Abstract
Oxidative glutamate toxicity is involved in diverse neurological disorders including epilepsy and ischemic stroke. Our present work aimed to assess protective effects of huperzine A (HupA) against oxidative glutamate toxicity in a mouse-derived hippocampal HT22 cells and explore its potential mechanisms. Cell survival and cell injury were analyzed by MTT method and LDH release assay, respectively. The production of ROS was measured by detection kits. Protein expressions of BDNF, phosphor-TrkB (p-TrkB), TrkB, phosphor-Akt (p-Akt), Akt, phosphor-mTOR (p-mTOR), mTOR, phosphor-p70s6 (p-p70s6) kinase, p70s6 kinase, Bcl-2, Bax, and β-actin were assayed via Western blot analysis. Enzyme-linked immunosorbent assay was employed to measure the contents of nerve growth factor, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). Our findings illustrated 10 μM HupA for 24 h significantly protected HT22 from cellular damage and suppressed the generation of ROS. Additionally, after treating with LY294002 or wortmannin [the selective inhibitors of phosphatidylinositol 3 kinase (PI3K)], HupA dramatically prevented the down-regulations of p-Akt, p-mTOR, and p-p70s6 kinase in HT22 cells under oxidative toxicity. Furthermore, it was observed that the protein levels of BDNF and p-TrkB were evidently enhanced after co-treatment with HupA and glutamate in HT22 cells. The elevations of p-Akt and p-mTOR were abrogated under toxic conditions after blockade of TrkB by TrkB IgG. Cellular apoptosis was significantly suppressed (decreased caspase-3 activity and enhanced Bcl-2 protein level) after HupA treatment. It was concluded that HupA attenuated oxidative glutamate toxicity in murine hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutamate toxicity is known to be involved in many neurological disorders including Alzheimer’s disease, ischemic stroke, Parkinson’s disease, epilepsy, and depression (Coyle and Puttfarcken 1993; Mao et al. 2015; Simonian and Coyle 1996). It was previously investigated that glutamate toxicity contributed to cell death mainly via N-methyl-D-aspartate (NMDA) receptor-activated excitotoxicity and non-receptor-mediated oxidative stress (Choi 1988; Mattson 2000). Specially, the neurons are susceptible to oxidative stress.
Oxidative glutamate damage was previously triggered by the high concentrations of glutamate, which prevented the synthesis of the intracellular anti-oxidant (glutathione) due to the breakdown of the cystine/glutamate antiporter, finally contributing to the elevated production of reactive oxygen species (ROS) and consequently induction of neuronal apoptosis (Jia et al. 2013; Murphy et al. 1989). It was found that the immortalized hippocampus-derived HT22 cells, which had the characteristics of lacking the functional ionotropic glutamate receptor (Jeong et al. 2010), were considered as a very useful cell model for investigating oxidative glutamate impairment excluding glutamate receptor-mediated excitotoxicity.
It is well known that neurotrophins are a family of proteins, which contain four major subtypes including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). There is ample evidence supporting that neurotrophins are closely associated with the development of nervous system including neuronal survival, differentiation, and apoptosis (Huang and Reichardt 2003). Indeed, activation of NGF was reported to attenuate oxidative stress damage in PC12 cells (Jackson et al. 1990) and be involved in the neuroprotection of huperzine A (HupA) against H2O2-induced damage in the human neuroblastoma cell line (SH-SY5Y) (Tang et al. 2005). In addition, BDNF also improves neurological function in adults (Wang et al. 2010). Activating endogenous BDNF activity could exert protective roles against ischemic, traumatic, and toxic brain lesions (Beck et al. 1994; Kazanis et al. 2004). In the meantime, pretreatment with BDNF could also evidently protect hippocampal neurons from glutamate toxicity and the protection might be linked with mediating phosphatidylinositol 3 kinase (PI3K) and Ras/mitogen-activated protein kinase (MAPK) pathways (Almeida et al. 2005). However, suppression of BDNF level or its effect was shown to aggravate neuronal death following ischemic insults in rats (Larsson et al. 1999). Taken together, these findings implicate that pharmacological strategies for augmenting neurotrophins level may provide a promising therapeutic potential of oxidative damage and glutamate toxicity.
HupA, an important lycopodium alkaloid purified from the plant Qian Ceng Ta (Huperzia serrata), is currently widely used for treating Alzheimer’s disease in clinical practices due to its well-known inhibitory effect on acetylcholinesterase (Wang et al. 2006). Additionally, a previous investigation also revealed that HupA attenuated oxidative injury via changing the expressions of apoptosis-regulatory genes in PC12 cells (Wang et al. 2001). Furthermore, Wang et al. found that HupA treatment significantly inhibited neuronal impairment in C6 rat glioma cells subjected to oxygen-glucose deprivation, and inhibition of NF-kappa B-mediated inflammatory pathway was involved in its neuroprotection (Wang and Tang 2007). However, it remains elusive whether HupA has protection against glutamate-induced oxidative damage in hippocampal HT22 cells. Besides, since neurotrophins play a critical role in cellular survival, we hypothesize that it involves in the HupA’s neuroprotection against oxidative glutamate damage. Therefore, our present study aimed to investigate the protective effect of HupA on immortalized hippocampus HT22 cells under glutamate exposure and further figure out its potential molecular mechanisms.
Materials and Methods
Chemicals and Reagents
HupA was provided by Tau Biotec (the purity of more than 98 %, Shanghai, China). Glutamate and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were from Sigma Chemical Co., (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), trypsin, and fetal bovine serum (FBS) were purchased from GIBCO Invitrogen (Carlsbad, CA, USA). LY294002, a specific inhibitor of PI3K, was acquired from Cell Signaling Technology (Beverly, MA, USA). Lactate dehydrogenase (LDH) release assay kit was bought from Jiancheng Bioengineering Institute (Nanjing, China). The BCA protein assay kit was supplied from Beyotime Institute of Biotechnology (Nantong, China). An enhanced chemiluminescence kit was from Pierce (Pierce, CA, USA). The anti-BDNF, anti-TrkB, anti-phospho-Akt, anti-Akt, anti-β-actin, anti-Bax, anti-Bcl-2, and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-phospho-TrkB was from Cell Signaling Technology (Beverly, MA, USA). Anti-TrkB IgG used for blocking TrkB receptor was bought from R&D Systems Inc. (Minneapolis, MN, USA).
Cell Culture and Drug Treatments
Immortalized mouse hippocampal HT22 cells (a gift from professor Wei-Lin Jin, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, China) were grown in DMEM containing 10 % (v/v) FBS and incubated in a humidified atmosphere (95 % air, 5 % CO2) at 37 °C. The human neuroblastoma SH-SY5Y cell line was acquired from Shanghai cell bank of Chinese Academy of Sciences (Shanghai, China) and cultivated in MEM/F12 medium supplemented with 10 % (v/v) FBS at 37 °C in 5 % CO2. After that, hippocampal HT22 cells were maintained with serum-free medium 2 h before drug treatments. They were interfered with anti-TrkB IgG or LY294002 prior to HupA treatment.
Cell Viability and LDH Release Assay
Cell viability of HT22 or SH-SY5Y cells was detected using MTT, a specific indicator of the mitochondrial activity of living cells, according to the previous reports (Wang et al. 2011). For short, cells were seeded into the 96-well plates with a density of about 1 × 104 cells per well and maintained in a humidified atmosphere (95 % air, 5 % CO2) at 37 °C overnight prior to conduction of experiments. After drug treatments, the cultures were washed with PBS for three times, and then the cells in each well were incubated with 10 μl of MTT (final concentration, 0.5 mg/ml) for 3 h at 37 °C. Subsequently, the culture medium was removed, and 100 μl of DMSO was added to dissolve the formazan crystals in living cells. Absorbance at 490 nm was read with a Sunrise RC microplate reader (Tecan Group, Maennedorf, Switzerland). Cell viability was represented as the percentage of control cells. The LDH release assay was employed to assess the membrane integrity after drug treatments and could evaluate cell injury. In our present study, HT22 cells were cultivated in 96-well plates at a density of 1 × 104 cells per well and grown to about 70 % confluence. After drug administrations, the amounts of LDH released into the media were measured using the commercial assay kits according to the manufacturer’s protocols.
ROS Measurement
Mouse hippocampal HT22 cells were cultivated at a density of 2.5 × 104 cells/well in 24-well plates. After adherence, cells were incubated with HupA for 24 h, followed by treatment with 5 mM glutamate for 12 h. After washing with PBS, cells were stained with 20 μM carboxy-H2DCFDA (Invitrogen) in DMEM for 1 h in a 37 °C incubator. Cells were observed under a laser-scanning confocal microscope (Nikon, Japan) with an excitation wavelength of 490 nm and an emission wavelength of 525 nm. Fluorescence intensity was calculated using a Multilabel counter (Perkin-Elmer 1420, MA, USA).
Western Blot Analyses
After drug treatments, cells were washed three times with ice-cold PBS and suspended in RIPA lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 10 % glycerol, 1 % Nonidet P-40, 5 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride, pH 7.5). After centrifugation at 13,200 g for 20 min at 4 °C, the supernatant was collected, and total protein concentration was determined by the commercial BCA assay kit. Proteins (30 μg) were separated on 8 or 10 % SDS-polyacryl-amide gels (SDS-PAGE) and then transferred electrophoretically onto nitrocellulose membranes. After blocking the membranes with 5 % fat-free milk for 1 h at room temperature, target proteins were immunodetected with the following primary antibodies: rabbit anti-BDNF (1:200), rabbit anti-phospho-TrkB (1:1000), rabbit anti-TrkB (1:500), rabbit anti-Bax (1:200), rabbit anti-Bcl-2 (1:200), rabbit anti-phospho-Akt (1:200), rabbit anti-Akt (1:300), and mouse anti-β-actin (1:2000), overnight at 4 °C. The membranes were then incubated with the second antibodies (1:5000) for 2 h at room temperature. Immunodetection was performed using an enhanced chemiluminescence kit, and the intensities of the protein bands were subsequently quantified using Quantity One software (BioRad, CA, USA). For the quantification of phosphorylated proteins, it was normalized to the total amount of proteins.
Determinations of NGF, BDNF, NT-3, and NT-4 by Enzyme-Linked Immunosorbent Assay (ELISA) Assay
After drug administration, HT22 cells from different groups were lysed in a cold buffer containing HEPES 25 mM, MgCl2·6H2O 5 mM, EDTA·2Na, 5 mM, pH 7.4, 0.5 % (v/w) Triton X-100, DTT 5 mM, PMSF 2 mM, Pepstation A 10 μg/mL and Leupeptin 10 μg/mL. The lysates were then centrifugated at 10,000 g for 10 min, and the supernatants were obtained. Protein contents were quantified by Coomassie blue method. The concentrations of NGF, BDNF, NT-3, and NT-4 were measured by respective commercial Emax® Immunoassay kits according to manufacturer’s instructions.
Caspase-3 Activity Assay
HT22 cells were cultivated on 6-well plates with a density of 2 × 105 cells per well, before pre-incubation of different drugs. Protein samples were prepared as indicated in Western blot analysis., 50 μg of total protein was added to a reaction buffer containing Ac-DEVD-pNA (2 mM), incubated at 37 °C for 4 h, and the absorbance of yellow pNA was determined by a spectrometer at 405 nm. The specific caspase-3 activity which was normalized for total protein in HT22 cells was then given as fold of the baseline caspase-3 activity of control group.
Statistics
All experiments mentioned above were repeated for at least three times. The data were represented as mean ± standard deviation (SD). Statistically significant differences were performed by the analysis of variance in SPSS 13.0 software (SPSS, Inc., Chicago, IL). A p value of less than 0.05 was considered statistically significant.
Results
HupA Attenuated Oxidative Damage in HT22 Cells Under Glutamate Exposure
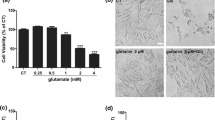
To evaluate the neuroprotection of HupA against oxidative glutamate toxicity in HT22 cells, the best concentrations of glutamate and HupA were firstly explored in our present investigation. The cell viability and LDH content in the media were determined by MTT method and LDH release assay kit according to manufacturers’ instructions. It was noteworthy that the cell viability of HT22 cells was significantly diminished in a dose- and time-dependent manner under glutamate stimulation (0–5 mM) (Fig. 1a). Nearly, a 70 % reduction of cell viability was found after glutamate exposure at the concentration of 5 mM for 12 h. Thus, the addition of glutamate of 5 mM for 12 h was selected in the subsequent experiments. Figure 1b reveals that HupA by the concentrations of 0–10 μM did not have any significant toxic effects on the cell viability of HT22 cells. It was also noted that 10 μM of HupA treatment for 24 h exerted the most evident protection to HT22 cells than any other concentration after glutamate exposure (Fig. 1c). LDH release assay also confirmed this phenomenon (Fig. 1d). In fact, the notion that huperzine A improved cell viability was also confirmed in the human neuroblastoma SH-SY5Y cell line, as displayed in supplementary data (S-Fig. 1).
HupA attenuated neuronal impairment in HT22 cells under glutamate stimulation. Cell viability of HT22 cells subjected to different concentrations of glutamate (0–5 mM) was analyzed by MTT method (a). Nearly, a 70 % reduction of cell viability was found after glutamate exposure at the concentration of 5 mM for 12 h. The addition of glutamate of 5 mM for 12 h was selected in the subsequent experiments. HupA by the concentrations of 0–10 μM did not have any significant toxic effects on the cell viability of HT22 cells (b). It was also noted that 10 μM of HupA treatment for 24 h evidently promoted the recovery of HT22 cells after glutamate exposure (c). LDH release assay also confirmed the protection of HupA against glutamate insults in HT22 cells (d). Results were presented as mean ± standard deviation (SD) (n = 8). ** p < 0.01 versus control group; ## p < 0.01 versus glutamate-treated group
HupA Suppressed Glutamate-Induced Generation of ROS in HT22 Cells
To investigate whether HupA had a protective effect on oxidative glutamate damage, the production of ROS was measured using corresponding detection kits in our present work. It was obvious to observe that treatment of HT22 cells with glutamate led to remarkable elevation of ROS (Fig. 2, p < 0.01). Nevertheless, pretreatment with HupA significantly prevented the increased ROS level induced by glutamate.
HupA suppressed the production of ROS in HT22 cells under glutamate stimulation. Cells were treated with 10 μM of HupA for 24 h, followed by treatment of 5 mM glutamate for 12 h. Results were presented as mean ± SD (n = 8). ** p < 0.01 versus control group; ## p < 0.01 versus glutamate-treated group
HupA Protected HT22 Cells from Oxidative Glutamate Toxicity via Activating PI3K/Akt/mTOR Signaling Pathway
To investigate whether PI3K/Akt/mTOR signaling pathway was involved in the HupA’s neuroprotection against HT22 cells after glutamate insults, LY294002, a specific inhibitor of PI3K, was employed in our current work. As displayed in Fig. 3a, pretreatment with 10 μM HupA remarkably augmented the cell viability of HT22 cells after glutamate exposure compared with the vehicle-treated group (p < 0.01). However, the protection of 10 μM HupA was obviously blocked by 10 μM LY294002 for 1 h, while administration of LY294002 alone did not influence the cell viability of HT22 cells. In addition, we further verified our results via adding another inhibitor of PI3K, namely, wortmannin prior to treatment with HupA and glutamate. Furthermore, we also investigated whether the PI3K downstream target, Akt was altered in murine HT22 cells subjected to the glutamate insults. Immunoblot analysis revealed that exposure of glutamate to HT22 cells exhibited the significant reductions of p-AktSer473 and p-AktThr308 (Fig. 3b–d). Nevertheless, this phenomenon was markedly reversed after HupA treatment, in comparison to the vehicle group. Interestingly, HupA-activated Akt phosphorylation in HT22 cells was completely abrogated after PI3 K was blocked by 10 μM LY294002 for 1 h or 100 nM wortmannin for 1 h. Meanwhile, an obvious decrease of p-mTOR protein was observed in HT22 cells in response to glutamate stimuli and HupA treatment significantly increased the phosphorylation of mTOR in HT22 cells (Fig. 3e, f). Blockade of PI3K by LY294002 or wortmannin evidently prevented HupA-activated p-mTOR protein levels in HT22 cells subjected to oxidative glutamate toxicity.
HupA protected HT22 cells from glutamate toxicity via activating PI3K/Akt/mTOR signaling pathway. The promotion of cell viability in HT22 cells after HupA treatment was blocked by 10 μM LY294002 for 1 h in response to glutamate stimuli (a). The suppression of HupA’s protection against glutamate toxicity was also confirmed by another PI3K inhibitor, wortmannin (100 nM for 1 h) (a). The representative immunoblot images of p-AktSer473 and p-AktThr308 were displayed, and the quantitative analysis was conducted using SPSS 13.0 software (b). c, d showed that the phosphorylations of AktSer473 and AktThr308 were both suppressed by PI3K inhibitors (LY294002 and wortmannin). HupA-activated phosphorylation of mTOR was also abrogated in HT22 cells after blockade of PI3K by LY294002 or wortmannin (e, f). Results were presented as mean ± SD (n = 8). ** p < 0.01 versus control group; ## p < 0.01 versus glutamate-treated group; ++ p < 0.01 versus glutamate-induced cell death in the presence of HupA
HupA Enhanced p70s6 Kinase Phosphorylation in HT22 Cells After Oxidative Glutamate Damage
The phosphorylation of p70s6 kinase was further determined in immortalized HT22 cells. It was noteworthy that there was a remarkable decrease of the p70s6 kinase phosphorylation in HT22 cells stimulated with 5 mM glutamate (Fig. 4). Pretreatment with HupA obviously enhanced p-p70s6 kinase protein level (p < 0.01). The elevated phosphorylated p70s6 kinase protein expression was evidently blocked by the mTOR inhibitor, namely, rapamycin (100 nM for 15 min) prior to the exposure to HupA (p < 0.01). Throughout our investigation, the total protein expression of p70s6 kinase was not changed among different groups.
HupA enhanced p70s6 kinase phosphorylation in HT22 cells after glutamate oxidative glutamate toxicity. The phosphorylation of p70s6 kinase was detected by Western blot analysis. After mTOR was suppressed by its specific inhibitor, rapamycin (100 nM for 15 min), the elevated phosphorylated p70s6 kinase protein expression induced by HupA was markedly prevented in HT22 cells under glutamate insults. Results were presented as mean ± SD (n = 8). ** p < 0.01 versus control group; ## p < 0.01 versus glutamate-treated group; ++ p < 0.01 versus glutamate-induced cell death in the presence of HupA
HupA-Stimulated PI3K/Akt/mTOR Signaling in HT22 Cells was Regulated by BDNF/TrkB Pathway Under Glutamate Exposure
Since neurotrophins were known to play a critical role in cell survival, ELISA analysis was employed to detect the protein expressions of the major neurotrophins, namely, NGF, BDNF, NT-3, and NT-4 in HT22 cells under glutamate insults. As seen in Fig. 5a, d, exposure of HT22 cells to glutamate exhibited evident reductions of NGF, BDNF, NT-3, and NT-4 (p < 0.01). However, HupA treatment only enhanced the protein level of BDNF in HT22 cells under glutamate damage (p < 0.01). The elevated BDNF protein level caused by HupA was also verified by immunoblot assay in HT22 cells after glutamate exposure (Fig. 6). It was well known that BDNF exerted protective effects against cell injury via binding and activating its specific receptor, TrkB. Subsequently, the phosphorylated forms of TrkB protein were detected in our current work. Obviously, HupA treatment activated phosphorylation of TrkB protein in HT22 cells after glutamate stimulation (Fig. 5e). To explore whether BDNF/TrkB pathway could regulate PI3 K/Akt/mTOR signaling in HT22 cells when treated with HupA, TrkB IgG was also used to inhibit TrkB receptor in our present study. It was found that the enhanced phosphorylations of Akt and mTOR proteins caused by HupA were both prevented in HT22 cells treated with 0.5 μg/ml TrkB IgG for 4 h before adding glutamate and HupA (p < 0.01, Fig. 5f–h). Nevertheless, adding TrkB IgG alone did not exert significant effects on the protein levels of p-Akt and p-mTOR in HT22 cells under glutamate stimulation.
HupA-stimulated PI3K/Akt/mTOR signaling in HT22 cells was regulated by BDNF/TrkB pathway under glutamate exposure. The protein contents of NGF (a), BDNF (b), NT-3 (c), and NT-4 (d) were measured via ELISA analysis and it was found that only BDNF content was significantly elevated in HT22 cells after co-treatment with HupA and glutamate. HupA treatment also reversed the decreased protein expression of phosphorylated TrkB in glutamate-stimulated HT22 cells (e). When treated with 0.5 μg/ml TrkB IgG for 4 h prior to the additions of glutamate and HupA, the enhanced phosphorylations of Akt (f, g) and mTOR (h) caused by HupA were found to be dramatically suppressed in HT22 cells. Results were presented as mean ± SD (n = 8). ** p < 0.01 versus control group; ## p < 0.01 versus glutamate-treated group; ++ p < 0.01 versus glutamate-induced cell death in the presence of HupA
HupA Modulated Apoptosis-Related Protein Levels in HT22 Cells Under Oxidative Glutamate Toxicity
Finally, the effects of HupA on apoptosis-related proteins were investigated in HT22 cells following glutamate insults in our present investigation. Caspase-3 was the major molecule responsible for apoptosis execution. Thus, caspase-3 activity was analyzed by colorimetric methods in the first place. Our results depicted that caspase-3 activity was found to be obviously elevated in neuronal HT22 cells during the stimulation of glutamate versus the control group and HupA significantly suppressed this index (p < 0.01, Fig. 7a). Meanwhile, Bcl-2 family proteins were critical to neuronal survival and we speculated that they are involved in the neuroprotection of HupA against oxidative glutamate toxicity in murine HT22 cells. Our Western blot results indicated that there was an evident decrease of Bcl-2 protein in HT22 cells after the incubation of 5 mM glutamate for 12 h, while the elevated Bax protein level was found compared to the control group (p < 0.01, Fig. 7b–d). However, HupA treatment remarkably augmented the protein level of Bcl-2 in HT22 cells stimulated with glutamate, although no significant alteration of Bax protein level was observed under the same experimental conditions.
HupA modulated apoptosis-related protein levels in HT22 cells under glutamate toxicity. a Revealed that caspase-3 activity was found to be obviously elevated in neuronal HT22 cells during the stimulation of glutamate and HupA significantly suppressed caspase-3 activity. b–d Revealed that HupA treatment remarkably augmented Bcl-2 protein expression, although no evident alteration of Bax protein was observed in HT22 cells after co-treatment with glutamate and HupA. Results were presented as mean ± SD (n = 8). ** p < 0.01 versus control group; ## p < 0.01 versus glutamate-treated group
Discussion
The major findings of our present investigation disclosed that HupA exerted neuroprotective effects against oxidative glutamate toxicity in immortalized hippocampal HT22 cells. What’s more, HupA treatment remarkably activated the phosphorylations of Akt and mTOR. Besides, the elevation of BDNF was found in HT22 cell co-treated with glutamate and HupA, while there were no alterations of NGF, NT-3, and NT-4 in cultured HT22 cells. In the meantime, after blockade of TrkB by TrkB IgG, the phosphorylated forms of Akt and mTOR were both dramatically abrogated in HT22 cells under glutamate insults. These findings suggest that BDNF/TrkB pathway was involved in the modulations of HupA on PI3 K/Akt/mTOR signaling.
It is well known that glutamate is the major excitatory neurotransmitter in the central nervous system and plays a critical role in the modulation of cognition and the development of CNS including synaptic plasticity (Danbolt 2001). However, excessive generation of glutamate contributes to marked cell damage and oxidative glutamate toxicity is associated with the pathogenesis of diverse neurological disorders such as epilepsy, ischemic stroke, and Alzheimer’s disease (Coyle and Puttfarcken 1993; Mao et al. 2015; Simonian and Coyle 1996). Therefore, the in vitro oxidative glutamate toxicity model can be used as a very useful model to assess the protective effects of drugs against neurological diseases. It was previously reported that extremely high concentration of glutamate could induce the evident oxidative cell damage (Jia et al. 2013; Murphy et al. 1989) and manipulation of oxidative injury might be beneficial to amelioration of glutamate-induced toxicity. In our present work, HT22 cells were employed as it lacked the functional ionotropic glutamate receptor and it was an ideal cell model for exploring the pathogenesis of oxidative glutamate damage excluding glutamate receptor-mediated excitotoxicity. Actually, oxidative injury was previously reported to be triggered in HT22 cells under glutamate stimulation, and the medicinal herb Gastrodia elata remarkably prevented oxidative glutamate toxicity (Han et al. 2014). Besides, several previous investigations illustrated that suppression of ROS production by various substances such as kaempferol (Yang et al. 2014), acetogenin A (Lee et al. 2015), pinacidil (Shukry et al. 2015), or herbal mixture (Ahn et al. 2015) could remarkably attenuate cell apoptosis in mouse hippocampal HT22 cells. Similarly, results from our current study illustrated that HupA pretreatment dramatically suppressed ROS accumulation and subsequent neuronal damage in HT22 cells. In fact, it was previously found that HupA exerted neuroprotective roles against Alzheimer and global cerebral ischemic animal models (Wang et al. 2011; Zhou et al. 2001). The dose range of HupA (0–100 μM) was also used to explore whether it had toxic effects on HT22 cells in our present work. It was obvious that HupA treatment by the concentration of 10 μM did not exert any significant toxic action on HT22 cells and this concentration caused the maximal neuroprotection against neurons in response to glutamate stimuli. In line with our results, Wang et al. reported that HupA at the concentration of 10 μM exhibited the most evident neuroprotection in SH-SY5Y cell models (Wang et al. 2011).
Neurotrophins are a family of proteins and have been demonstrated to induce the survival, differentiation, and function of neurons in the CNS (Huang and Reichardt 2003). Generally, there are at least four subtypes of neurotrophins, namely, NGF, BDNF, NT-3, and NT-4. In our current work, BDNF was observed to be evidently enhanced after co-treatment with HupA and glutamate, while no alterations of NGF, NT-3, and NT-4 were found in murine HT22 cells. Furthermore, TrkB, a high affinity receptor for BDNF was also explored in neuronal HT22 cells under oxidative toxic conditions. It was illustrated that the phosphorylation of TrkB (activated form of TrkB) was significantly decreased in mouse hippocampal HT22 cells subjected to glutamate insults and this phenomenon was evidently reversed after HupA treatment. Particularly, BDNF/TrkB has been shown to activate PI3 K/Akt signaling pathway, subsequently contributing to neuronal survival under a wide range of circumstances (Brunet et al. 2001). To figure out whether HupA protected HT22 cells from oxidative glutamate toxicity via mediating BDNF/TrkB-PI3 K/Akt signaling pathway, we further detected the phosphorylations of Akt, mTOR, and p70s6 kinase in the presence of TrkB IgG or PI3 K inhibitor LY294002. Our present study showed that inhibition of TrkB by TrkB IgG prevented the elevated phosphorylation of Akt. In the meantime, pharmacological suppression of PI3 K by LY294002 significantly abrogated the increased phosphorylations of mTOR and p70s6 kinase after HupA treatment. Furthermore, the inhibitions of TrkB by TrkB IgG or PI3 K by wortmannin also verified our findings as mentioned above. Taken together, these results indicated that HupA protected against oxidative glutamate toxicity in hippocampal HT22 cells and its neuroprotection might be at least modulated by BDNF/TrkB-dependent PI3 K/TrkB/mTOR signaling pathway. Similarly, a previous investigation illustrated that BDNF/TrkB signaling could activate PI3K/TrkB pathway and subsequently suppressed cellular apoptosis following ischemic insults (Yao et al. 2012).
There is compelling evidence supporting that cellular apoptosis is triggered by two major pathways, namely, death receptor and cell stress (Bao et al. 2013). These pathways are dependent on caspase-3 activation, and caspase-3 is considered as an executioner molecule in the pathogenesis of apoptosis (Mao et al. 2012). Bcl-2 family proteins have been also reported to be involved in the modulation of cell apoptosis. It is well known that Bcl-2 functions in preventing apoptosis, while Bax serves as a proapoptotic molecule (Mao et al. 2012). In fact, it was previously investigated that mitochondrial Bcl-2 protein level was significantly diminished, while elevated Bax level was observed after exposure to high concentration of glutamate (10 mM) in hippocampal HT22 neurons, finally contributing to oxidative stress-induced cell apoptosis (Yang et al. 2012). In consistent with our current work, lower glutamate exposure (5 mM) resulted in evident reduction of Bcl-2 and increased Bax protein level. Furthermore, the altered expressions of Bcl-2 and Bax caused by glutamate were remarkably reversed by HupA treatment. These findings hinted that the neuroprotection of HupA against glutamate toxicity might involve the modulation of Bcl-2 and Bax proteins in hippocampal HT22 cells.
Conclusions
It was concluded that HupA effectively ameliorated oxidative glutamate toxicity in immortalized hippocampal HT22 cells and its neuroprotection might be associated with modulating PI3K/Akt/mTOR signaling dependent of BDNF/TrkB pathway. The molecular mechanism can be served as the potential therapeutic targets for attenuating glutamate toxicity-associated neurological disorders, including epilepsy, ischemic stroke, depression, and Alzheimer’s disease in future. Further investigations are essential to validate our findings.
References
Ahn SM et al (2015) Neuroprotection and spatial memory enhancement of four herbal mixture extract in HT22 hippocampal cells and a mouse model of focal cerebral ischemia. BMC Complement Altern Med 15:202. doi:10.1186/s12906-015-0741-1
Almeida RD et al (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12:1329–1343. doi:10.1038/sj.cdd.4401662
Bao MH, Zhang YW, Zhou HH (2013) Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway. J Ethnopharmacol 146:543–551. doi:10.1016/j.jep.2013.01.019
Beck T, Lindholm D, Castren E, Wree A (1994) Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J Cereb Blood Flow Metab 14:689–692. doi:10.1038/jcbfm.1994.86
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Han YJ et al (2014) Gastrodia elata shows neuroprotective effects via activation of PI3K signaling against oxidative glutamate toxicity in HT22 cells. Am J Chin Med 42:1007–1019. doi:10.1142/s0192415x14500633
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. doi:10.1146/annurev.biochem.72.121801.161629
Jackson GR, Apffel L, Werrbach-Perez K, Perez-Polo JR (1990) Role of nerve growth factor in oxidant-antioxidant balance and neuronal injury. I. Stimulation of hydrogen peroxide resistance. J Neurosci Res 25:360–368. doi:10.1002/jnr.490250313
Jeong GS, Byun E, Li B, Lee DS, Kim YC, An RB (2010) Neuroprotective effects of constituents of the root bark of Dictamnus dasycarpus in mouse hippocampal cells. Arch Pharm Res 33:1269–1275. doi:10.1007/s12272-010-0818-9
Jia J et al (2013) Differential mechanisms underlying neuroprotection of hydrogen sulfide donors against oxidative stress. Neurochem Int 62:1072–1078. doi:10.1016/j.neuint.2013.04.001
Kazanis I, Giannakopoulou M, Philippidis H, Stylianopoulou F (2004) Alterations in IGF-I, BDNF and NT-3 levels following experimental brain trauma and the effect of IGF-I administration. Exp Neurol 186:221–234. doi:10.1016/j.expneurol.2003.12.004
Larsson E, Nanobashvili A, Kokaia Z, Lindvall O (1999) Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab 19:1220–1228. doi:10.1097/00004647-199911000-00006
Lee DS, Cha BY, Woo JT, Kim YC, Jang JH (2015) Acerogenin A from nikoense maxim prevents oxidative stress-induced neuronal cell death through Acer Nrf2-mediated heme oxygenase-1 expression in mouse hippocampal HT22 Cell Line. Molecules 20:12545–12557. doi:10.3390/molecules200712545
Mao X et al (2012) Topiramate attenuates cerebral ischemia/reperfusion injury in gerbils via activating GABAergic signaling and inhibiting astrogliosis. Neurochem Int 60:39–46. doi:10.1016/j.neuint.2011.10.015
Mao XY, Cao YG, Ji Z, Zhou HH, Liu ZQ, Sun HL (2015) Topiramate protects against glutamate excitotoxicity via activating BDNF/TrkB-dependent ERK pathway in rodent hippocampal neurons. Prog Neuropsychopharmacol Biol Psychiatry 60:11–17. doi:10.1016/j.pnpbp.2015.01.015
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129. doi:10.1038/35040009
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2:1547–1558
Shukry M, Kamal T, Ali R, Farrag F, Almadaly E, Saleh AA, Abu El-Magd M (2015) Pinacidil and levamisole prevent glutamate-induced death of hippocampal neuronal cells through reducing ROS production. Neurol Res. doi:10.1179/1743132815y.0000000077
Simonian NA, Coyle JT (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106. doi:10.1146/annurev.pa.36.040196.000503
Tang LL, Wang R, Tang XC (2005) Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur J Pharmacol 519:9–15. doi:10.1016/j.ejphar.2005.06.026
Wang ZF, Tang XC (2007) Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation-induced injury. FEBS Lett 581:596–602. doi:10.1016/j.febslet.2007.01.016
Wang R, Xiao XQ, Tang XC (2001) Huperzine A attenuates hydrogen peroxide-induced apoptosis by regulating expression of apoptosis-related genes in rat PC12 cells. NeuroReport 12:2629–2634
Wang R, Yan H, Tang XC (2006) Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 27:1–26. doi:10.1111/j.1745-7254.2006.00255.x
Wang R et al (2010) Curcumin produces neuroprotective effects via activating brain-derived neurotrophic factor/TrkB-dependent MAPK and PI-3K cascades in rodent cortical neurons. Prog Neuropsychopharmacol Biol Psychiatry 34:147–153. doi:10.1016/j.pnpbp.2009.10.016
Wang CY et al (2011) Huperzine A activates Wnt/beta-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 36:1073–1089. doi:10.1038/npp.2010.245
Yang EJ et al (2012) Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochem Biophys Res Commun 421:658–664. doi:10.1016/j.bbrc.2012.04.053
Yang EJ, Kim GS, Jun M, Song KS (2014) Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct 5:1395–1402. doi:10.1039/c4fo00068d
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX (2012) Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res 37:2777–2786. doi:10.1007/s11064-012-0871-5
Zhou J, Zhang HY, Tang XC (2001) Huperzine A attenuates cognitive deficits and hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett 313:137–140
Acknowledgments
This project was partly funded by the National Natural Science Foundation of China (No. 81302750).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
These authors declared no potential conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10571_2015_276_MOESM1_ESM.tiff
Supplementary material 1 S-Fig. 1 HupA attenuated neuronal impairment in human neuroblastoma SH-SY5Y cells under glutamate stimulation. SH-SY5Y cells was subjected to different concentrations of glutamate (2, 4, 6, 8 and 10 mM) for 24 h and cell viability was measured by MTT method (a). Nearly a 30% reduction of cell viability was found after glutamate exposure at the concentration of 10 mM for 24 h. HupA by the concentrations of 0-10 μM did not have any significant toxic effects on the cell viability of SH-SY5Y cells (b). It was also noted that 10 μM of HupA treatment for 24 h evidently promoted the recovery of SH-SY5Y cells after glutamate exposure (c). Results were expressed as mean ± standard deviation (SD) (n = 8). ** p<0.01 versus control group; ## p<0.01 versus glutamate-treated group. (TIFF 306 kb)
Rights and permissions
About this article
Cite this article
Mao, XY., Zhou, HH., Li, X. et al. Huperzine A Alleviates Oxidative Glutamate Toxicity in Hippocampal HT22 Cells via Activating BDNF/TrkB-Dependent PI3K/Akt/mTOR Signaling Pathway. Cell Mol Neurobiol 36, 915–925 (2016). https://doi.org/10.1007/s10571-015-0276-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0276-5