Abstract
The morphology, crystallinity, crystallite size, and production yield of bacterial cellulose (BC) produced with six different carbon sources (glucose, fructose, lactose, maltitol, sucralose, and xylitol) in static and agitated fermentation conditions by five strains of Komagataeibacter xylinus (KX, TISTR 086, 428, 975, and 1011) which are locally available, were studied. In static condition, the BC pellicle was formed as a membrane sheet at the medium surface exposed to air, while in agitated condition, the spherical or asterisk-like shape BC particles were obtained in the culture media. The XRD and FT-IR analyses found no significant differences in the cellulose crystallinity, crystallite size or polymorphic distribution within the carbon sources. However, changes in crystallinity and mass fraction of the Iα allomorph were observed in BC produced from the different bacterial strains and incubation conditions. The BC samples produced by the same bacterial strain with the varying culture conditions showed the alteration of physical properties more clearly than the BC samples prepared by the opposite situation. These findings suggested that the strains of bacteria and fermentation conditions strongly affected on the physical structures of BC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant biopolymer found in nature and is produced by a wide variety of organisms, ranging from plant to algae and prokaryotic organisms (Delmer and Amor 1995; Klemm et al. 2005). However, bacterial cellulose (BC) has been more interesting and useful due to its higher purity and highly crystalline nanostructure. BC structures are formed by extracellular-excreted nanofibers produced by various species of bacteria such as Acetobacter, Aerobacter, Azotobacter, Agrobacterium, Achromobacter, Gluconacetobacter, Rhizobium, Sarcina and Salmonella (Brown 2004; Jahn et al. 2011; Morgan et al. 2013). Among the mentioned genera, the most effective cellulose producing specie is Gluconacetobacter xylinus (formerly Acetobacter xylinum) which is often used as model organisms in bacterial cellulose production studies (Keshk et al. 2006; Nguyen et al. 2008). At present, G. xylinus is reclassified as Komagataeibacter xylinus according to its 16S rRNA gene sequences (Yamada et al. 2012). K. xylinus is an aerobic gram negative bacterium, has ability in producing cellulose extracellularly at temperatures between 25 and 30 °C and pH from 3 to 7, using glucose, fructose, sucrose, mannitol, and other carbohydrates, as carbon sources (Castro et al. 2011).
BC has distinctive characteristics including high mechanical strength, high purity, and extremely fine cellulose fiber network structure of around 100 nm and 100 µm in diameter and length, respectively. This ribbon-like network structure is consisted of bundles of microfibrils of 2-4 nm in diameter (Gindl and Keckes 2004; Hirai et al. 2002; Iguchi et al. 2000; Nakagaito et al. 2005). In its native state, BC is a water-swollen network of cellulose nanofibers. BC has coherent 3D network structure with porosity, high water holding capability, and high biocompatibility. Due to these special characteristics, BC has become a very useful biomaterial in many applications such as paper making (Yamanaka et al. 1989), membrane (Iguchi et al. 2000), food industry (Budhiono et al. 1999), and biomedical materials (Chang and Zhang 2011).
The BC structure and production yield can be affected by several factors including types of BC producing bacterial strains, fermentative media, carbon sources, and growth conditions which can be altered to obtain BC with desirable properties. The BC production yield is mostly influenced by the relation of types of bacterial strain and carbon sources used. For example, Gluconacetobacter hansenii NCIM 2529 produced the highest BC yield from a sucrose containing medium (Mohite et al. 2013), whereas Gluconacetobacter xylinus ATCC 53524 and PTCC 1734 had the highest ability in BC production when mannitol was used as a carbon source (Mohammadkazemi et al. 2015; Ruka et al. 2012). The determination of an optimal medium and an appropriate set of growth conditions for high levels of BC production would be beneficial for implementing this technology to an industrial scale.
Interestingly, the BC morphology and characteristic properties are greatly affected by different cultivation methods, static and agitated. In the static condition, a BC pellicle in a gelatinous form is produced at the surface of the culture medium. In contrast, various sizes (10 µm–1 mm) and shapes (spherical, ellipsoidal, stellate or fibrous) of well-dispersed BC in the culture medium are synthesized in the agitated condition, depending on types of BC producing bacterial strains (Dudman 1960; Hestrin and Schramm 1954; Yoshinaga et al. 1997). The BC produced through the agitated cultivation method displayed some microstructural changes which were the decrease in degree of polymerization, crystallinity index, cellulose Iα content, and crystallite size, but the increase in water holding capacity as compared to static cultivation (Czaja et al. 2004; Krystynowicz et al. 2002; Watanabe et al. 1998). Consequently, the BC with different characteristics suitable for different applications can be produced by varying these variable factors.
In this study, the aim was to evaluate the effect of various BC producing bacterial strains, carbon sources, and fermentation conditions on the BC production. The synthesis and structural characteristics of BC produced with six different carbon sources in static and agitated cultures by five different strains of K. xylinus, which are commercially available in Thailand, were investigated. The BC production yield was measured as the dry weight of cellulose within the volume of medium in liter (g/L). The morphology of resulting BCs was examined by the field emission scanning electron microscopy. To characterize the effects of variable factors on the crystalline arrangement of glucan chains within microfibrils and their crystallite size, the X-ray diffraction was used. Furthermore, the estimation of Iα and Iβ cellulose fractions in BC samples from different culture conditions was carried out using the FT-IR spectroscopy. The obtaining results would be useful database for other researchers to select optimal BC production conditions for their desired BC applications.
Materials and methods
Bacterial strains
Four strains of K. xylinus; K. xylinus TISTR 086, 428, 975, and 1011 (K086, K428, K975, and K1011, respectively) were obtained from the Thailand Institute of Scientific and Technological Research, and one K. xylinus (KX) was obtained from the Institute of Food Research and Product Development, Kasetsart University, Thailand. Each of K. xylinus strains was cultured on glucose yeast extract (GYE) agar containing 100 g d-glucose, 10 g yeast extract, 5 g peptone, 20 g CaCO3, 25 g agar per liter at 30 °C for 3 days. Working cultures were routinely prepared on GYE and stored at 4 °C until use. Chemicals for microbiology and other chemicals used were purchased from Sigma-Aldrich.
Media
The glucose yeast extract broth (GYB) was selected from the literature and modified for the present study. The GYB consisted of 50 g glucose and 5 g yeast extract in one-liter solution. Glucose (GC), fructose (FT), lactose (LT), maltitol (MT), sucralose (SC), and xylitol (XL) were used as different carbon sources in the modified GYB media. Before use, all the media were autoclaved at 121 °C for 15 min. The pH was adjusted to 5.0 with HCl or NaOH.
Growth conditions
For preparation of each K. xylinus strain seed culture, a single colony from a working plate of GYE agar was selected and inoculated in 10 mL of each of six modified GYB media. These seed cultures were incubated for 7 days at 30 °C under static condition. Following growth, bacterial cells were separated from cellulose pellicles in the seed cultures by vigorously shaking; as a result, the cell suspension for inoculation was obtained. Cultures were grown in 250-mL erlenmeyer flasks containing 100 mL of media and inoculated with 5% (v/v) of the cell suspension. The BC production was studied in two conditions: under static condition and agitated condition in which cultures were agitated in a shaking incubator at speed of 150 rpm. The incubation period was 7 days at 30 °C for both static and agitated conditions.
BC purification
After incubation, the BC pellicles and particles were harvested from the cultures and rinsed with distilled water to remove any residual media. The BC products were washed with 2% w/v NaOH at 80 °C for 1 h, and then washed repeatedly with distilled water until a neutral pH was obtained.
Dry weight and yield of BC
The BC production was investigated as the dry weight of cellulose within the volume of medium in liter (g/L). The dry weight of BC was determined by weighing the dried BC pellicles and pellets which were air dried in a desiccator at room temperature for 3 days until reaching constant weight.
Fourier transform infrared spectroscopy (FT-IR)
Each BC sample was air-dried on a glass slide in the form of a thin film. FT-IR spectra were obtained using an ATR Nicolet iD7 FT-IR spectrometer. All spectra were scanned between 4000 and 400 cm−1 with 128 convolutions at a resolution of 4 cm−1. Baselines for each sample spectrum were normalized using the Spectrum software. The fα fraction of the samples was calculated by the following equation (Yamamoto et al. 1996):
where \( f_{ \propto }^{IR} \) of cellulose can be calculated as Aα/(Aα + Aβ) and Aα and Aβ are absorbencies at 750 and 710 cm−1, respectively.
X-ray diffraction (XRD)
X-ray diffraction diagrams of dried BC samples were recorded using a Rigaku Model SmartLab 4800 diffractometer with the CuKα radiation wave length (λ = 1.54 Å), generated at a voltage of 40 kV and a filament emission of 30 mA. Samples were scanned from 5–40° 2θ-range at scan speed of 2°/min and scan step of 0.02°. The crystallinity index (CrI) and crystallite size (CrS) were calculated based on X-ray diffraction measurements. Crystallinity index was calculated from the following equation:
where I110 is the overall intensity of the peak at 2θ about 22.7° and Iam is the intensity of the baseline at 2θ about 18° (Mihranyan et al. 2004). The CrS was determined using the Scherrer equation as following (Cheng et al. 2009):
where K is the shape factor (0.9), λ is the X-ray wavelength (1.54 Å), β is the full width at half maximum height (FWHM), and θ is the Bragg’s angle.
Field emission scanning electron microscopy (FE-SEM)
The dried BC samples were sputter coated with platinum in preparation for FE-SEM imaging. The field-emission SEM Hitachi S-4800 model was used, operating at accelerated voltage of 5 kV and magnification of 20 k.
Results and discussion
Production and yield of BC
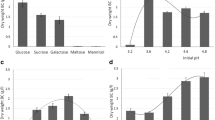
The culture medium used in this study was the GYB which was a simple medium containing only glucose (or other sugar) as a carbon source, and yeast extract as a protein and mineral source for bacterial growth and bacterial cellulose production. The amounts of BC production from various types of carbon sources by five strains of K. xylinus were measured after 7 days incubation. In the static condition, the bacterial strains KX (1.14–1.84 g/L) and K975 (1.11–1.55 g/L) were comparably able to produce BC pellicles in the highest amount in all carbon sources, then followed by the K1011 (0.57–1.46 g/L); while the K086 (0.14–0.39 g/L) and K428 (0.09–0.22 g/L) had the lowest abilities in BC production as shown in Fig. 1a. The KX strain is generally used as a culture feed in Nata de Coco dessert productions in Thailand. The result of high BC production yield could confirm the benefit of the KX use in the commercial level. Moreover, the KX might be substituted by the K975 with the same high productivity. The highest amounts of BC produced by KX and K975 were 1.84 and 1.55 g/L, respectively when fermenting with glucose.
Bacterial cellulose is synthesized by microorganisms using a metabolic pool of hexose phosphate which is obtained directly by the phosphorylation of exogenous hexoses, and indirectly through the pentose cycle and gluconeogenic pathway (Colvin and Leppard 1977; Ross et al. 1991; Schramm et al. 1957). Six sugars were selected to study the capability of five strains of K. xylinus in the BC biosynthesis with different carbon sources. Glucose and fructose are hexose monosaccharides with the same chemical formula (C6H12O6) but different in their ring structures. Xylitol is a sugar alcohol and monosaccharide (C5H12O5) derived from xylose (pentose sugar). Lactose is a disaccharide consisting of galactose and glucose. Maltitol is a sugar alcohol and disaccharide (C12H24O11) obtained from the hydrogenation of maltose. Sucralose is a selective chlorinated sucrose, disaccharide (C12H19Cl3O8). All five strains were able to grow and produce BC in the GYB medium with these six sugars. Glucose and fructose gave high BC yields in all strains. Even though these two sugars are utilized by the same pathway for BC synthesis (Schramm et al. 1957), glucose gave the highest amount of produced BC when comparing in each bacterial strains. This phenomenon potentially resulted from the higher ability of glucose in promoting cell growth, consequently high BC yields can be obtained since BC production correlates to cell growth (Ross et al. 1991). Interestingly, maltitol was able to give significantly high BC amounts in the strains KX and K975. The possible reason was maltitol is composed of two molecules of glucose which might be consumed effectively by these two bacterial strains. In the xylitol medium, all strains produced low to moderate levels of BC, except the KX was able to synthesize BC in high amount. In contrast, the sucralose medium gave the lowest BC productions in all five bacterial strains due to its chlorinated modified structure which may not be suitable for bacterial growth (Omran et al. 2013), resulting in low BC production.
Besides the types of microorganism and carbon sources that could influence on the production of bacterial cellulose, the production methods had significant impacts as well. Two methods of BC production, static and agitated fermentation methods, were used in this research. As can be seen from Fig. 1b, the production of BC by three K. xylinus strains (KX, K1011, and K975) were highly influenced by the fermentation method. In the agitated condition, the BC productivity of K1011 and K975 increased significantly. It is generally known that the aerobic bacteria such as K. xylinus can proliferate better at the air/solution interface where oxygen is readily supplied, than in the culture nutrient-rich and oxygen-lean liquid media (Iguchi et al. 2000; Lee and Zhao 1999). In static fermentation, inside the BC pellicles may have concentration gradients of oxygen and nutrients (including carbon sources). This finding confirmed that the oxygen supply significantly influences on the cellulose production (Hestrin and Schramm 1954). The agitated condition could increase oxygen diffusion into the medium, resulting in the higher productivity of K975 and K1011 which the highest BC produced went up to 3.54 and 4.69 g/L, respectively. On the contrary, this action could induce the emergence of mutant cells which their ability in cellulose biosynthesis are depleted, causing a diminish in the production of cellulose. This consequence might explain the decrease in BC production yield of KX under agitated culture.
Morphology of BC
The BC production by different fermentation methods provided the BC with different morphology and properties. Under static condition, all K. xylinus strains formed pellicles at the surface of the culture medium. After 7 days incubation, KX, K1011, and K925 produced smooth and thick BC sheets; but K428 and K086 gave rough and thin BC pellicles which showed their low ability in BC biosynthesis. Nevertheless, the variation of carbon sources did not impact the morphology of BC produced by each strain. Examples of optical images of cellulose produced in the high BC-achieving carbon sources were presented in Fig. 2. The micro-architecture of cellulose pellicles was investigated by FE-SEM as shown in Fig. 3, the FE-SEM images of the obtained pellicles revealed no apparent difference in the morphological appearances and fibril diameters, as the cellulose produced under all conditions displayed the retention of its nanosized-interwoven structure.
In the agitated condition, the BC products were not in pellicle sheet forms, but appeared in forms of spherical shape and irregular granules that were well dispersed in culture media. The BC particles were accumulated in the agitated media with different sizes and shapes, including solid sphere-like BC (Fig. 2f, g, h, and j), and flocky asterisk-like BC (Fig. 2i). After purification with 2% NaOH solution at 80 °C, the resulting particles were white and semi-transparent appearances with diameters of 5–20 mm. The FE-SEM images as shown in Fig. 3f–j (Agitated-BC) illustrated that the BC microfibrils were in disorderly reticulated structures and consisted of 50–100 nm width ultrafine fibrils which were similar in size, not dependent of the different bacterial strains. The fibril arrangement of agitated BCs was looser and larger in pore size. There were some changes in the surface structures, the microfibrils were appeared in stretchable woven structures with the larger cracks webbing between cellulose microfibrils. The stretching cellulose microfibrils and larger holes between woven cellulose microfibrils may be a result of the disrupted microfibril formation by the agitated treatment during the fermentation process. According to their distinctive properties, the sphere-like BCs with the tenuous woven microfibrils have been found to be useful as adsorbents for heavy metal ions and sewage treatment (Zhu et al. 2011). In addition, according to their good biocompatibility, they might be useful in development of specific biomedical materials, such as bone type tissue scaffolds, bioseparation, immobilized reaction, and cell suspension culture (Hu and Catchmark 2011).
Crystallinity and crystallite size
Effect of bacterial strains
The XRD and FT-IR techniques were performed to characterize the crystalline structure and the mass fractions of cellulose Iα and Iβ of the BC samples produced in the chosen media. Cellulose is a homogeneous polycrystalline macromolecular compound composing of crystalline (ordered) and amorphous (less ordered) regions (Bi et al. 2014). Cellulose I is the naturally mostly found form of cellulose which consists of parallel chains (Delmer and Amor 1995), and occurs in two distinct allomorphs, meta-stable state celluloses Iα and stable state celluloses Iβ (Atalla and Vanderhart 1984). The XRD analysis results of bacterial cellulose harvested after being cultivated for 7 days were shown Fig. 4. There were three main diffraction peaks at 2θ = 14.5°, 16.7° and 22.7° which can be corresponded to the (100), (010), and (110) planes of cellulose Iα, or the (\(1\bar{1}0\)), (110), and (200) planes of cellulose Iβ (French 2014). Due to the co-existing peak positions of two allomorphs, it is difficult to differentiate the two allomorphs by only determining the XRD peak positions. Nevertheless, the intensity of the 14.5° peak was higher than the 16.7° peak, indicating the unique properties of typical BC samples produced by K. xylinus which contain mostly cellulose Iα (Tokoh et al. 1998). For the shapes of cellulose crystallites, they are in square cross-sectional shape if the intensities of the (100) and (010) reflections are nearly equal; however, the slightly stronger (100) reflection in Fig. 4a and b suggested that the shape of cellulose crystallites were rectangular (Bi et al. 2014). The shape of the cellulose crystallites has been speculated that it is affected by the arrangement of the cellulose synthesis enzymes at the BC producing bacteria cell wall membrane (Tsekos 1999). Furthermore, the crystallite size and crystallinity index of produced BC samples were calculated from the resulting XRD peaks, and given in Table 1. In general, bacterial cellulose has small crystallite sizes and high crystallinity, in which in this study they were in range of 4.7–6.8 nm and 55–81%, respectively. Interestingly, the difference of bacterial strains profoundly influenced on these BC structural characteristics. Under static condition, the K011 and K975 produced the largest crystallites about 6.0–6.8 nm, followed by the KX (5.7–6.6 nm) and K428 (5.1–5.3 nm), and the K086 gave the smallest crystallites of 4.7–5.0 nm. The BC produced by K1011, K975, and KX showed higher crystallinities than BC produced by K428 and K086, with values of up to 80, 81, 81, 64, and 60%, respectively. These results suggested that the three strains of K. xylinus; KX, K1011, and K975 had better ability in BC production and crystallization, leading to the higher crystallinity of BC microfibrils than those from the other two strains; K428 and K086.
In addition, the types of cellulose producing organisms have effects on the ratio of cellulose Iα and Iβ produced. The FT-IR spectroscopy was performed to determine the exact values of mass fractions of these two allomorphs. As shown in Fig. 5, the regions of the FT-IR spectra peaks at 750 and 710 cm-1 were assigned to Iα and Iβ fractions, respectively. The cellulose Iα contents (%) of all BC samples were shown in Table 1. The results demonstrated that the variation in cellulose Iα contents of the BC produced by five different bacterial strains in this study were small, with the values ranged from 73 to 82%. It confirmed that the BC is composed of high cellulose Iα contents, whereas plant cellulose is rich in cellulose Iβ (Atalla and Vanderhart 1984).
Effect of carbon sources
For K. xylinus bacteria, the change in the carbon sources has also been shown to affect the characteristics of BC produced (Klemm et al. 2005). From the Table 1, the crystallite size and Iα content data did not differ greatly between carbon sources among each bacterial strain; oppositely, the crystallinity of the cellulose produced in the different carbon sources was apparently different. Especially in the case of the KX strain, the highest crystallinity BC (81%) was obtained from the BC production with the glucose-containing medium, while the other sugar media provided the crystallinity of cellulose about 60–69%. This could be explained by the KX can efficiently use glucose as a carbon source to produce and crystallize BC microfibril more perfectly, resulting in the higher crystallinity (Benziman et al. 1980).
Effect of growth conditions
For the purposes of comparison of the microstructural changes in cellulose samples from both different culture conditions and estimation of the influence of the shaking condition on disturbance in the crystallization process, the XRD technique was determined. Analysis of the reflections corresponding to all three peaks in those XRD profiles in Fig. 4b revealed that the peaks of the cellulose samples from agitated culture were shifted to wider angles. Additionally, when comparing of 2θ angle values, the (100) and (010) reflections of the agitated BC samples were positioned closer together than in the cellulose profile from the static BC. These d-spacing changes appeared to represent that the proportion of Iα and Iβ cellulose allomorphs was altered, as reported previously (Czaja et al. 2004; Watanabe et al. 1998). Likewise, from the calculation based on the FT-IR spectra, the cellulose Iα contents of agitated BC were lesser to the value range of 67 to 77%. Interestingly, the change in cellulose Iα contents of the K428 was very small, the values decreased from 73–80 to 70–76%. This result agreed with the previous study which reported that the cellulose Iα content in the flocky asterisk-like BC was higher than the one found in the solid sphere-like BC (Bi et al. 2014). This finding suggested that the crystallization of cellulose microfibril structures of produced BCs could be affected by their macrostructure morphology.
Moreover, the percent crystallinity of cellulose grown under agitated culture was lower than the cellulose synthesized under static condition, suggesting that the agitation could interfere the aggregation of BC microfibrils causing the lower crystallinity. The crystallinities of BC produced by the K975, K1011, K428, and K086 decreased to the range of 61–66%, 53–74%, 46–55%, and 48–53%, respectively. The considerable reduction of crystallinity was found in the BC produced by the KX, the values decreased from 60–81 to 41–52% which could confirm that the agitation caused the mutation of the KX strain resulting in the loss of BC production ability.
The results also demonstrated the existence of smaller crystallite sizes in cellulose from agitated culture. The stress occurred during agitation seemed to interrupt greatly in the process of nascent microfibrils crystallization; therefore, it was more favorable to form smaller size microfibrils and increase the cellulose Iβ contents (more stable allomorph) in the BC production under stressful conditions. This hypothesis reconciled with previous reports (Ruka et al. 2012; Yamamoto et al. 1996).
Conclusion
In this study, the structural characteristics of BC produced by five strains of K. xylinus available in Thailand with six categories of carbon sources (glucose, fructose, lactose, maltitol, sucralose, and xylitol) in static and agitated culture conditions were investigated. Some conclusions were as follows:
-
The yield of production and morphology of BC were mainly affected by types of bacterial producing strains and methods of fermentation. Whereas the difference of carbon sources had small effects on the BC yield depending on each K. xylinus strains.
-
Static fermentation method produced bacterial cellulose in the pellicle form, while agitated fermentation method generated fragmented-cellulose particles with predominantly spherical shape. Agitation caused woven cellulose microfibrils become looser and form larger porosity.
-
One of K. xylinus strains used was the KX provided by the Institute of Food Research and Product Development, Kasetsart University, Thailand which has been known to produce high yield of cellulose and popularly used in food industry in Thailand. This KX was able to highly biosynthesize BC from all carbon sources in the static condition but not the agitated condition.
-
The K975 had comparable ability of BC production as the KX, and was capable of producing BC in the agitated condition.
-
The K1011 was suitable for BC production in the agitated condition since its BC yield dramatically increased when incubated in the agitated culture comparing to the static one.
-
The K428 and K086 although had low BC yield in the static condition, their BC particles produced in the agitated condition were fascinating because of their small-size morphology which can provide large surface area. Especially, the asterisk-like BC particles made by the K428 would give extra-large surface area that is good for absorption ability.
References
Atalla RH, Vanderhart DL (1984) Native cellulose: a composite of two cistinct crystalline forms. Science 223:283–285. https://doi.org/10.1126/science.223.4633.283
Benziman M, Haigler CH, Brown RM, White AR, Cooper KM (1980) Cellulose biogenesis: polymerization and crystallization are coupled processes in Acetobacter xylinum. Proc Natl Acad Sci U S A 77:6678–6682
Bi JC, Liu SX, Li CF, Li J, Liu LX, Deng J, Yang YC (2014) Morphology and structure characterization of bacterial celluloses produced by different strains in agitated culture. J Appl Microbiol 117:1305–1311. https://doi.org/10.1111/jam.12619
Brown RM (2004) Cellulose structure and biosynthesis: what is in store for the 21st century? J Polym Sci Part A Polym Chem 42:487–495. https://doi.org/10.1002/pola.10877
Budhiono A, Rosidi B, Taher H, Iguchi M (1999) Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohydr Polym 40:137–143. https://doi.org/10.1016/S0144-8617(99)00050-8
Castro C, Zuluaga R, Putaux J-L, Caro G, Mondragon I, Gañán P (2011) Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr Polym 84:96–102. https://doi.org/10.1016/j.carbpol.2010.10.072
Chang C, Zhang L (2011) Cellulose-based hydrogels: present status and application prospects. Carbohydr Polym 84:40–53. https://doi.org/10.1016/j.carbpol.2010.12.023
Cheng K-C, Catchmark JM, Demirci A (2009) Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J Biol Eng 3:12. https://doi.org/10.1186/1754-1611-3-12
Colvin JR, Leppard GG (1977) The biosynthesis of cellulose by Acetobacter xylinum and Acetobacter acetigenus. Can J Microbiol 23:701–709. https://doi.org/10.1139/m77-105
Czaja W, Romanovicz D, Rm Brown (2004) Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 11:403–411. https://doi.org/10.1023/b:cell.0000046412.11983.61
Delmer DP, Amor Y (1995) Cellulose biosynthesis. Plant Cell 7:987–1000
Dudman WF (1960) Cellulose production by Acetobacter strains in submerged culture. Microbiology 22:25–39. https://doi.org/10.1099/00221287-22-1-25
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Gindl W, Keckes J (2004) Tensile properties of cellulose acetate butyrate composites reinforced with bacterial cellulose. Compos Sci Technol 64:2407–2413. https://doi.org/10.1016/j.compscitech.2004.05.001
Hestrin S, Schramm M (1954) Synthesis of cellulose by acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58:345–352
Hirai A, Tsuji M, Horii F (2002) TEM study of band-like cellulose assemblies produced by acetobacter xylinum at 4 C. Cellulose 9:105–113. https://doi.org/10.1023/a:1020195205030
Hu Y, Catchmark JM (2011) Integration of cellulases into bacterial cellulose: toward bioabsorbable cellulose composites. J Biomed Mater Res Part B 97B:114–123. https://doi.org/10.1002/jbm.b.31792
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci 35:261–270. https://doi.org/10.1023/a:1004775229149
Jahn CE, Selimi DA, Barak JD, Charkowski AO (2011) The Dickeya dadantii biofilm matrix consists of cellulose nanofibres, and is an emergent property dependent upon the type III secretion system and the cellulose synthesis operon. Microbiology 157:2733–2744. https://doi.org/10.1099/mic.0.051003-0
Keshk S, Razek T, Sameshima K (2006) Bacterial cellulose production from beet molasses. Afr J Biotechnol 5:1519–1523
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainableraw material. Angew Chem Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Krystynowicz A, Czaja W, Wiktorowska-Jezierska A, Gonçalves-Miśkiewicz M, Turkiewicz M, Bielecki S (2002) Factors affecting the yield and properties of bacterial cellulose. J Ind Microbiol Biotech 29:189–195. https://doi.org/10.1038/sj.jim.7000303
Lee H, Zhao X (1999) Effects of mixing conditions on the production of microbial cellulose by Acetobacter xylinum. Biotechnol Bioprocess Eng 4:41–45. https://doi.org/10.1007/bf02931912
Mihranyan A, Llagostera AP, Karmhag R, Strømme M, Ek R (2004) Moisture sorption by cellulose powders of varying crystallinity. Int J Pharm 269:433–442. https://doi.org/10.1016/j.ijpharm.2003.09.030
Mohammadkazemi F, Azin M, Ashori A (2015) Production of bacterial cellulose using different carbon sources and culture media. Carbohydr Polym 117:518–523. https://doi.org/10.1016/j.carbpol.2014.10.008
Mohite B, Salunke B, Patil S (2013) Enhanced production ofbacterial cellulose by using Gluconacetobacter hansenii NCIM 2529 strain under shaking conditions. Appl Biochem Biotechnol 169:1497–1511. https://doi.org/10.1007/s12010-013-0092-7
Morgan JLW, Strumillo J, Zimmer J (2013) Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493:181–186. https://doi.org/10.1038/nature11744
Nakagaito AN, Iwamoto S, Yano H (2005) Bacterial cellulose: the ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl Phys A 80:93–97. https://doi.org/10.1007/s00339-004-2932-3
Nguyen VT, Gidley MJ, Dykes GA (2008) Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol 25:471–478. https://doi.org/10.1016/j.fm.2008.01.004
Omran A, Ahearn G, Bowers D, Swenson J, Coughlin C (2013) Metabolic effects of sucralose on environmental bacteria. J Toxicol 2013:6. https://doi.org/10.1155/2013/372986
Ross P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55:35–58
Ruka DR, Simon GP, Dean KM (2012) Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr Polym 89:613–622. https://doi.org/10.1016/j.carbpol.2012.03.059
Schramm M, Gromet Z, Hestrin S (1957) Synthesis of cellulose by Acetobacter xylinum. 3. Substrates and inhibitors. Biochem J 67:669–679
Tokoh C, Takabe K, Fujita M, Saiki H (1998) Cellulose synthesized by Acetobacter xylinum in the presence of acetyl glucomannan. Cellulose 5:249–261. https://doi.org/10.1023/a:1009211927183
Tsekos I (1999) The sites of cellulose synthesis in algae: diversity and evolution of cellulose-synthesizing enzyme complexes. J Phycol 35:635–655. https://doi.org/10.1046/j.1529-8817.1999.3540635.x
Watanabe K, Tabuchi M, Morinaga Y, Yoshinaga F (1998) Structural features andproperties of bacterial cellulose produced in agitated culture. Cellulose 5:187–200. https://doi.org/10.1023/a:1009272904582
Yamada Y, Yukphan P, Lan Vu HT, Muramatsu Y, Ochaikul D, Tanasupawat S, Nakagawa Y (2012) Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J Gen Appl Microbiol 58:397–404. https://doi.org/10.2323/jgam.58.397
Yamamoto H, Horii F, Hirai A (1996) In situ crystallization of bacterial cellulose II. Influences of different polymeric additives on the formation of celluloses Iα and Iβ at the early stage of incubation. Cellulose 3:229–242. https://doi.org/10.1007/bf02228804
Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S, Nishi Y, Uryu M (1989) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 24:3141–3145. https://doi.org/10.1007/bf01139032
Yoshinaga F, Tonouchi N, Watanabe K (1997) Research progress in production of bacterial cellulose by aeration and agitation culture and its application as a new industrial material. Biosci Biotechnol Biochem 61:219–224. https://doi.org/10.1271/bbb.61.219
Zhu H, Jia S, Wan T, Jia Y, Yang H, Li J, Yan L, Zhong C (2011) Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr Polym 86:1558–1564. https://doi.org/10.1016/j.carbpol.2011.06.061
Acknowledgments
The Petroleum and Petrochemical College, Chulalongkorn University was thanked for supporting. This research was funded by the Doctoral Degree Chulalongkorn University 100th Year Birthday Anniversary Scholarship, and the Ratchadapisek Sompoch Endowment Fund (2016), Chulalongkorn University (CU-59-026-AM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singhsa, P., Narain, R. & Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 25, 1571–1581 (2018). https://doi.org/10.1007/s10570-018-1699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1699-1