2n = 33 was the same as previously reported by Gorman and Gress (1970); nevertheless, these authors reported that the putative Y chromosome in this species is metacentric, while we noticed that the chromosome is acrocentric with a prominent secondary constriction, which was probably misinterpreted as a centromere in the original work.
Comparative painting
Here, we report the results based on painting with the probes corresponding to GJA1, 2, 3, 6, 10, 11 and 12 chromosome pairs. We tested also single-chromosome probes covering the other small chromosomes, but the signal was not identifiable in all species and these results are thus not reported here.
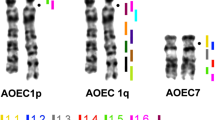
In O. monilis (2n = 38, Diplodactylidae), GJA chromosomes 1, 2, 3, 6, 10 and 11 were conserved in toto, and the chromosomes homologous to GJA1 and GJA2 were subtelocentric in O. monilis, while those homologous with GJA 3, 6, 10, 11 were acrocentric in O. monilis. The GJA12 probe painted just a band of a large subtelocentric chromosome of O. monilis (Figs. 2 and 3).
Examples of chromosome painting. The first column shows the hybridization of the probes with metaphases of the same species, Gekko japonicus, as described by Trifonov et al. (2011). In the next three columns, images of FISH of the probe derived from flow-sorted chromosomes of G. japonicus on metaphases of Oedura monilis, Tarentola annularis and Paroedura bastardi are shown. Numbers refer to the numbers of chromosomes/probes of G. japonicus. Arrows point to the FISH signals
Phylogenetic tree of studied species according to Pyron et al. (2013) with visualization of FISH signals on chromosomes. The times of the divergence follow Gamble et al. (2011). The pictograms of the chromosomes represent chromosomal pairs in all cases but hybridization of GJA10 on Lialis burtonis, where the probe hybridized with just a single chromosome. Data for species from the genera Gekko and Hemidactylus from Trifonov et al. (2011) are included
In L. burtonis (2n = 34 in females, 33 in males, Pygopodidae), GJA1, 3 and 6 were conserved in toto but were all acrocentric in L. burtonis. We were not able to identify any strong hybridization signal in FISH with the GJA2 probe in this species. The GJA11 probe painted just the p arm of the largest, metacentric chromosome of L. burtonis. The q arm of the same chromosome of L. burtonis was painted by the GJA10 probe but only in one homologue from the chromosomal pair (Fig. 3). This strange pattern was found on all metaphases of the single available male individual. The GJA12 probe painted just one band near the centromeric region of the second largest, metacentric chromosome of L. burtonis (Fig. 3).
In C. variegatus (2n = 32, Eublepharidae), the species with all-acrocentric chromosomes, GJA1, 2, 3, 6, 10 and 11, were conserved in toto. The GJA12 probe painted just one band on a large acrocentric chromosome of C. variegatus (Fig. 3).
The karyotype of T. annularis (2n = 42, Phyllodactylidae) also consists only of acrocentric chromosomes. The chromosomes homologous with GJA1, 3, 6, 10, 11 and 12 were conserved in toto in this species. The signal after hybridization with the GJA2 covered two chromosomes in T. annularis (Figs. 2 and 3).
In P. bastardi (2n = 34, Gekkonidae), GJA1, 2, 6, 10 and 11 were conserved in toto. The chromosomal pairs homologous with GJA1, 6, 10 and 11 are acrocentric, while the pair homologous with GJA2 is metacentric in P. bastardi. The signal of GJA3 probe painted the q arm of the largest, metacentric pair of PBA. The GJA12 probe painted a single band on an acrocentric chromosome of P. bastardi (Figs. 2 and 3).
In A. persica (2n = 42, Gekkonidae), the chromosomes GJA1, 3, 6, 10, 11 and 12 were conserved in toto as acrocentric or submetacentric chromosomes with the exception of the metacentric pair homologous to GJA6. The GJA2 probe painted two acrocentric chromosomes in A. persica (Fig. 3).
Discussion
Up to date, molecular cytogenetic data on chromosome homology for gekkotan lizard were restricted to a narrow taxonomic group, i.e. relatively closely related genera Gekko and Hemidactylus (Trifonov et al. 2011), or chromosomes homologous with the avian Z chromosome (Kawai et al. 2009; Pokorná et al. 2011; Matsubara et al. 2014b). These studies supported the large chromosome conservation in geckos reported in studies performed before the onset of molecular cytogenetic approaches (King 1987a,b; King and Megdon 1990; King 1990) and revealed only a limited number of interchromosomal rearrangements. This genomic feature is now confirmed by the expanded dataset presented here.
Gekkotan lizards are a very ancient group; the deepest split of Pygopodidae + Diplodactylidae + Carphodactylidae versus Eublepharidae + Sphaerodactylidae + Gekkonidae + Phyllodactylidae was estimated to be older than 140 MY (Gamble et al. 2011) and the divergence times of particular lineages within these two groups are substantial as well. According to Gamble et al. (2011), even the youngest split between gekkotan families, i.e. between Pygopodidae and Carphodactylidae, occurred already in the Cretaceous period. Despite these ancient divergences, our results demonstrate that most chromosomes are still conserved in toto across the whole gekkotan lineages. This conservation of chromosomes in geckos concurs with recent molecular cytogenetic evidence for high chromosome conservation in all sauropsids (e.g. Griffin et al. 2007; Pokorná et al. 2011, 2012; Kasai et al. 2012; Uno et al. 2012). A high chromosome conservation in geckos could also be expected from relatively stable number of chromosomes in karyotypes in some lineages (e.g. Diplodactylidae, King 1987b; most members of Eublepharidae, Pokorná et al. 2010), while chromosomal evolution was probably accelerated in other gekkotan lineages, for example, in the gekkonid genus Gehyra (King 1987b) or Hemidactylus (Trifonov et al. 2011).
The results showed that many chromosomes remained conserved in toto through the long evolutionary history of geckos, although they changed radically in their morphology (Fig. 3). For example, GJA1 is a metacentric chromosome which is syntenic with just a single subtelocentric or acrocentric chromosome in most geckos in our sample, especially in species with the more basal position such as O. monilis, L. burtonis and C. variegatus. Similarly, GJA2 is a metacentric chromosome, but its homologues are subtelocentric in O. monilis or acrocentric in C. variegatus, and acrocentric GJA6 is homologous with the metacentric chromosome in A. persica (Fig. 3). These changes in chromosomal morphology suggest that intrachromosomal rearrangements including pericentric inversion or centromere reposition were relatively common in geckos (see also Shibaike et al. 2010 or Trifonov et al. 2011 within the genus Gekko). Based on a comparison of whole genome sequences, Skinner and Griffin (2011) showed that intrachromosomal rearrangements were not rare in birds despite their low rate of interchromosomal rearrangements, which can be a general trend in sauropsids (Alföldi et al. 2011; Rovatsos et al. 2014c). Our analyses were not able to detect intrachromosomal rearrangements not involving conspicuous alterations in chromosome morphology, and detection of these in geckos will require different techniques. However, this limited evidence of intrachromosomal changes suggests that the situation in geckos may be comparable to the avian lineage.
All interchromosomal rearrangements we detected in geckos could be explained by chromosome fusions/fissions (see also Trifonov et al. 2011 within the genus Hemidactylus). One of the major aims of this project was to search for chromosomal rearrangements serving as putative synapomorphies for resolution of gekkotan phylogenetic relationships. Nevertheless, four of the interchromosomal rearrangements revealed in geckos by molecular cytogenetic techniques were not phylogenetically informative, as they are apomorphies of single included representatives, i.e. fusion involving chromosome homologous with GJA3 in P. bastardi, previously confirmed fusion of GJA1q and GJA5p in H. turcicus by Trifonov et al. (2011) and fusions involving chromosomes homologous with GJA10 and GJA11 in L. burtonis. It is interesting that the GJA10 probe painted a part of just a single homologue in a chromosomal pair in our male individual of L. burtonis, a species with neo-sex chromosomes (2n = 33 in males, 2n = 34 in females; Gorman and Gress 1970). However, the identified sex chromosomes are much smaller than the chromosome-bearing signal of GJA10 probe (Gorman and Gress 1970). More material would be needed to fully explain the localization results of the GJA10 probe in this species.
Split of the ancestral chromosome homologous with GJA1 into two acrocentric chromosomes seems to be a synapomorphy of the studied members of the genus Hemidactylus (H. turcicus, H. platyurus and H. frenatus; Trifonov et al. 2011).
Part of the genome homologous with GJA12 formed just a band on a pair of a bigger chromosomal pair in the ancestral gekkotan karyotype. It is present in members of the basal families Diplodactylidae (O. monilis), Pygopodidae (L. burtonis) and Eublepharidae (C. variegatus). This ancestral situation is also observed in P. bastardi, but not in the other members of the family Gekkonidae or in T. annularis (Phyllodactylidae), where the GJA12 probe paints a whole sole chromosome pair. This finding challenges the view that all karyotypes of the genus Gekko including GJA (2n = 38) are the same as the ancestral gekkotan karyotype (cf. Trifonov et al. 2011), which was suggested to be composed of the same number of chromosomes (e.g. Gorman 1973; Pokorná et al. 2010). The interpretation of the phylogenetic distribution of the interchromosomal rearrangements involving homologues of GJA12 is equivocal, and the most parsimonious interpretation of the distribution of chromosomal rearrangements is not concordant with the proposed phylogenetic relationships in geckos (cf. Gamble et al. 2008a, b, 2012; Fig. 3). Most importantly, the character state concerning GJA12 homologue is the same as the reconstructed ancestral gekkotan situation in P. bastardi (Gekkonidae), while the derived condition found otherwise in the members of the family Gekkonidae was also found in T. annularis (Phyllodactylidae). This observation questions the monophyly of the family Gekkonidae with respect to the family Phyllodactylidae defined by Gamble et al. (2008a). It is important to note that although the statistical support for the monophyly of the family was strong and the monophyly was repeatedly shown in different phylogenetic reconstructions (Gamble et al. 2008a, 2011, 2012), up to date, there is no known morphological character and only a few synapomorphies in gene sequences support the monophyly of the family Phyllodactylidae. Based on morphology of adhesive toe pads, the genus Paroedura was traditionally considered to be closely related to the genus Phyllodactylus (e.g. Dixon and Kroll 1974). The cytogenetic data presented here suggest that T. annularis could be more closely related to the genera Agamura, Gekko and Hemidactylus than the genus Paroedura, which is in total agreement with the phylogenetic relationships among these genera reconstructed by analysis of morphological characters (Kluge and Nussbaum 1995).
Part of the genome homologous with GJA2 probably formed a single-chromosome pair in the ancestral karyotype of Gekkota. This situation is present in members of the basal families Diplodactylidae (O. monilis) and Eublepharidae (C. variegatus), as well as in the members of the genus Gekko, in H. frenatus and P. bastardi (all Gekkonidae), although here the homologous part of the genomes forms a metacentric pair. Fission of GJA2 into two acrocentric chromosomes can be found in T. annularis (Phyllodactylidae), A. persica, H. turcicus and H. platyurus (all Gekkonidae). Trifonov et al. (2011) considered fission of the chromosome homologous with GJA2 and also GJA5 as putative synapomorphies of H. turcicus and H. platyurus within the genus Hemidactylus, leaving H. frenatus as sister to the H. turcicus-H. platyurus clade. Nevertheless, this interpretation is in contrast to the phylogeny of the genus Hemidactylus, where H. platyurus and H. frenatus are members of the monophyletic “Tropical Asian clade”, while H. turcicus is a member of the “Arid clade” (Carranza and Arnold 2006; see also Bansal and Karanth 2010 for the same topology), as well as with the yet alternative topology by Pyron et al. (2013) (Fig. 3). More character changes are required for the interpretation of the chromosome rearrangements concerning the chromosome homologous to GJA2 in geckos, and some character states must be homoplasic in this case. Robertsonian rearrangements are known to have relatively high levels of homoplasy. Homoplasy is also in agreement with the distribution of character states across the species included in our study. Polymorphism is present within the genus Hemidactylus, which is very likely monophyletic (e.g. Gamble et al. 2012; Pyron et al. 2013; Šmíd et al. 2013). Therefore, at least one of the character states must be derived within the genus Hemidactylus leading to homoplasic formation of the state present in its out-groups. Our preferred scenario is that the chromosome homologous to GJA2 was ancestrally acrocentric, or subtelocentric as recently found in O. monilis and C. variegatus, and that it evolved into the metacentric chromosome currently present in P. bastardi and members of the genus Gekko and H. frenatus. This chromosome could be independently split into two acrocentrics in the ancestor of T. annularis, A. persica, H. turcicus and H. platurus. Such reuse of a chromosome breakpoint leading to a homoplasic chromosome configuration was for instance demonstrated by us in Unidentata (Pokorná et al. 2012). More taxonomic sampling in future studies will be needed to resolve the situation more precisely.
In summary, our study demonstrates relatively high chromosome conservatism across the ancient group of gekkotan lizards. We documented that many changes in chromosomal morphology across geckos can be attributed to intrachromosomal rearrangements. However, we found a relatively small number of interchromosomal rearrangements. The observed rearrangements are not in agreement with the monophyly of the family Gekkonidae with respect to the family Phyllodactylidae. The results also pointed to homoplasy in the evolution of gecko karyotypes.
Abbreviations
- GJA:
-
Gekko japonicus
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative polymerase chain reaction
- DOP-PCR:
-
Degenerate oligonucleotide primed PCR
- FISH:
-
Fluorescence in situ hybridization
- dUTP:
-
Deoxy-uridine-5′-triphosphate
- SSC:
-
Saline sodium citrate
- DAPI:
-
4′,6-Diamidino-2-phenylindole
References
Alföldi J, Di Palma F, Grabherr M, Williams C, Kong L, Russell EP, Lowe CB, Glor RE, Jaffe JD, Ray DA, Boissinot S, Shedlock AM, Botka C, Castoe TA, Colbourne JK, Fujita MK, Godinez- Moreno R, Ten-Hallers BF, Haussler D, Heger A, Heiman D, Janes DE, Johnson J, De Jong PJ, Koriabine MY, Lara M, Novick PA, Organ CL, Peach SE, Poe S, Pollock DD, De Queiroz K, Sanger T, Searle S, Smith JD, Smith Z, Swofford R, Turner-Maier J, Young JS, Zadissa A, Edwards SV, Glenn TC, Schneider CJ, Losos JB, Lander ES, Breen M, Ponting CP, Lindblad-Toh K (2011) The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477:587–591
Aprea G, Andreone F, Fulgione D, Petraccioli A, Odierna G (2013) Chromosomal rearrangements occurred repeatedly and independently during species diversification in Malagasy geckos, genus Paroedura. Afr Zool 48:96–108
Bansal R, Karanth KP (2010) Molecular phylogeny of Hemidactylus geckos (Squamata: Gekkonidae) of the Indian subcontinent reveals a unique Indian radiation and an Indian origin of Asian house geckos. Mol Phylogenet Evol 57:459–465
Bauer AM, Masroor R, Titus-Mcquillan J, Heinicke MP, Daza JD, Jackman TR (2013) A preliminary phylogeny of the Palearctic naked-toed geckos (Reptilia: Squamata: Gekkonidae) with taxonomic implications. Zootaxa 3599:301–324
Carranza S, Arnold EN (2006) Systematics, biogeography, and evolution of Hemidactylus geckos (Reptilia: Gekkonidae) elucidated using mitochondrial DNA sequences. Mol Phylogenet Evol 38:531–545
Červenka J, Kratochvíl L, Frynta D (2008) Phylogeny and taxonomy of the Middle Eastern geckos of the genus Cyrtopodion and their selected relatives. Zootaxa 1931:25–36
Deakin JE, Ezaz T (2014) Tracing the evolution of amniote chromosomes. Chromosoma 123:201–216
Dixon JR, Kroll JC (1974) Resurrection of the generic name Paroedura for the phyllodactyline geckos of Madagascar, and description of a new species. Copeia 1974:24–30
Estes R, de Queiroz K, Gauthier J (1988) Phylogenetic relationships within Squamata. In: Estes R, Pregill J (eds) Phylogenetic relationships of the lizard families. Stanford University Press, Stanford, pp 119–281
Ferguson-Smith MA, Trifonov V (2007) Mammalian karyotype evolution. Nat Rev Genet 8:950–962
Gamble T, Bauer AM, Greenbaum E, Jackman TR (2008a) Out of the blue: a novel, trans-Atlantic clade of gecko lizards (Gekkota, Squamata). Zool Scr 37:355–366
Gamble T, Bauer AM, Greenbaum E, Jackman TR (2008b) Evidence for Gondwanan vicariance in an ancient clade of gecko lizards. J Biogeogr 35:88–104
Gamble T, Bauer AM, Colli GR, Greenbaum E, Jackman TR, Vitt LJ, Simons AM (2011) Coming to America: multiple origins of New World geckos. J Evol Biol 24:231–244
Gamble T, Greenbaum E, Jackman TR, Russell AP, Bauer AM (2012) Repeated origin and loss of adhesive toepads in geckos. PLoS One 7:e39429
Gamble T, Geneva AJ, Glor RE, Zarkower D (2014) Anolis sex chromosomes are derived from a single ancestral pair. Evolution 68:1027–1041
Gorman GC (1973) The chromosomes of the Reptilia, a cytotaxonomic interpretation. In: Chiarelli AB, Capanna E (eds) Cytotaxonomy and vertebrate evolution. Academic, New York, pp 349–424
Gorman GC, Gress F (1970) Sex chromosomes of a pygopodid lizard, Lialis burtonis. Experientia 26:206–207
Graphodatsky AS, Trifonov VA, Stanyon R (2011) The genome diversity and karyotype evolution of mammals. Mol Cytogenet 4:22
Griffin DK, Robertson LBW, Tempest HG, Skinner BM (2007) The evolution of the avian genome as revealed by comparative molecular cytogenetics. Cytogenet Genome Res 117:64–77
Grismer LL (1988) The phylogeny, taxonomy, classification and biogeography of eublepharid geckos (Reptilia: Squamata). In: Estes R, Pregill J (eds) Phylogenetic relationships within Squamata. Stanford University Press, Stanford, pp 369–469
Han D, Zhou K, Bauer AM (2004) Phylogenetic relationships among gekkotan lizards inferred from C‐mos nuclear DNA sequences and a new classification of the Gekkota. Biol J Linn Soc 83:353–368
Hillier LW, Miller W, Birney E, Warren W, Hardison RC, Ponting CP, … Dodgson JB (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432: 695–716
Johnson Pokorná M, Rovatsos M, Kratochvíl L (2014) Sex chromosomes and karyotype of the (nearly) mythical creature, the Gila monster, Heloderma suspectum (Squamata: Helodermatidae). PLoS One 9:e104716
Kasai F, O’Brien PCM, Martin S, Ferguson-Smith MA (2012) Extensive homology of chicken macrochromosomes in the karyotypes of Trachemys scripta elegans and Crocodylus niloticus revealed by chromosome painting despite long divergence times. Cytogenet Genome Res 136:303–307
Kawai A, Ishijima J, Nishida C, Kosaka A, Ota H, Kohno SI, Matsuda Y (2009) The ZW sex chromosomes of Gekko hokouensis (Gekkonidae, Squamata) represent highly conserved homology with those of avian species. Chromosoma 118:43–51
King M (1987a) Monophyleticism and polyphyleticism in the Gekkonidae: a chromosomal perspective. Aust J Zool 35:641–654
King M (1987b) Chromosomal evolution in the Diplodactylinae (Gekkonidae, Reptilia). 1. Evolutionary relationships and patterns of change. Aust J Zool 35:507–531
King M (1990) Chromosomal and immunogenetic data: a new perspective on the origin of Australia’s reptiles, in Olmo E (ed) Cytogenetics of amphibians and reptiles pp 153–180
King M, Mengdon G (1990) Chromosomal evolution in the Diplodactylidae (Gekkonidae: Reptilia) II. Chromosomal variability between New Caledonian species. Aust J Zool 38:219–226
Kluge AG (1983) Cladistic relationships among gekkonid lizards. Copeia 1983:465–475
Kluge AG, Nussbaum RA (1995) A review of African-Madagascan gekkonid lizard phylogeny and biogeography (Squamata). Misc Publ Mus Zool Mich 183:1–28
Koubová M, Johnson Pokorná M, Rovatsos M, Farkačová K, Altmanová M, Kratochvíl L (2014) Sex determination in Madagascar geckos of the genus Paroedura (Squamata: Gekkonidae): are differentiated sex chromosomes indeed so evolutionary stable? Chromosome Res 22:441–452
Manilo VV (1996) Peculiarities of the karyotypes in the family Gekkonidae (Sauria, Reptilia). Communication 3. Genus Tenuidactylus. Vestnik Zool 32:31–37
Matsubara K, Tarui H, Toriba M, Yamada K, Nishida-Umehara C, Agata K, Matsuda Y (2006) Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc Natl Acad Sci U S A 103:18190–18195
Matsubara K, Sarre SD, Georges A, Matsuda Y, Graves JAM, Ezaz T (2014a) Highly differentiated ZW sex microchromosomes in the Australian Varanus species evolved through rapid amplification of repetitive sequences. PLoS One 9:e95226
Matsubara K, Gamble T, Matsuda Y, Zarkower D, Sarre SD, Georges A, Graves JAM, Ezaz T (2014b) Non-homologous sex chromosomes in two geckos (Gekkonidae: Gekkota) with female heterogamety. Cytogenet Genome Res 143:251–258
Oguiura N, Ferrarezzi H, Batistic RF (2010) Cytogenetics and molecular data in snakes: a phylogenetic approach. Cytogenet Genome Res 127:128–142
Pokorná M, Rábová M, Ráb P, Ferguson-Smith MA, Rens W, Kratochvíl L (2010) Differentiation of sex chromosomes and karyotypic evolution in the eye-lid geckos (Squamata: Gekkota: Eublepharidae), a group with different modes of sex determination. Chromosome Res 18:809–820
Pokorná M, Giovannotti M, Kratochvíl L, Kasai F, Trifonov VA, O’Brien PC, Caputo V, Olmo E, Ferguson-Smith MA, Rens W (2011) Strong conservation of the bird Z chromosome in reptilian genomes is revealed by comparative painting despite 275 million years divergence. Chromosoma 120:455–468
Pokorná M, Giovannotti M, Kratochvíl L, Caputo V, Olmo E, Ferguson-Smith MA, Rens W (2012) Conservation of chromosomes syntenic with avian autosomes in squamate reptiles revealed by comparative chromosome painting. Chromosoma 121:409–418
Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 13:93
Rens W, O’Brien PCM, Yang F, Graves JAM, Ferguson-Smith MA (1999) Karyotype relationships between four distantly related marsupials revealed by reciprocal chromosome painting. Chromosome Res 7:461–474
Rens W, Fu B, O’Brien PCM, Ferguson-Smith MA (2006) Cross-species chromosome painting. Nat Protoc 1:783–790
Rovatsos M, Altmanová M, Pokorná M, Kratochvíl L (2014a) Conserved sex chromosomes across adaptively radiated Anolis lizards. Evolution 68:2079–2085
Rovatsos M, Pokorná M, Altmanová M, Kratochvíl L (2014b) Cretaceous park of sex determination: sex chromosomes are conserved across iguanas. Biol Lett 10:20131093
Rovatsos M, Altmanová M, Johnson Pokorná M, Kratochvíl L (2014c) Novel X-linked genes revealed by qPCR in the green anole, Anolis carolinensis. G3:114
Shibaike Y, Takahashi Y, Arikura I, Iiizumi R, Kitakawa S, Sakai M, Imaoka C, Shiro H, Tanaka H, Akakubo N, Nakano M, Watanabe M, Ohne K, Kubota S, Kohno S, Ota H (2010) Chromosome evolution in the lizard genus Gekko (Gekkonidae, Squamata, Reptilia) in the east Asian islands. Cytogenet Genome Res 127:182–190
Skinner BM, Griffin DK (2011) Intrachromosomal rearrangements in avian genome evolution: evidence for regions prone to breakpoints. Heredity 108:37–41
Šmíd J, Carranza S, Kratochvíl L, Gvoždík V, Nasher AK, Moravec J (2013) Out of Arabia: a complex biogeographic history of multiple vicariance and dispersal events in the gecko genus Hemidactylus (Reptilia: Gekkonidae). PLoS One 8:e64018
Srikulnath K, Nishida C, Matsubara K, Uno Y, Thongpan A, Suputtitada S, Apisitwanich S, Matsuda Y (2009) Karyotypic evolution in squamate reptiles: comparative gene mapping revealed highly conserved linkage homology between the butterfly lizard (Leiolepis reevesii rubritaeniata, Agamidae, Lacertilia) and the Japanese four-striped rat snake (Elaphe quadrivirgata, Colubridae, Serpentes). Chromosome Res 17:975–986
Srikulnath K, Matsubara K, Uno Y, Nishida C, Olsson M, Matsuda Y (2014) Identification of the linkage group of the Z sex chromosomes of the sand lizard (Lacerta agilis, Lacertidae) and elucidation of karyotype evolution in lacertid lizards. Chromosoma 123:563–575
Townsend TM, Larson A, Louis E, Macey JR (2004) Molecular phylogenetics of Squamata: the position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Syst Biol 53:735–757
Trifonov VA, Giovannotti M, O’Brien PC, Wallduck M, Lovell F, Rens W, Parise-Maltempi PP, Caputo V, Ferguson-Smith MA (2011) Chromosomal evolution in Gekkonidae. I. Chromosome painting between Gekko and Hemidactylus species reveals phylogenetic relationships within the group. Chromosome Res 19:843–855
Uetz P, Hošek J (eds) (2014) The reptile database. Available at http://www.reptile-database.org. Accessed August 1, 2014
Underwood G (1954) On the classification and evolution of geckos. Proc Zool Soc 124:469–492
Uno Y, Nishida C, Tarui H, Ishishita S, Takagi C, Nishimura O, Ishijima J, Ota H, Kosaka A, Matsubara K, Murakami Y, Kuratani S, Ueno N, Agata K, Matsuda Y (2012) Inference of the protokaryotypes of amniotes and tetrapods and the evolutionary processes of microchromosomes from comparative gene mapping. PLoS One 7:e53027
Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Künstner A, Searle S, White S, Vilella AJ, Fairley S, Heger A, Kong L, Ponting CP, Jarvis ED, Mello CV, Minx P, Lovell P, Velho TA, Ferris M, Balakrishnan CN, Sinha S, Blatti C, London SE, Li Y, Lin YC, George J, Sweedler J, Southey B, Gunaratne P, Watson M, Nam K, Backström N, Smeds L, Nabholz B, Itoh Y, Whitney O, Pfenning AR, Howard J, Völker M, Skinner BM, Griffin DK, Ye L, McLaren WM, Flicek P, Quesada V, Velasco G, Lopez-Otin C, Puente XS, Olender T, Lancet D, Smit AF, Hubley R, Konkel MK, Walker JA, Batzer MA, Gu W, Pollock DD, Chen L, Cheng Z, Eichler EE, Stapley J, Slate J, Ekblom R, Birkhead T, Burke T, Burt D, Scharff C, Adam I, Richard H, Sultan M, Soldatov A, Lehrach H, Edwards SV, Yang SP, Li X, Graves T, Fulton L, Nelson J, Chinwalla A, Hou S, Mardis ER, Lancet D (2010) The genome of a songbird. Nature 464:757–762
Wiens JJ, Hutter CR, Mulcahy DG, Noonan BP, Townsend TM, Sites JW, Reeder TW (2012) Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol Lett 8:1043–1046
Yang F, Carter NP, Shi L, Ferguson-Smith MA (1995) A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103:642–652
Yaseen AE, Hassan HA, Kawashti IS (1995) Chromosomes of three Egyptian lizards of the family Gekkonidae (Squamata: Reptilia). Cytologia 60:233–242
Acknowledgments
The authors would like to express their gratitude to P. Ráb for the ongoing support, to J. Červenka for animal care and two anonymous reviewers for constructive comments. The project was supported by Czech Science Foundation (project no. 506/10/0718).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fengtang Yang
Rights and permissions
About this article
Cite this article
Pokorná, M.J., Trifonov, V.A., Rens, W. et al. Low rate of interchromosomal rearrangements during old radiation of gekkotan lizards (Squamata: Gekkota). Chromosome Res 23, 299–309 (2015). https://doi.org/10.1007/s10577-015-9468-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-015-9468-6