Abstract
The perineurium serves as a selective, metabolically active diffusion barrier in the peripheral nervous system, which is composed of perineurial cells joined together by tight junctions (TJs). Not only are these junctions known to play an essential role in maintaining cellular polarity and tissue integrity, but also limit the paracellular diffusion of certain molecules and ions, whereas loss of TJs barrier function is imperative for tumour growth, invasion and metastasis. Hence, a detailed study on the barrier function of perineurial cells may provide insights into the molecular mechanism of perineural invasion (PNI). In this study, we aimed to develop an efficient procedure for the establishment of perineurial cell lines as a tool for investigating the physiology and pathophysiology of the peripheral nerve barriers. Herein, the isolation, expansion, characterization and maintenance of perineurial cell lines under favourable conditions are presented. Furthermore, the analysis of the phenotypic features of these perineurial cells as well as the barrier function for the study of PNI are described. Such techniques may provide a valuable means for the functional and molecular investigation of perineurial cells, and in particular may elucidate the pathogenesis and progression of PNI, and other peripheral nerve disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perineural invasion (PNI) refers to invasion and metastasis of tumour cells along a nerve or membrane structure around nerve fibres. PNI is one of the markers of a malignant tumour and a distinctive mechanism of malignant tumour metastasis, which often leads to incomplete tumour resection and postoperative tumour recurrence and seriously affects the prognosis of patients (Chen et al. 2019). Liebig et al. first defined PNI as a condition in which a tumour is close to nerve fibres and surrounding the periphery of nerve fibres by at least 33% or in which tumour cells have invaded into any of three layers of connective tissue enveloping nerve fibres; this definition also constitutes the pathological diagnostic criteria for the occurrence of PNI (Liebig et al. 2009). It is known that PNI is a complex process involving multiple cytokines and signal pathways resulting from interaction with and promotion of tumour cells by the tumour microenvironment (Liang et al. 2016). Common types of tumours involving perineural invasion include prostate cancer, pancreatic cancer, stomach cancer, adenoid cystic carcinoma, etc. (Deng et al. 2014; Lubig et al. 2018; Patel et al. 2018; Zhang et al. 2018). Tumours with perineural invasion often have a poor prognosis; therefore, further study of the mechanism of tumour perineural invasion has very important clinical value for earlier and more accurate determination of whether patients have perineural invasion, or even for blocking or reversing the PNI of tumours.
Nerve fibres of the peripheral nervous system gather together to form nerves, and the dense connective tissue surrounding a nerve has three layers; i.e. epineurium, perineurium and endoneurium from outside to inside (Reina et al. 2000). The cell barrier composed of perineurial cells is the first layer of cell barrier structure for defence against perineural invasion by tumours. The study of the mechanism of interaction between tumours and perineurial cells is of great value for further exploration of PNI mechanisms in salivary adenoid cystic carcinoma.
Perineurial cells are derived from mesenchymal cells. They constitute the perineurium and create a physical barrier to maintain the integrity and stability of the nerve's internal environment; they moreover limit entry by bioactive proteins, infectious pathogens and haematogenous cells (Erlandson 1991). The interaction between tumours and the perineurium is of great significance for further exploration of the early mechanism of PNI in tumours. However, there are few studies of the perineurial barrier at present, and a complete system of perineurial cell culture is lacking. In vitro studies of the relationship between nerve tumours and the perineurium are limited. This study mainly does the following: investigates culture methods and conditions for perineurial cells; identifies perineurial cells; discusses their functional features; develops plans for establishing a methodology for separation, culture and identification of perineurial cells; and conveys a certain understanding to provide reference value for further investigation of the role of perineurial cells in tumour perineural invasion.
Methods
Cell Culture
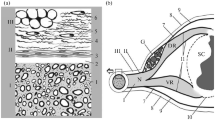
RSC96 (iCell Bioscience Inc, Shanghai, China), a rat Schwann cell line, was cultured in DMEM (Gibco, Carlsbad, CA, USA) medium containing 10% foetal bovine serum (FBS). SACC-83 (From Prof. Jingsong Li of Department of Oral and Maxillofacial Surgery, SUN YAT-SEN MEMORIAL HOSPITAL, SUN YAT-SEN UNIVERSITY), an adenoid cystic carcinoma cell line, was cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) containing 10% FBS (Gibco, Carlsbad, CA, USA). Regarding the primary culture of perineurial cells, the Institutional Animal Care and Use Committee of Southern Medical University approved this study. Three-week-old Sprague–Dawley rats were deeply anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 15 mg/kg xylazine, until the absence of a deep tendon reflex (no foot or paw withdrawal in response to pinching). Each rat was sacrificed by cervical dislocation and disinfected by immersion in 75% alcohol, and then decapitated and fixed in the prone position. The spinal cord was removed by severing the spinal canal with microscissors, exposing the dorsal root ganglion (DRG) (Fig. 1). The DRG was rinsed with PBS and digested by trypsin (30 min), then the supernatant was removed and the DRG tissue was digested by trypsin again (30 min) after centrifugation (300 g, 3 min). After digestion, cells were dispersed by repeated pipetting with a 1-mL pipette and then cultured in a Petri dish for 3 h (37 °C, 5% CO2). Finally, the supernatant, tissue mass and non-adherent cells were removed, and adherent cells were cultured in DMEM (DMEM medium containing 10% foetal bovine serum) or Basal Epithelial Medium (BEM; consisted of a 3:1 mixture of Ham's F12 and DMEM supplemented with 2.5% FBS, 10 ng/mL Epidermal Growth Factor, 0.4 μg/mL Hydrocortisone and 0.5 μg/mL insulin) or ECM (Sciencell, San Diego, California, USA) mediums, respectively. All cultures were supplemented with 100 UI/mL penicillin and 100 μg/mL streptomycin and placed in an incubator (37 °C, 5% CO2). Cell growth was observed under a microscope.
Perineurial cells in primary culture and verification. a The position of dorsal root ganglion (DRG) is indicated by arrow. b Cell morphology of perineurial cells was observed under inverted microscope: Primary perineurial cells showed a flat, polygonal appearance (A: × 40; B: × 100); The first-generation (P1) perineurial cells were more slender than the primary cells (C: × 40; D: × 100). c Identification of perineurial cells by immunocytochemical staining under light microscope (× 400): perineurial cells were positive for CLDN1 (A), GLUT1 (B), EMA (C) and negative for CD57 (D), S-100 (E). d Identification of perineurial cells by flow cytometry: perineurial cells were positive for CD29 and negative for CD31, CD34
Immunocytochemistry
The second generation of perineurial cells were harvested, prepared into cell suspension after digestion by trypsin and inoculated into a 24-well plate with a slide in advance. After cells reached 50% of the slide, they were fixed with 4% paraformaldehyde. Hydrogen peroxide (30%) and methanol were mixed at a ratio of 1:50, dropped on the slide surface and incubated at 37 °C for 20 min to inactivate endogenous peroxidase. Confining liquid was added and blocked at room temperature for 20 min. Cells were incubated with rabbit anti-human OCLN (Abcam, Cambridge, England), CLDN1 (Abcam, Cambridge, England), GLUT1 (Abcam, Cambridge, England), EMA (Cell Signaling Technology, Beverly, MA, USA), S-100 (Cell Signaling Technology, Beverly, MA, USA) and CD57 (Cell Signaling Technology, Beverly, MA, USA) antibodies (1:50 dilution) overnight at 4 °C and washed three times with PBS. Cells were then incubated with HRP-conjugated or Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:1000 dilution) at room temperature for 1 h. HRP activity was detected by diaminobenzidine tetrahydrochloride (DAB) staining, and immunofluorescence staining was performed to stain nuclei using 1 mg/L DAPI. Cells were then imaged under an optical or a fluorescent microscope.
MTT Assay
DMEM, BEM and ECM mediums were used in primary culture to culture perineurial cells. Perineurial cells in logarithmic growth phase were inoculated into a 96-well plate at a density of 5 × 103/mL. DMEM, BEM and ECM mediums were adopted separately and incubated at 37 °C and 5% CO2, using days as a unit. MTT staining solution was added for 7 consecutive days. The supernatant was drawn up after continuous culture for 4 h; DMSO was added and the preparation was shaken thoroughly on a shaker. The absorbance value was measured with a microplate reader at 570 nm wavelength. Each well was repeated at least three times; cell proliferation curves were drawn according to the obtained OD values.
Flow Cytometry
Logarithmic perineurial cells cultured in DMEM, BEM and ECM mediums were prepared into cell suspensions, centrifuged and resuspended, washed twice with PBS with the supernatant discarded, fixed with 70% ethanol at 4 °C overnight, washed twice with PBS to remove ethanol, incubated at 37 °C for 30 min and by 10 μL of RNase at 37 ℃ for 20 min, stained with PI staining solution and analysed by flow cytometry.
Cell Tubule Formation Assay
A pre-cooled pipette tip was used to spread Matrigel on ice in a 96-well plate at 50 μL per well; the plate was placed in a 37 °C, 5% CO2 incubator for 1 h and cell suspension was added (2 × 104 cells/well). After 6 h, 12 h, 18 h, 24 h and 36 h, the tubular arrangement of cells and the number and integrity of tubular structures were observed under a microscope and photographed.
Electron Microscope
The second generation of perineurial cells were harvested, prepared into cell suspension and inoculated into a 24-well plate with a slide in advance. After cells reached 50% of the slide, they were postfixed with 2.5% glutaraldehyde for 2 h, washed with 0.1 M phosphate-buffered saline and then exposed to 1% osmium tetroxide for 2 h. Subsequently, they were washed several times with PBS, dehydrated using an alcohol gradient and embedded in Epon resin. Randomly selected, ultrathin sections were stained with uranyl acetate and lead citrate and then examined using a transmission electron microscope (H-7650, HITACHI, Tokyo, Japan).
Transmembrane Electrical Resistance Measurements
Perineurial cells and RSC96 were, respectively, seeded onto collagen-coated 12-mm-diameter polycarbonate Transwell permeable support cell culture inserts (0.4 pore size). Then, cells were cultured for 24 h until the formation of tight monolayers. The resistance values were measured with a resistance metre at 24 h, 48 h and 72 h.
Cell Monolayer Permeability Assays
Permeability across cell monolayers was measured using gelatin-coated Transwell units. Perineurial cells and RSC96 were separately plated and then cultured for 24 h until the formation of tight monolayers. Then, FITC-labelled dextran (1 mg/mL of fluorescein isothiocyanate-dextran, molecular mass: 10 kDa; Sigma-Aldrich, USA) was added to the top of each chamber. After 30-min treatment with FITC-dextran, 100 μL of each receiver plate well solution was collected, and fluorescence was evaluated using a SpectraMax M5 multifunctional microplate reader. The fluorescence due to FITC-dextran was determined using the Gen5 program at an excitation wavelength of 485 nm and an emission wavelength of 527 nm. Values are expressed as fluorescence arbitrary units (a.u). The experiment was repeated three times.
Boyden Chamber Assay
Perineurial cells and RSC96 were separately plated and cultured as above until tight monolayers formed. Then, SACC-83 cells were labelled with calcein (500 nM), following the manufacturer’s instructions. Calcein-AM labelled SACC-83 cells were added to each monolayer at a density of 1.5 × 105 cells/dish. After 12 h of migration, cells in each upper chamber were removed with cotton swabs. SACC-83 cells which crossed the membrane of each Transwell unit were counted in five different visual fields under an IX71 fluorescence microscope equipped with a 20X objective. The experiment was repeated three times.
Statistical Analysis
Assay data were statistically plotted with GraphPad Prism 5.0, and related data analysis was performed with SPSS 19.0 statistical software. Pairwise comparisons of data between groups were performed using the independent sample t test. The statistical significance for resistance data analysis and permeability assays was determined by one-way analysis of variance (ANOVA), followed by multiple pairwise comparisons with Tukey’s post hoc tests. Differences were considered statistically significant at P < 0.05.
Results
Culture and Identification of Perineurial Cells
After 3 days of primary culture, the distribution of perineurial cells was observed with an inverted microscope. Cells appeared flat and polygonal (Fig. 1b), which was consistent with the morphological features of endothelial cells. One week later (after cell passage), the first-generation (P1) perineurial cells were more slender than primary cells (Fig. 1b), and cells were tightly connected into a sheet. Immunocytochemical staining results (Fig. 1c) showed that CLDN1, GLUT1 and EMA from the primary culture perineurial cells were positively expressed, the cytoplasm was brown and the staining was darker. CLDN1, GLUT1 and EMA were used as identification indexes for perineurial cells. Additionally, the expression of S-100 and CD57 was negative, distinguishing these cells from Schwann cells. In conclusion, these primary cultured cells were proven to be perineurial cells (Hirose et al. 2003; Peltonen et al. 1987). Flow cytometry results (Fig. 1d) showed negative expression of endothelial cell markers CD31 and CD34 in these cells, which indicated that although the morphology of perineurial cells was similar to that of endothelial cells, it was not identical to endothelial cells. The mesenchymal marker CD29 was positively expressed, suggesting that perineurial cells may be derived from mesenchymal cells.
To select the most suitable medium, DMEM, BEM and ECM mediums were separately used to culture perineurial cells. It can be seen from the figure (Fig. 2a) that after 3 days of primary culture, the ECM medium contained the greatest number of cells. Morphology of cells in ECM and BEM mediums was clearer than for those in DMEM medium, and cells were arranged more closely and were polygonal. Cells in DMEM medium were relatively slender, loosely arranged and the number of cells was relatively low. After passage, the number and morphology of cells cultured in different mediums also differed. The cell arrangement in BEM and DMEM mediums was relatively loose compared to that in the ECM medium, and numbers were also low.
Effect of different media compositions on proliferation of perineurial cells. a Cell morphology and proliferative capacity of perineurial cells cultured in different media were observed under inverted microscope (× 100): ECM medium had the largest number of cells. Cell morphology of ECM and BEM mediums was clearer than that of DMEM medium, and cells were arranged more closely and polygonal, while cells in DMEM medium were relatively slender, loosely arranged, and the number of cells was relatively small. b The proliferative capacity of perineurial cells cultured in different media was assessed using MTT assay (**P < 0.01): The OD value of ECM group was significantly higher than that of DMEM group and BEM group. c The distribution of perineurial cells cultured in different media as percentage in each cell cycle was examined by flow cytometry
Proliferative Capacity of Perineurial Cells Cultured in Different Mediums
MTT was used to detect changes in the proliferative capacity of perineurial cells cultured in different mediums, and the results showed that the OD value of the ECM group was significantly higher than that of the DMEM group and the BEM group over time (P < 0.01). Additionally, the BEM group had a higher OD value than did the DMEM group (P < 0.01) (Fig. 2b). Flow cytometry was adopted to detect the cell cycle of perineurial cells cultured in different mediums (Fig. 2c). The results showed that the percentage of cells in either G2 or S phase in the ECM group was significantly higher than in the BEM and DMEM groups, and the percentage of cells in either G2 or S phase in the BEM group was significantly higher than in the DMEM group (P < 0.05). It is suggested that, compared with DMEM medium and BEM medium, the cell proliferation capacity of perineurial cells was significantly enhanced when cells were cultured with ECM medium. In addition, compared with DMEM medium, BEM medium showed stronger proliferation capacity of perineurial cells.
Perineurial Cells had the Capacity to form Tubules In Vitro
Interestingly, it was found that when cultured cells were sparse, perineurial cells were arranged into a cobweb-like structure, forming a single cavity-like structure (Fig. 3a). Combining the features of perineurial cells that could surround the nerve tract, it was speculated that perineurial cells had the capacity to form tubules. A tubule-forming assay showed that perineurial cells had the capacity to form tubules (Fig. 3b). Lumina began to form at 6 h and gradually expanded and developed at 12–24 h. They began to shrink after 24 h until becoming almost completely filled with proliferating cells at 36 h.
Tube formation ability of perineurial cells. a Observed under inverted microscope, perineurial cells were arranged into a cobweb-like structure, forming a single cavity-like structure (A: × 100; B: × 200). b In vitro tube formation assay of perineurial cells (× 40): lumen began to form at 6 h (A), and gradually expanded and formed at 12–24 h (B, C). It began to shrink after 24 h until it was almost completely filled with proliferating cells at 36 h (D)
Perineurial Cells Exhibit Barrier Capacity
Transmission electron microscopy (Fig. 4a) showed that tight junctions (TJs) were formed between perineurial cells and were complete, and the desmosome structure could be seen. The cell immunofluorescence chemistry experiment (Fig. 4b) showed that OCLN was positively expressed in perineurial cells and mainly distributed around the cell membranes, with perineurial cells arranged closely. To verify the barrier functioning of perineurial cells, a transmembrane electrical resistance experiment, cell monolayer permeability assay and Boyden chamber assay were performed to compare perineurial cells with their adjacent Schwann cells.
Verification of barrier function in perineurial cells. a Transmission electron images of perineurial cells showed the presence of tight junctions indicated by the arrows (× 10,000). b Expression of OCLN in perineurial cells: OCLN was positive expressed in perineurial cells and mainly distributed around the cell membrane. c Transmembrane electrical resistance experiment showed that the resistance value of the barrier formed by perineurial cells was significantly higher than that of Schwann cell group (**P < 0.01). d Cell monolayer permeability assay showed that macromolecular substances (FITC-dextran) penetrated the cell barrier of perineurial cells group were significantly less than Schwann cells group (***P < 0.001). e Boyden chamber assay showed that the metastasis number of SACC-83 cells labelled with calcein-AM in Schwann cell group was significantly higher than that in the perineurial cells group
Electrical resistance is a measure of the barrier property of the epithelium, where decreased resistance indicates an increase in permeability. The transmembrane electrical resistance experiment showed that the resistance value of the barrier formed by perineurial cells was significantly higher than that of the Schwann cell group (Fig. 4c). The cell monolayer permeability assay (Fig. 4d) showed that the OD value of perineurial cells was significantly lower than that of the Schwann cell group, indicating that macromolecular substances that penetrated the cell barrier were significantly less than for the Schwann cells. The Boyden chamber assay (Fig. 4e) showed that the number of metastatic SACC-83 cells labelled with calcein-AM in the Schwann cell group was significantly higher than in the perineurial cells group. Therefore, it may be inferred that among the various layers of the nerve, perineurial cells are the main player in the nerve barrier function.
Discussion
PNI is one of the main features of many tumours, as well as a key pathological feature of many malignancies, including head–neck carcinoma, pancreatic cancer, colon cancer, rectal cancer, prostate cancer and gastric cancer. It is called the fourth pathway of tumour metastasis. The other three are direct invasion of surrounding tissues, lymphatic metastasis and haematogenous metastasis (Amit et al. 2016). PNI is closely related to survival rate and recurrence rate in cancer patients, and directly affects the prognosis of patients. For many malignant tumours, the occurrence of perineural invasion is often a sign of poor prognosis and reduced chance of survival (Gasparini et al. 2019; Kraus et al. 2019; Nozawa et al. 2019).
Of note, PNI occurrence is not the result of a single factor. Indeed, its formation is considered to be a continuous multistep process and associated with various influencing factors. Schwann cells have in recent years received increasing attention in relation to PNI process. It has been found that these cells can promote the survival of damaged nerve cells and regenerate the axons by secreting a variety of neurotrophic factors, which themselves have been implicated in promoting PNI and cancer invasion. Previous studies have shown that Schwann cells can migrate inward to tumours before PNI occurs, and promote tumour invasion by directly contacting cancer cells and guiding them to migrate toward the nerves (Deborde et al. 2016).
The perineural nerves are enclosed by three layers of connective tissue from the outside to the inside, the epineurium, perineurium and endoneurium. The perineurium is the outermost barrier system of nerves with cellular structure. It is composed of perineurial cells, which form tight junctions and surround the axon in a concentric circle with a certain defensive effect against tumour perineural invasion (Weerasuriya and Mizisin 2011), while the endoneurium surrounds individual axons and Schwann cells forming the myelin. Compared with Schwann cells, perineurial cells have stronger barrier capacity. They are the main player of nervous barrier function and the first line of defence against PNI by tumours (Reina et al. 2003). When tumours invade a nerve, it is possible that the secretion of certain substances damages the tight junctions between perineurial cells and weakens the resistance of the perineurium barrier, thereby facilitating invasion of the nerve (Mizisin and Weerasuriya 2011). Studying the perineurium barrier will help to further elucidate the mechanism of PNI. The basis for studying the relationship between the perineurium barrier and tumour perineural invasion is first to establish a complete methodology for separation, culture and identification of perineurial cells, and second, to understand some features of perineurial cells, so that the mechanism can be better researched.
When studying the culture conditions of perineurial cells, three different culture mediums, ECM, BEM and DMEM, were used in order to select the most suitable culture medium. Cell cycle and cell reproductive activity in different mediums were tested through the MTT cell proliferation assay and flow cytometry. ECM is specialized for endothelial cells, BEM is suitable for the growth of epithelial cells and DMEM is widely suitable for the cultivation of a variety of cells, especially for mesenchymal cells. Interestingly, it was found that ECM was more suitable for perineurial cells than was DMEM, and their growth was best in ECM. Flow cytometry confirmed that perineurial cells were derived from mesenchymal cells. Therefore, it may be speculated that although perineurial cells are derived from neurogenic mesenchymal cells, they might be different from other mesenchymal cells and exhibit special features (Sasse et al. 2015).
As for the identification of perineurial cells, some researchers previously proposed that CLDN1, GLUT1 and EMA were proteins specifically expressed by perineurial cells which could be used for the identification of this cell type (Izquierdo et al. 2012). Since such cells were cultured from the DRG of SD rats, Schwann cells were also present in addition to perineurial cells (Deborde and Wong 2017). S-100 and CD57 are considered to be specific proteins of Schwann cells (Chen et al. 2012), and have been used as markers to distinguish perineurial cells from Schwann cells (Al-Ghanem et al. 2013).
Interestingly, it was found that perineurial cells in a matrix-free cell culture dish could form a tubular-like structure. A tubule formation assay was performed subsequently to prove that perineurial cells could form tubules in vitro, which provided new ideas for exploring in vitro study models of perineurium formation.
In addition to perineurial cells, there are also Schwann cells in each layer of nerve structure. Perineurial cells were compared with Schwann cells, and the result showed that the barrier capacity of perineurial cells was much higher than that of Schwann cells, and Schwann cells had almost no barrier function. It is suggested that the barrier function of the nerve mainly derives from perineurium, not from Schwann cells. OCLN was an important cytoskeleton protein that formed tight junctions in cells and was expressed in perineurial cells and distributed in the cell membrane, suggesting that perineurial cells had tight junctions and certain barrier capacity (Pummi et al. 2006). In addition, it was observed with electron microscopy that tight junctions were formed between perineurial cells and desmosome structure was visible, indicating that perineurium has barrier capacity and may resist tumour invasion to a certain extent. Studying the mechanism of damage to the perineurium has significance for further exploration of the mechanism of tumour perineural invasion.
At present, some scholars have suggested that tumour perineural invasion is related to damage to the perineurium barrier (Liu et al. 2018; Morris et al. 2017). As one of the important messengers of the tumour microenvironment (Madeo et al. 2018), what role do exosomes play in the tumour microenvironment? According to some of our previous studies, tumour-derived exosomes act on perineurial cells and can damage tight junctions between cells, weaken the barrier between cells and thereby promote tumour invasion. However, research on the perineurium is still relatively sparse, and the method for culturing perineurial cells has not yet been reported. In this article, a reliable method for separation, culture and identification of perineurial cells is presented, and perineurial cells are verified to have capacity to form tubules. Tight junctions can be formed between perineurial cells, creating a barrier function. These findings provide a specific experimental basis for continued investigation of the mechanism of perineural invasion of tumours.
Although rat perineural fibroblasts, R1710-SC (ScienCell, San Diego, California, USA) are currently commercially available, until now there has been no direct experimental evidence that demonstrates R1710-SC can form tight junction structure, as described in the product instruction of R1710-SC, as well as references (Ariza et al. 1988; Erlandson 1991; Salzer 1999) cited in this instruction and other publications. By contrast, our data have demonstrated that the perineurial cells, cultured in our optimized conditions, can form tight junction that creates an intercellular barrier, as well as have the capacity of tube formation in vitro. Hence, the perineurial cells cultured by the method mentioned above could be used as a good cellular model to investigate the barrier function of perineurium in vitro.
References
Al-Ghanem R, Ramos-Pleguezuelos FM, Pérez-Darosa SI, Galicia-Bulnes JM, Cabrerizo-Carvajal F, El-Rubaidi OA (2013) Olfactory ensheathing cell tumour: case report and literature review. Neurocirugia (Astur) 24(3):130–134. https://doi.org/10.1016/j.neucir.2012.07.004
Amit M, Na'ara S, Gil Z (2016) Mechanisms of cancer dissemination along nerves. Nat Rev Cancer 16(6):399–408. https://doi.org/10.1038/nrc.2016.38
Ariza A, Bilbao JM, Rosai J (1988) Immunohistochemical detection of epithelial membrane antigen in normal perineurial cells and perineurioma. Am J Surg Pathol 12(9):678–683. https://doi.org/10.1097/00000478-198809000-00004
Chen W, Dong S, Zhou J, Sun M (2012) Investigation of myoepithelial cell differentiation into Schwann-like cells in salivary adenoid cystic carcinoma associated with perineural invasion. Mol Med Rep 6(4):755–759. https://doi.org/10.3892/mmr.2012.1003
Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ (2019) Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res 9(1):1–21
Deborde S, Wong RJ (2017) How Schwann cells facilitate cancer progression in nerves. Cell Mol Life Sci 74(24):4405–4420. https://doi.org/10.1007/s00018-017-2578-x
Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, Chernichenko N, Lee SY, Barajas F, Chen CH, Bakst R, Wong RJ (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 126(4):1538–1554. https://doi.org/10.1172/jci82658
Deng J, You Q, Gao Y, Yu Q, Zhao P, Zheng Y, Fang W, Xu N, Teng L (2014) Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS ONE 9(2):e88907. https://doi.org/10.1371/journal.pone.0088907
Erlandson RA (1991) The enigmatic perineurial cell and its participation in tumors and in tumorlike entities. Ultrastruct Pathol 15(4–5):335–351. https://doi.org/10.3109/01913129109016243
Gasparini G, Pellegatta M, Crippa S, Lena MS, Belfiori G, Doglioni C, Taveggia C, Falconi M (2019) Nerves and pancreatic cancer: new insights into a dangerous relationship. Cancers (Basel). https://doi.org/10.3390/cancers11070893
Hirose T, Tani T, Shimada T, Ishizawa K, Shimada S, Sano T (2003) Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol 16(4):293–298. https://doi.org/10.1097/01.Mp.0000062654.83617.B7
Izquierdo F, Suárez-Vilela D, Honrado E (2012) Perineurial cells in granular cell tumors and neoplasms with perineural invasion: an immunohistochemical study. Am J Dermatopathol 34(8):800–809. https://doi.org/10.1097/DAD.0b013e31824ba93b
Kraus RD, Barsky A, Ji L, Garcia Santos PM, Cheng N, Groshen S, Vapiwala N, Ballas LK (2019) The perineural invasion paradox: is perineural invasion an independent prognostic indicator of biochemical recurrence risk in patients with pT2N0R0 Prostate Cancer? A multi-institutional study. Adv Radiat Oncol 4(1):96–102. https://doi.org/10.1016/j.adro.2018.09.006
Liang D, Shi S, Xu J, Zhang B, Qin Y, Ji S, Xu W, Liu J, Liu L, Liu C, Long J, Yu X (2016) New insights into perineural invasion of pancreatic cancer: more than pain. Biochim Biophys Acta 1865(2):111–122. https://doi.org/10.1016/j.bbcan.2016.01.002
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115(15):3379–3391. https://doi.org/10.1002/cncr.24396
Liu Q, Wang X, Yi S (2018) Pathophysiological changes of physical barriers of peripheral nerves after injury. Front Neurosci 12:597. https://doi.org/10.3389/fnins.2018.00597
Lubig S, Thiesler T, Müller S, Vorreuther R, Leipner N, Kristiansen G (2018) Quantitative perineural invasion is a prognostic marker in prostate cancer. Pathology 50(3):298–304. https://doi.org/10.1016/j.pathol.2017.09.013
Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, Grossberg AJ, Muirhead D, Rickel AP, Hong Z, Zhao J, Vermeer PD (2018) Cancer exosomes induce tumor innervation. Nat Commun 9(1):4284. https://doi.org/10.1038/s41467-018-06640-0
Mizisin AP, Weerasuriya A (2011) Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol 121(3):291–312. https://doi.org/10.1007/s00401-010-0783-x
Morris AD, Lewis GM, Kucenas S (2017) Perineurial glial plasticity and the role of TGF-β in the development of the blood-nerve barrier. J Neurosci 37(18):4790–4807. https://doi.org/10.1523/jneurosci.2875-16.2017
Nozawa H, Morikawa T, Kawai K, Hata K, Tanaka T, Nishikawa T, Sasaki K, Shuno Y, Kaneko M, Hiyoshi M, Emoto S, Ishihara S (2019) Obstruction is associated with perineural invasion in T3/T4 colon cancer. Colorectal Dis 21(8):917–924. https://doi.org/10.1111/codi.14655
Patel BN, Olcott E, Jeffrey RB (2018) Extrapancreatic perineural invasion in pancreatic adenocarcinoma. Abdom Radiol (NY) 43(2):323–331. https://doi.org/10.1007/s00261-017-1343-9
Peltonen J, Jaakkola S, Virtanen I, Pelliniemi L (1987) Perineurial cells in culture. An immunocytochemical and electron microscopic study. Lab Invest 57(5):480–488
Pummi KP, Aho HJ, Laato MK, Peltonen JT, Peltonen SA (2006) Tight junction proteins and perineurial cells in neurofibromas. J Histochem Cytochem 54(1):53–61. https://doi.org/10.1369/jhc.5A6671.2005
Reina MA, López A, Villanueva MC, de Andrés JA, León GI (2000) Morphology of peripheral nerves, their sheaths, and their vascularization. Rev Esp Anestesiol Reanim 47(10):464–475
Reina MA, López A, Villanueva MC, De Andrés JA, Machés F (2003) The blood-nerve barrier in peripheral nerves. Rev Esp Anestesiol Reanim 50(2):80–86
Salzer JL (1999) Creating barriers: a new role for Schwann cells and Desert hedgehog. Neuron 23(4):627–629. https://doi.org/10.1016/s0896-6273(02)23442-x
Sasse S, Neuert H, Klämbt C (2015) Differentiation of Drosophila glial cells. Wiley Interdiscip Rev Dev Biol 4(6):623–636. https://doi.org/10.1002/wdev.198
Weerasuriya A, Mizisin AP (2011) The blood-nerve barrier: structure and functional significance. Methods Mol Biol 686:149–173. https://doi.org/10.1007/978-1-60761-938-3_6
Zhang M, Liang XH, Tang YL (2018) Advances in the molecular mechanisms of perineural invasion in salivary adenoid cystic carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi 36(2):204–211. https://doi.org/10.7518/hxkq.2018.02.017
Acknowledgements
We acknowledge Prof. Jingsong Li (Department of Oral and Maxillofacial Surgery, SUN YAT-SEN MEMORIAL HOSPITAL, SUN YAT-SEN UNIVERSITY for kindly providing SACC-83 cell line. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81500870, 81971902), the Natural Science Foundation of Guangdong Province, China (Grant No. 2016A030313590) for Dr. Jin Hou and Foundation Project of Director of Nanfang Hospital of Southern Medical University for Dr. Xuemin Yin (Grant No. 2017B013).
Author information
Authors and Affiliations
Contributions
JH, XY & XY conceived and planned the experiments; XY, XL, XL & JZ carried out the experiments; XY, XL, JZ, YZ, JH & XY contributed to the analysis of the data; XL, XY & JH contributed to the final version of the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research Involving Human and/or Animal Rights
No human participants or animals were involved in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, X., Liu, X., Zhang, Y. et al. Establishment of a Convenient System for the Culture and Study of Perineurium Barrier In Vitro. Cell Mol Neurobiol 42, 807–816 (2022). https://doi.org/10.1007/s10571-020-00978-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00978-0