Abstract
Bone cancer pain (BCP), which is induced by primary or metastatic bone cancer, remains a clinically challenging problem due to the poor understanding of its mechanisms. Sirtuin 1 (SIRT1) plays an important role in various pain models. Intrathecal administration of SRT1720, a SIRT1 activator, attenuates BCP in a rat model. However, the expression and activity of SIRT1 during the development and maintenance of BCP remain unknown. Furthermore, the underlying mechanism of SIRT1 in BCP remains ambiguous. In this study, we detected the time course of SIRT1 expression and activity in the spinal cord of mice with BCP and examined whether SRT1720 alleviated BCP by inhibiting metabotropic glutamatergic receptor (mGluR) 1/5 expression. In addition, we downregulated spinal SIRT1 expression in normal mice through an intrathecal injection of AAV-SIRT1-shRNA and then assessed pain behavior and mGluR1/5 expression. Mice with BCP developed significant mechanical allodynia and spontaneous flinching, accompanied by decreased levels of the SIRT1 protein, mRNA, and activity in the spinal cord. The SRT1720 treatment produced an analgesic effect on tumor-bearing mice and decreased the spinal levels of the mGluR1/5 protein and mRNA. In contrast, the AAV-SIRT1-shRNA treatment induced pain behavior in normal mice and increased the spinal levels of the mGluR1/5 protein and mRNA. The results suggested a critical role for SIRT1 in the development and maintenance of BCP and further indicated that activation of SIRT1 in the spinal cord by SRT1720 functionally reverses BCP in mice by inhibiting mGluR1/5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone cancer pain (BCP), one of the most common types of cancer-related pain, exerts a severe effect on the quality of life of patients (Jimenez Andrade and Mantyh 2010; Meuser et al. 2001). However, the current therapies for BCP are ineffective or have significant unwanted side effects, such as analgesic tolerance and somnolence (McNicol et al. 2003). Therefore, the identification of novel therapies and the elucidation of the mechanism of BCP are important for pain relief.
Sirtuin 1 (SIRT1), a type of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, has been reported to play critical roles in inflammatory pain (Wang et al. 2016) and neuropathic pain (Gui et al. 2018; Shao et al. 2014). In a recent study by Li et al., intrathecal treatment with a SIRT1 agonist reversed pain behaviors in rats with BCP (Li et al. 2018). Nevertheless, the expression and activity of SIRT1 during the development and maintenance of BCP remain unknown. Furthermore, the underlying mechanisms of SIRT1 in BCP have not been clarified.
Metabotropic glutamatergic receptor (mGluR) 1 and mGluR5, members of group I mGluRs, play crucial roles in central sensitization and chronic pain (Chiechio and Nicoletti 2012; Osikowicz et al. 2013). Group I mGluRs expressed in the spinal cord exert pronociceptive effects (Azkue et al. 2003; Gabra et al. 2007). Intrathecal administration of an mGluR5 antagonist attenuates BCP by inhibiting spinal astrocyte activation (Ren et al. 2012). Moreover, a recent study showed that SIRT1-mediated epigenetic regulation of mGluR1/5 expressions was involved in the development of neuropathic pain in rats with type 2 diabetes (Zhou et al. 2017). Accordingly, we hypothesized that SIRT1 attenuates BCP by inhibiting mGluR1/5 expression.
In the present study, the time course of SIRT1 expression and activity in the spinal cord of mice with BCP was detected. Furthermore, we examined whether SRT1720, a SIRT1 agonist, alleviated BCP by inhibiting mGluR1/5 expression. In addition, we downregulated spinal SIRT1 expression in normal mice through an intrathecal injection of AAV-SIRT1-shRNA and then assessed pain behavior and mGluR1/5 expression.
Methods
Experimental Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Science and Technology of China and were conducted according to the ethical guidelines for the use of experimental animals (Zimmermann 1983). All efforts were made to minimize both the suffering and number of animals used in this study. Experiments were performed on male C3H/HeN mice (age, 4 to 6 weeks; weight, 20 to 25 g; Vital River Laboratory Animal Technology Co., Ltd., Beijing, China, SCXK JING 2012–0001). The mice were housed in groups of five to a cage at a temperature of 21 ± 1 °C on a 12-h dark/light schedule and provided food pellets and water ad libitum.
Cell Culture and Implantation
Osteosarcoma NCTC 2472 cells (American Type Culture Collection, ATCC, 2087787) were incubated in NCTC 135 medium (Sigma-Aldrich, St. Louis, MO) containing 10% horse serum (Gibco, Grand Island, NY) at 37 °C in a 5% CO2 and 95% air atmosphere (Thermo Forma, Marietta, OH) and passaged twice per week according to the ATCC recommendations.
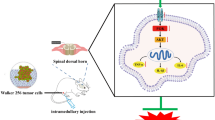
The method for inducing BCP was established as previously described by Schwei et al. (Schwei et al. 1999). On the day of surgery, mice were anesthetized with an intraperitoneal injection of 50 mg/kg pentobarbital sodium (1% in normal saline), and a right knee arthrotomy was performed. Then, a 25-μL microsyringe was used to inject 20 μL of α-minimum essential medium (α-MEM) containing 0 or 2 × 105 NCTC 2472 cells into the intramedullary space of the right femur, which corresponded to sham or tumor-bearing mice, respectively. Subsequently, the injection site was sealed with bone wax, followed by copious irrigation with normal saline. Finally, the wound was sutured closed.
Drug Preparation and Intrathecal Administration
SRT1720 (Selleck Chemical, Houston, TX), a selective SIRT1 agonist, was dissolved in 20% dimethyl sulfoxide (DMSO) and administered intrathecally at a dose of 5 μg/5 μL from days 14 to 16 after tumor cell inoculation for 3 consecutive days. The vehicle treatment was 20% DMSO. AAV-SIRT1-shRNA with a target sequence of 5′-GCGGGAATCCAAAGGATAATT-3′ (Hanbio, Shanghai, China) was administered intrathecally at a titer of 1.4 × 1012 vg/mL in a bolus of 5 μL. Meanwhile, AAV-GFP served as a negative control.
Manual intrathecal injections were performed between the L5 and L6 lumbar space in unanesthetized mice according to a method described by Hylden and Wilcox (Hylden and Wilcox 1980). The injection was performed using a glass microsyringe with a 25-gauge needle. Each mouse was injected with a volume of 5 μL. A successful puncture was confirmed by the tail-flicking behavior of the mouse.
Pain Behavior Tests
All tests were performed during the light phase. Mice were allowed to habituate for at least 30 min prior to each test. All behavioral tests were conducted by experimenters who were blinded to the treatment groups.
Mechanical Allodynia
Mechanical allodynia was assessed using von Frey filaments (North Coast Medical, Morgan Hill, CA) as previously described by Chaplan et al. (Chaplan et al. 1994). The mice were placed in individual transparent Plexiglas compartments (10 cm × 10 cm × 15 cm) on a metal mesh floor (graticule: 0.5 cm × 0.5 cm). A set of von Frey filaments (0.16 g, 0.4 g, 0.6 g, 1.0 g, 1.4 g, and 2.0 g) was applied to the right hind paw of each mouse. The filaments were pressed vertically against the plantar surface with such sufficient force to cause a slight bend against the paw and held in place for 6–8 s. Stimuli were presented at 10-min intervals. Brisk withdrawal or paw flinching was considered positive responses. Each mouse was tested five times at each stimulus strength. The von Frey filament with the lowest strength that produced three or more positive responses was regarded as the paw withdrawal mechanical threshold (PWMT).
Spontaneous Lifting Behavior
Mice were placed in individual Plexiglas compartments (10 cm × 10 cm × 15 cm) and observed for 2 min to quantify the number of spontaneous flinches (NSF) of the right hind paw. Each lift of the right hindlimb that was not related to walking or grooming was considered one flinch. Each mouse was tested five times (Luger et al. 2002).
Bone Histology
A previously described histological method was used to examine bone destruction (Han et al. 2018). Mice were anesthetized and perfused on day 21 after tumor cell inoculation. The right femur of each mouse was removed and decalcified for 24 h. Then, the bones were rinsed, dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin and eosin to reveal the extent of tumor infiltration and bone destruction.
Assay of SIRT1 Activity
A SIRT1 fluorometric kit (Abcam, Cambridge, United Kingdom) was used to measure SIRT1 activity. First, proteins were extracted from the spinal cord. Then, the nuclear extract was purified by immunoprecipitation with a rabbit anti-SIRT1 antibody (Elabscience Biotechnology, Wuhan, China) and Protein A Agarose Beads (Cell Signaling Technology Inc., Beverly, MA). The reaction mixture that contained SIRT1 assay buffer, Fluoro-Substrate peptide solution, NAD, ddH2O, Developer and SIRT1-Protein A Agarose beads was added, and the NAD-dependent deacetylase activity was measured according to the manufacturer’s instructions. Finally, the fluorescence intensity was recorded continuously for 30 min at 2-min intervals with excitation at 355 nm and emission at 460 nm using an automatic microplate reader (Thermo Scientific, Waltham, MA).
Quantitative Real-Time Polymerase Chain Reaction (Real-Time PCR)
The L3–L5 lumbar spinal cord segments of sacrificed mice were frozen in liquid nitrogen and stored at − 80 °C. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA) and transcribed into cDNAs using a Reverse Transcription-Polymerase Chain Reaction Kit (Vazyme Biotech, Nanjing, China). The cDNAs were used as a template for PCR amplification with Taq Plus DNA Polymerase (TIANGEN, Beijing, China) and primers. Each sample was analyzed in triplicate. The following primer sequences were used: SIRT1: 5′-ATCGTTACATATTCCACGGTGCT-3′ (forward) and 5′-CACTTTCATCTTCCAAGGGTTCT-3′ (reverse); Grm1: 5′-CCAATGGGGGAATCACAAT-3′ (forward) and 5′-ATGGCATAGATGGCGTTGA-3′ (reverse); Grm5: 5′-GTTTGCACAGGAGAACAGCA-3′ (forward) and 5′-GTCCAAAAGTTTCCGCCCAT-3′ (reverse); and β-actin: 5′-CACGATGGAGGGGCCGGACTCATC′ (forward) and 5′-TAAAGACCTCTATGCCAACACAGT-3′ (reverse). The relative expression levels of SIRT1, Grm1, and Grm5 were normalized to β-actin.
Western Blotting
Mice were sacrificed by decapitation under deep anesthesia with pentobarbital sodium (1% in normal saline, 50 mg/kg, i.p.). The lumbar spinal cords and ipsilateral L3–L5 dorsal root ganglia (DRG) were harvested quickly and stored in liquid nitrogen. Tissue samples were homogenized in RIPA lysis buffer supplemented with protease inhibitor cocktails. The homogenate was centrifuged at 12,000 rpm for 30 min at 4 °C, and the supernatant was collected. Protein concentrations were determined using BCA Protein Assay Kit (Kaiji Biotechnology, Nanjing, China). Protein lysates (40 μg) were separated using SDS-PAGE (8% gels) and subsequently transferred to polyvinylidene fluoride membranes (Millipore Corporation, MA) at 200 mA for 120 min. Membranes were blocked with 5% non-fat milk for 2 h at room temperature and then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-SIRT1 (1:1000; Affinity, USA), rabbit anti-mGluR1 (1:1000; Affinity, USA), or rabbit anti-mGluR5 (1:1000; Affinity, USA). The membrane was then washed six times with Tris-buffered saline-Tween and incubated with a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:50,000, Elabscience, Wuhan, China) for 2 h at room temperature. Immunoblots were developed using the ECL system (Santa Cruz Biotechnology, CA) and visualized with Kodak BioMax MR X-ray film (Kodak, New York, NY). Images of the protein bands on Western blots were recorded and analyzed using Quantity One v4.40 software (Bio-Rad). β-actin served as a loading control.
Statistical Analysis
Data are presented as mean ± SD. Animals were randomly assigned to different treatment groups. The pain behavior presented in Fig. 1 was analyzed using two-way analysis of variance (ANOVA). Levels of SIRT1 protein in the DRG were compared between the sham and BCP groups using independent two-sample t tests. Other data were analyzed using one-way ANOVA followed by the least significant difference (LSD) post hoc test. P values < 0.05 were considered statistically significant.
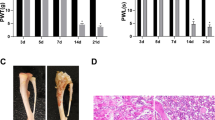
Validation of the mouse model of bone cancer pain (BCP). a Changes in the paw withdrawal mechanical threshold in response to von Frey filaments over the indicated time course. b Changes in the number of spontaneous flinches that occurred within 2 min over the indicated time course. Pain behavior was assessed at 0, 4, 7, 10, 14, 21 days after surgery in tumor-bearing and sham mice. All data are presented as mean ± SD. n = 8 mice in each group. **P < 0.01 compared with the sham group. c A significant amount of cortical bone had been infiltrated and eroded by the tumor in mice with BCP (hematoxylin–eosin staining, 200 ×). d No obvious bone destruction was observed in sham mice (hematoxylin–eosin staining, 200 ×)
Results
Validation of Mouse Models of BCP
On days 0 and 4 after implantation, the PWMT and NSF of BCP and sham mice did not show obvious differences. However, the mice with BCP exhibited a significant increase in PWMT and a decrease in NSF on days 7, 10, 14, and 21 after implantation compared with the sham mice (P < 0.01) (Fig. 1a, b), indicating that mice with BCP developed mechanical allodynia and spontaneous flinching.
Furthermore, the histological method was used to examine bone destruction. On day 21 after tumor cell implantation, tumor growth and bone destruction were observed. The tumor significantly infiltrated and eroded the cortical bone in mice with BCP, while no obvious bone destruction was observed in sham mice (Fig. 1c, d).
Downregulation of Spinal SIRT1 Expression and Activity in Mice with BCP
We first analyzed the time course of the expression of the SIRT1 protein in the spinal cord of mice with BCP to determine the role of SIRT1 in BCP. As shown in Fig. 2a–d, the levels of the SIRT1 protein, mRNA, and activity were decreased in the spinal cord of mice with BCP on days 7, 14, and 21 after implantation compared with the levels in sham mice (P < 0.01). Moreover, as shown in Fig. 2e ,f, the levels of the SIRT1 protein were also decreased in the DRG of mice with BCP on day 14 compared with the levels in sham mice (P < 0.01). These results suggested a potential association between SIRT1 function and BCP.
Time course of SIRT1 expression and activity in the spinal cord and expression of the SIRT1 protein in the DRG of mice with BCP. On days 7, 14, and 21 after tumor cell implantation. a, b Western blot showing the levels of the SIRT1 protein in the spinal cord. c Real-time PCR analysis of the SIRT1 mRNA in the spinal cord. d A SIRT1 fluorometric kit was used to detect SIRT1 activity in the spinal cord. e, f Western blot showing levels of the SIRT1 protein in the DRG on day 14 after tumor cell inoculation. Data are presented as mean ± SD. n = 6 mice in each group. **P < 0.01 compared with the sham group
Upregulation of Spinal SIRT1 Expression Reversed Pain Behavior
Mice were divided into four groups (n = 6 mice each), namely, the sham group, BCP group, DMSO group, and SIRT1720 group. Drugs were intrathecally administered from days 14 to 16 after inoculation for 3 consecutive days, as described in a previous study (Zhou et al. 2017). Behavioral tests were performed from days 1 to 4 after intrathecal administration. Analyses of the levels of the SIRT1 protein, mRNA, and activity were conducted on day 1 after intrathecal administration. As shown in Fig. 3a–d, intrathecal administration of SRT1720 increased the levels of the SIRT1 protein, mRNA, and activity in mice with BCP. Furthermore, SRT1720 produced a significant increase in PWMT and a significant decrease in NSF compared with the BCP group. The analgesic effect of repeated intrathecal injections of SRT1720 on BCP persisted for approximately 3 days (Fig. 3e, f).
Effect of intrathecal injections of SRT1720 on SIRT1 expression during BCP. Drugs were intrathecally administered from days 14 to 16 after inoculation for 3 consecutive days. Behavioral tests were performed from day 1 to day 4 after the intrathecal administration. Analyses of the levels of the SIRT1 protein, mRNA, and activity were conducted on day 1 after intrathecal administration. a, b Western blots showing levels of the SIRT1 protein. c Real-time PCR analysis of the SIRT1 mRNA. d A SIRT1 fluorometric kit was used to detect SIRT1 activity. e Paw withdrawal mechanical threshold in different groups after intrathecal administration. f The number of spontaneous flinches in different groups after intrathecal administration. Data are presented as mean ± SD. n = 6 mice in each group. **P < 0.01 compared with the sham group; #P < 0.05 and ##P < 0.01 compared with the BCP group
Upregulation of Spinal SIRT1 Expression Inhibited mGluR1/5 Expression in the Spinal Cord
As shown in Fig. 4a–f, the levels of the mGluR1/5 protein and mRNA were significantly increased in the BCP and DMSO groups compared with the sham group (P < 0.01). Furthermore, the levels of the mGluR1/5 protein and mRNA were significantly decreased in the SRT1720 group compared with the BCP group (P < 0.01).
Effect of intrathecal injections of SRT1720 on the levels of the mGluR1/5 protein and mRNA in the spinal cord of mice with BCP. a, b Western blots showing the levels of the mGluR1 protein. c, d Western blots showing the levels of the mGluR5 protein. e Real-time PCR analysis of the mGluR1 mRNA. f Real-time PCR analysis of the mGluR5 mRNA. Data are presented as mean ± SD. n = 6 mice in each group. **P < 0.01 compared with the sham group; ##P < 0.01 compared with the BCP group
Downregulation of Spinal SIRT1 Expression Induced Pain Behavior
Mice were randomly divided into three groups (n = 6 mice each), namely, the naive group, AAV-GFP control group, and SIRT1 shRNA group. Behavioral tests were performed on day 21 after the intrathecal injection. Then, mice were sacrificed to measure levels of the SIRT1 protein, mRNA, and activity in the spinal cord. As shown in Fig. 5a–d, the intrathecal administration of AAV-SIRT1-shRNA decreased the levels of the SIRT1 protein, mRNA, and activity. Meanwhile, it induced mechanical allodynia (Fig. 5e) and spontaneous flinching (Fig. 5f) in normal mice. Based on these results, the downregulation of SIRT1 is sufficient to induce pain behavior in healthy animals.
Effect of intrathecal injections of AAV-SIRT1-shRNA on pain behavior in normal mice. a, b Western blots showing the levels of the SIRT1 protein. c Real-time PCR analysis of the SIRT1 mRNA. d A SIRT1 fluorometric kit was used to detect SIRT1 activity. e Paw withdrawal mechanical threshold in naive, AAV-GFP control, and SIRT1 shRNA groups. f The number of spontaneous flinches in naive, AAV-GFP control, and SIRT1 shRNA groups. Data are presented as mean ± SD. n = 6 mice in each group. **P < 0.01 compared with the naive group
Downregulation of Spinal SIRT1 Expression Increased mGluR1/5 Expression in the Spinal Cord
As shown in Fig. 6a–f, the levels of the mGluR1/5 protein and mRNA were significantly increased in AAV-SIRT1-shRNA-treated mice compared with naive and AAV-GFP control mice (P < 0.01).
Effect of intrathecal injections of AAV-SIRT1-shRNA on the levels of the mGluR1/5 protein and mRNA in the spinal cord of normal mice. a, b Western blots showing the levels of the mGluR1 protein. c, d Western blots showing the levels of the mGluR5 protein. e Real-time PCR analysis of the mGluR1 mRNA. f Real-time PCR analysis of the mGluR5 mRNA. Data are presented as mean ± SD. n = 6 mice in each group. **P < 0.01 compared with the naive group
Discussion
BCP remains a clinically challenging problem, and the underlying mechanisms are poorly understood. Based on recent evidence, epigenetic modifications, including histone modifications and DNA methylation, regulate the expression of pain-related genes (Liang et al. 2015; Lutz et al. 2014; Zhang et al. 2011), contributing to the development and maintenance of chronic pain. Furthermore, increased expression of DNA methyltransferase (DNMT) 3a in the dorsal horn contributes to BCP by silencing Kv1.2 expression (Miao et al. 2017). The suppression of histone deacetylase (HDAC) 2 expression in the spinal cord attenuates mechanical hyperalgesia and restores KCC2 expression in a rat model of BCP (Hou et al. 2018). In addition, four HDAC inhibitors have been approved by the Food and Drug Administration (FDA) as treatments for some types of cancer (Manal et al. 2016). Thus, epigenetic modifications may be potential new targets for cancer pain management. SIRT1, an NAD+-dependent histone deacetylase, has been reported to play a critical role in BCP (Li et al. 2018; Zhu et al. 2017). However, the expression and activity of SIRT1 during the development and maintenance of BCP remain unknown.
In the present study, the implantation of osteosarcoma NCTC 2472 cells into the right femur of male C3H/HeN mice produced mechanical allodynia and spontaneous flinching on day 7. On days 14 and 21 after tumor cell implantation, the severity of pain behavior was further increased. These results were consistent with previous studies and the clinical spontaneous and evoked pain experienced by patients with bone cancer. Based on these findings, we chose the 14th day after implantation to initiate the intrathecal injections of SRT1720. In addition, histology showed that the tumor had significantly infiltrated and eroded the cortical bone in mice with BCP on day 21 after implantation. In summary, the behavioral and histological results indicated the successful establishment of a mouse model of BCP.
To the best of our knowledge, this report is the first to show the time course of SIRT1 expression and activity in the spinal cord of mice with BCP. Consistent with the results of the pain behavior assessment, the levels of the SIRT1 protein, mRNA, and activity were significantly decreased in the spinal cord of mice with BCP on days 7, 14, and 21 after implantation. Moreover, the activation of SIRT1 by SRT1720 suppressed mechanical allodynia and spontaneous flinching in tumor-bearing mice. In contrast, knockdown of spinal SIRT1 expression by Ad-SIRT1-shRNA induced pain behaviors in normal mice. Therefore, SIRT1 is involved in the development and maintenance of BCP and may represent a viable new target for pain relief in patients with BCP.
Glutamate is the major excitatory neurotransmitter in the mammalian nervous system and exerts its action through ionotropic and metabotropic receptors. Metabotropic glutamatergic receptors (mGluRs) have been reported to play critical roles in pain transmission and central sensitization (Dai et al. 2017; Dolan and Nolan 2000; Johnson et al. 2017; Muguruza et al. 2016). Eight mGluRs, mGluR1 to mGluR8, have been identified to date. These receptors are subdivided into three groups based on sequence identity, pharmacology, and signal transduction (Conn and Pin 1997). Group I mGluRs (mGluR1 and mGluR5) mainly lead to phospholipase C (PLC) activation, while group II (mGluR2 and mGluR3) and group III receptors (mGluR 4, 6, 7, and 8) predominantly inhibit adenylate cyclase (AC) (Neugebauer 2002).
Based on accumulating evidence, the inhibition of mGluR1 and mGluR5 may exert analgesic effects. Systemic administration of mGluR1 (Satow et al. 2008; Varty et al. 2005; Zhu et al. 2008) and mGluR5 (Satow et al. 2008; Varty et al. 2005; Zammataro et al. 2011) antagonists attenuates both mechanical and thermal hypersensitivity in subjects with a broad range of pain conditions, from inflammatory pain to long-lasting chronic pain. The administration of an mGluR5 antagonist at peripheral afferent endings also inhibits visceral nociception (Lindstrom et al. 2008). As mGluR1/5 are expressed at high levels in the spinal cord (Jia et al. 1999; Tang and Sim 1999), the intrathecal administration of the mGluR1/5 antagonists also produces antinociceptive effects on various models of inflammatory (Karim et al. 2001), neuropathic (Yashpal et al. 2001), and bone cancer pain (Dai et al. 2017; Ren et al. 2012). In addition, mGluR5 antagonists reduce anxiety in naive animals, a comorbidity that is often associated with chronic pain states (Varty et al. 2005). However, the activation of mGluR1/5 also exerts antinociceptive effects. In the periaqueductal gray matter, the activation of mGluR1/5 produces antinociceptive effects by activating the descending antinociceptive pathway from the periaqueductal gray matter (Maione et al. 1998, 2000). These contradictory results may be attributed to the different sites of activation. Thus, mGluR1 and mGluR5 play very crucial roles in pain modulation, as they not only produce pronociceptive effects but also exert antinociceptive effects.
Several underlying mechanisms by which spinal mGluR1/5 contribute to modulating pain have been identified. First, mGluR5 increase intracellular Ca2+ levels, resulting in Src and protein kinase C activation, as well as N-methyl-d-aspartate phosphorylation, which increases synaptic transmission (Guo et al. 2004). Second, mGluR1/5 agonists activate ERK1/2 signaling to enhance pain sensitivity. Moreover, ERK1/2 activation by mGluR5 leads to the phosphorylation of Kv4.2-containing potassium channels, resulting in increased dorsal horn neuron excitability (Hu et al. 2007). Therefore, downregulation of spinal mGluR1/5 expression decreases synaptic transmission, pain sensitivity, and the excitability of dorsal horn neurons.
In the present study, levels of the mGluR1/5 protein and mRNA were significantly increased in the spinal cord of tumor-bearing mice compared with sham mice, indicating critical roles for mGluR1/5 in the development and maintenance of BCP. These results are consistent with a previous study (Ren et al. 2012). Similar to the behavioral changes, the increased levels of the mGluR1/5 protein and mRNA were decreased by the intrathecal administration of SRT1720. In contrast, the levels of the mGluR1/5 protein and mRNA were increased in AAV-SIRT1-shRNA-treated mice compared with control mice.
Moreover, the level of the SIRT1 protein was decreased in the DRG of mice with BCP, indicating that SIRT1 expressed in the DRG was involved in the pathophysiology of BCP. Notably, mGluR1 and mGluR5 are expressed in DRG (Carlton and Hargett 2007; Masuoka et al. 2016). The level of the mGluR5 protein is significantly increased in the DRG of a rat model of diabetic neuropathic pain (Li et al. 2010). The intrathecal administration of SRT1720 also altered SIRT1 expression in the DRG. Accordingly, the upregulation of SIRT1 in the DRG, which may inhibit mGluR1/5 expression, potentially contributed to the antinociceptive effects of the intrathecal injection of SRT1720. However, our present study only focuses on spinal SIRT1 expression during the development and maintenance of BCP. The mechanism by which SIRT1 expressed in the DRG or supraspinal areas contributes to the pathophysiology of BCP will be analyzed in our ongoing research. In summary, these findings are evidence that SIRT1 probably attenuates BCP by inhibiting mGluR1/5 expression in the mouse spinal cord.
However, our study has a limitation in that we did not determine the mechanism by which SIRT1 inhibits mGluR1/5 expression due to technical constraints. In subjects with type 2 diabetes mellitus-induced neuropathic pain, SIRT1 activation alleviates pain behavior and epigenetically downregulates mGluR1/5 expression. Therefore, further studies are needed to determine whether SIRT1 mGluR1/5 expression in a mouse model of BCP by modulating deacetylation.
Conclusions
Our present results indicate a critical role for spinal SIRT1 in the development and maintenance of BCP and further suggest that the activation of spinal SIRT1 by SRT1720 functionally attenuates BCP in mice, probably by inhibiting mGluR1/5 expression. This study reveals some of the mechanisms of BCP and may provide new insights into the clinical treatment of BCP.
References
Azkue JJ, Liu XG, Zimmermann M, Sandkuhler J (2003) Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain 106:373–379
Carlton SM, Hargett GL (2007) Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol 501:780–789. https://doi.org/10.1002/cne.21285
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Chiechio S, Nicoletti F (2012) Metabotropic glutamate receptors and the control of chronic pain. Curr Opin Pharmacol 12:28–34. https://doi.org/10.1016/j.coph.2011.10.010
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. https://doi.org/10.1146/annurev.pharmtox.37.1.205
Dai WL, Yan B, Jiang N, Wu JJ, Liu XF, Liu JH, Yu BY (2017) Simultaneous inhibition of NMDA and mGlu1/5 receptors by levo-corydalmine in rat spinal cord attenuates bone cancer pain. Int J Cancer 141:805–815. https://doi.org/10.1002/ijc.30780
Dolan S, Nolan AM (2000) Behavioural evidence supporting a differential role for group I and II metabotropic glutamate receptors in spinal nociceptive transmission. Neuropharmacology 39:1132–1138
Gabra BH, Kessler FK, Ritter JK, Dewey WL, Smith FL (2007) Decrease in N-methyl-d-aspartic acid receptor-NR2B subunit levels by intrathecal short-hairpin RNA blocks group I metabotropic glutamate receptor-mediated hyperalgesia. J Pharmacol Exp Ther 322:186–194. https://doi.org/10.1124/jpet.107.120071
Gui Y, Zhang J, Chen L, Duan S, Tang J, Xu W, Li A (2018) Icariin, a flavonoid with anti-cancer effects, alleviated paclitaxel-induced neuropathic pain in a SIRT1-dependent manner. Mol Pain 14:1744806918768970. https://doi.org/10.1177/1744806918768970
Guo W et al (2004) Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci: Off J Soc Neurosci 24:9161–9173. https://doi.org/10.1523/jneurosci.3422-04.2004
Han MM, Yang CW, Cheung CW, Li J (2018) Blockage of spinal endothelin A receptors attenuates bone cancer pain via regulation of the Akt/ERK signaling pathway in mice. Neuropeptides 68:36–42. https://doi.org/10.1016/j.npep.2018.01.003
Hou X et al (2018) Suppression of HDAC2 in spinal cord alleviates mechanical hyperalgesia and restores KCC2 expression in a rat model of bone cancer pain. Neuroscience 377:138–149. https://doi.org/10.1016/j.neuroscience.2018.02.026
Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RWT (2007) Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci: Off J Soc Neurosci 27:13181–13191. https://doi.org/10.1523/jneurosci.0269-07.2007
Hylden JL, Wilcox GL (1980) Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67:313–316
Jia H, Rustioni A, Valtschanoff JG (1999) Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol 410:627–642
Jimenez Andrade JM, Mantyh P (2010) Cancer pain: from the development of mouse models to human clinical trials. In: Kruger L, Light AR (eds) Translational pain research. CRC Press, Boca Raton, FL
Johnson MP et al (2017) Broad spectrum efficacy with LY2969822, an oral prodrug of metabotropic glutamate 2/3 receptor agonist LY2934747, in rodent pain models. Br J Pharmacol 174:822–835. https://doi.org/10.1111/bph.13740
Karim F, Wang CC, Gereau RWT (2001) Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci: Off J Soc Neurosci 21:3771–3779
Li JQ, Chen SR, Chen H, Cai YQ, Pan HL (2010) Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem 112:162–172. https://doi.org/10.1111/j.1471-4159.2009.06437.x
Li MY et al (2018) SIRT1 activation by SRT1720 attenuates bone cancer pain via preventing Drp1-mediated mitochondrial fission. Biochim Biophys Acta Mol Basis Dis 1865:587–598. https://doi.org/10.1016/j.bbadis.2018.12.017
Liang L, Lutz BM, Bekker A, Tao YX (2015) Epigenetic regulation of chronic pain. Epigenomics 7:235–245. https://doi.org/10.2217/epi.14.75
Lindstrom E et al (2008) Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain 137:295–305. https://doi.org/10.1016/j.pain.2007.09.008
Luger NM et al (2002) Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs. inflammatory pain. Pain 99:397–406. https://doi.org/10.1016/s0304-3959(02)00102-1
Lutz BM, Bekker A, Tao YX (2014) Noncoding RNAs: new players in chronic pain. Anesthesiology 121:409–417. https://doi.org/10.1097/ALN.0000000000000265
Maione S, Marabese I, Leyva J, Palazzo E, de Novellis V, Rossi F (1998) Characterisation of mGluRs which modulate nociception in the PAG of the mouse. Neuropharmacology 37:1475–1483
Maione S, Oliva P, Marabese I, Palazzo E, Rossi F, Berrino L, Filippelli A (2000) Periaqueductal gray matter metabotropic glutamate receptors modulate formalin-induced nociception. Pain 85:183–189
Manal M, Chandrasekar MJ, Gomathi Priya J, Nanjan MJ (2016) Inhibitors of histone deacetylase as antitumor agents: a critical review. Bioorg Chem 67:18–42. https://doi.org/10.1016/j.bioorg.2016.05.005
Masuoka T et al (2016) Long-term activation of group I metabotropic glutamate receptors increases functional TRPV1-expressing neurons in mouse dorsal root Ganglia. Front Cell Neurosci 10:79. https://doi.org/10.3389/fncel.2016.00079
McNicol E et al (2003) Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain: Off J Am Pain Soc 4:231–256
Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S (2001) Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 93:247–257
Miao XR et al (2017) DNMT3a contributes to the development and maintenance of bone cancer pain by silencing Kv1.2 expression in spinal cord dorsal horn. Mol Pain 13:1744806917740681. https://doi.org/10.1177/1744806917740681
Muguruza C, Meana JJ, Callado LF (2016) Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front Pharmacol 7:130. https://doi.org/10.3389/fphar.2016.00130
Neugebauer V (2002) Metabotropic glutamate receptors–important modulators of nociception and pain behavior. Pain 98:1–8
Osikowicz M, Mika J, Przewlocka B (2013) The glutamatergic system as a target for neuropathic pain relief. Exp Physiol 98:372–384. https://doi.org/10.1113/expphysiol.2012.069922
Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL (2012) Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology 116:122–132. https://doi.org/10.1097/aln.0b013e31823de68d
Satow A et al (2008) Pharmacological effects of the metabotropic glutamate receptor 1 antagonist compared with those of the metabotropic glutamate receptor 5 antagonist and metabotropic glutamate receptor 2/3 agonist in rodents: detailed investigations with a selective allosteric metabotropic glutamate receptor 1 antagonist, FTIDC [4-[1-(2-fluoropyridine-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methy l-3,6-dihydropyridine-1(2H)-carboxamide]. J Pharmacol Exp Ther 326:577–586. https://doi.org/10.1124/jpet.108.138107
Schwei MJ et al (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci: Off J Soc Neurosci 19:10886–10897
Shao H et al (2014) Spinal SIRT1 activation attenuates neuropathic pain in mice. PLoS ONE 9:e100938. https://doi.org/10.1371/journal.pone.0100938
Tang FR, Sim MK (1999) Pre- and/or post-synaptic localisation of metabotropic glutamate receptor 1alpha (mGluR1alpha) and 2/3 (mGluR2/3) in the rat spinal cord. Neurosci Res 34:73–78
Varty GB et al (2005) The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology 179:207–217. https://doi.org/10.1007/s00213-005-2143-4
Wang LL et al (2016) Resveratrol attenuates inflammatory hyperalgesia by inhibiting glial activation in mice spinal cords. Mol Med Rep 13:4051–4057. https://doi.org/10.3892/mmr.2016.5027
Yashpal K, Fisher K, Chabot JG, Coderre TJ (2001) Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain 94:17–29
Zammataro M, Chiechio S, Montana MC, Traficante A, Copani A, Nicoletti F, Gereau RWT (2011) mGlu2 metabotropic glutamate receptors restrain inflammatory pain and mediate the analgesic activity of dual mGlu2/mGlu3 receptor agonists. Mol Pain 7:6. https://doi.org/10.1186/1744-8069-7-6
Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ (2011) Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 17:1448–1455. https://doi.org/10.1038/nm.2442
Zhou CH, Zhang MX, Zhou SS, Li H, Gao J, Du L, Yin XX (2017) SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain 158:130–139. https://doi.org/10.1097/j.pain.0000000000000739
Zhu CZ et al (2008) Analgesic activity of metabotropic glutamate receptor 1 antagonists on spontaneous post-operative pain in rats. Eur J Pharmacol 580:314–321. https://doi.org/10.1016/j.ejphar.2007.09.047
Zhu H, Ding J, Wu J, Liu T, Liang J, Tang Q, Jiao M (2017) Resveratrol attenuates bone cancer pain through regulating the expression levels of ASIC3 and activating cell autophagy. Acta Biochim Biophys Sin (Shanghai). https://doi.org/10.1093/abbs/gmx103
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
Funding
This study was supported by a grant from the Natural Science Foundation of Anhui Province of China (Grant No. 1908085MH251).
Author information
Authors and Affiliations
Contributions
CY and JL conceived and designed the study. CY performed the SIRT1 activity assay and real-time PCR and edited the manuscript. FK performed Western blotting, intrathecal injections, and pain behavior tests. SW performed bone histology and analyzed the data. MH prepared animals and cells and established models of bone cancer pain. ZZ contributes to revising the manuscript. ZZ and JL coordinated and directed the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to declare.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiments presented in this manuscript comply with the current laws of the country in which they were performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Kang, F., Wang, S. et al. SIRT1 Activation Attenuates Bone Cancer Pain by Inhibiting mGluR1/5. Cell Mol Neurobiol 39, 1165–1175 (2019). https://doi.org/10.1007/s10571-019-00710-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00710-7